Co-Clinical Imaging Metadata Information (CIMI) for Cancer Research to Promote Open Science, Standardization, and Reproducibility in Preclinical Imaging
Abstract
:1. Introduction
2. Methods
2.1. Design of the Survey
2.2. DICOM Viewing Tool
3. Results
3.1. Animal Identification
3.2. Oncology Animal Models
3.3. Animal Feeding, Environment, and Housing
3.4. Protocol Items
3.5. Imaging Related Metadata
3.6. DICOM Analysis Tool
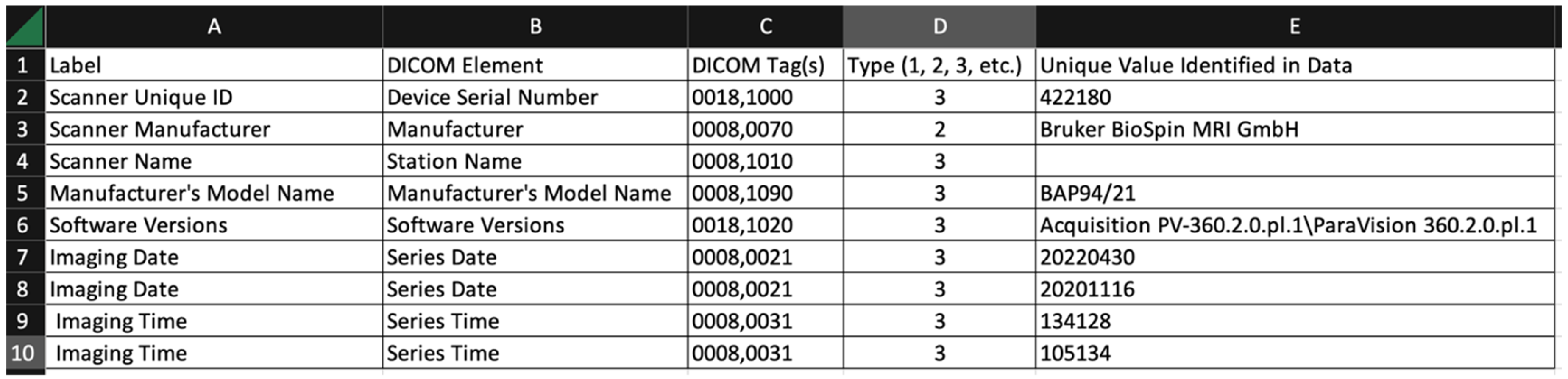
- Label: arbitrary string defined by the user of the tool. The user might choose the strings used in the survey, or might choose different strings for a different task;
- DICOM element: the name of the DICOM element (from the DICOM standard);
- DICOM tag(s): one or more tags used to extract data from the DICOM file. Multiple tags are needed when the item is encoded in a DICOM sequence;
- Type (1, 2, 3, etc.): This is the DICOM data element type. The data element type of an attribute of an information object definition or an attribute of a SOP class definition is used to specify whether that attribute is mandatory or optional. The data element type also indicates whether an attribute is conditional (only mandatory under certain conditions);
- Unique value identified in data: The software uses a simple scheme to report only unique values in this summary output. The tool does not repeat values that have been identified over multiple image sets.
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hormuth, D.A., 2nd; Sorace, A.G.; Virostko, J.; Abramson, R.G.; Bhujwalla, Z.M.; Enriquez-Navas, P.; Gillies, R.; Hazle, J.D.; Mason, R.P.; Quarles, C.C.; et al. Translating preclinical MRI methods to clinical oncology. J. Magn. Reson. Imaging 2019, 50, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Van de Donk, P.P.; Oosting, S.F.; Knapen, D.G.; van der Wekken, A.J.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Groot, D.A.; de Vries, E.G. Molecular imaging to support cancer immunotherapy. J. Immunother. Cancer 2022, 10, e004949. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Venkatraman, S.; Myint, K.Z.; Janvilisri, T.; Wongprasert, K.; Kumkate, S.; Bates, D.O.; Tohtong, R. Co-Clinical Trials: An Innovative Drug Development Platform for Cholangiocarcinoma. Pharmaceuticals 2021, 14, 51. [Google Scholar] [CrossRef]
- Clohessy, J.G.; Pandolfi, P.P. Mouse hospital and co-clinical trial project—From bench to bedside. Nat. Rev. Clin. Oncol. 2015, 12, 491–498. [Google Scholar] [CrossRef]
- Genta, S.; Coburn, B.; Cescon, D.W.; Spreafico, A. Patient-derived cancer models: Valuable platforms for anticancer drug testing. Front. Oncol. 2022, 12, 976065. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Samsa, G.; Samsa, L. A Guide to Reproducibility in Preclinical Research. Acad. Med. 2019, 94, 47–52. [Google Scholar] [CrossRef]
- McDougald, W.; Vanhove, C.; Lehnert, A.; Lewellen, B.; Wright, J.; Mingarelli, M.; Corral, C.; Schneider, J.; Plein, S.; Newby, D.; et al. Standardization of preclinical PET/CT imaging to improve quantitative accuracy, precision and reproducibility: A multi-center study. J. Nucl. Med. 2019, 61, 461–468. [Google Scholar] [CrossRef]
- Workman, P.; Aboagye, E.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef]
- Workman, P.; Balmain, A.; Hickman, J.A.; McNally, N.J.; Rohas, A.M.; Mitchison, N.A.; Pierrepoint, C.G.; Raymond, R.; Rowlatt, C.; Stephens, T.C.; et al. UKCCCR guidelines for the welfare of animals in experimental neoplasia. Lab. Anim. 1988, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Meehan, T.F.; Conte, N.; Goldstein, T.; Inghirami, G.; Murakami, M.A.; Brabetz, S.; Gu, Z.; Wiser, J.A.; Dunn, P.; Begley, D.A.; et al. PDX-MI: Minimal Information for Patient-Derived Tumor Xenograft Models. Cancer Res. 2017, 77, e62–e66. [Google Scholar] [CrossRef]
- Shoghi, K.I.; Badea, C.T.; Blocker, S.J.; Chenevert, T.L.; Laforest, R.; Lewis, M.T.; Luker, G.D.; Manning, H.C.; Marcus, D.S.; Mowery, Y.M.; et al. Co-Clinical Imaging Resource Program (CIRP): Bridging the Translational Divide to Advance Precision Medicine. Tomography 2020, 6, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Kalen, J.D.; Clunie, D.A.; Liu, Y.; Tatum, J.L.; Jacobs, P.M.; Kirby, J.; Freymann, J.B.; Wagner, U.; Smith, K.E.; Suloway, C.; et al. Design and Implementation of the Pre-Clinical DICOM Standard in Multi-Cohort Murine Studies. Tomography 2021, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- The Co-Clinical Imaging Research Resources Program. 8 May. Available online: https://ncihub.cancer.gov/groups/cirphub (accessed on 3 May 2023).
- Tseng, J.R.; Dandekar, M.; Subbarayan, M.; Cheng, Z.; Park, J.M.; Louie, S.; Gambhir, S.S. Reproducibility of 3′-deoxy-3′-(18)F-fluorothymidine microPET studies in tumor xenografts in mice. J. Nucl. Med. 2005, 46, 1851–1857. [Google Scholar] [PubMed]
- Dandekar, M.; Tseng, J.R.; Gambhir, S.S. Reproducibility of 18F-FDG microPET studies in mouse tumor xenografts. J. Nucl. Med. 2007, 48, 602–607. [Google Scholar] [CrossRef]
- Fueger, B.J.; Czernin, J.; Hildebrandt, I.; Tran, C.; Halpern, B.S.; Stout, D.; Phelps, M.E.; Weber, W.A. Impact of animal handling on the results of 18F-FDG PET studies in mice. J. Nucl. Med. 2006, 47, 999–1006. [Google Scholar]
- Hartley, C.J.; Reddy, A.K.; Madala, S.; Michael, L.H.; Entman, M.L.; Taffet, G.E. Effects of isoflurane on coronary blood flow velocity in young, old and ApoE(-/-) mice measured by Doppler ultrasound. Ultrasound Med. Biol. 2007, 33, 512–521. [Google Scholar] [CrossRef]
- Hildebrandt, I.J.; Su, H.; Weber, W.A. Anesthesia and other considerations for in vivo imaging of small animals. Ilar J. 2008, 49, 17–26. [Google Scholar] [CrossRef]
- Kober, F.; Iltis, I.; Cozzone, P.J.; Bernard, M. Cine-MRI assessment of cardiac function in mice anesthetized with ketamine/xylazine and isoflurane. Magma 2004, 17, 157–161. [Google Scholar] [CrossRef]
- Kober, F.; Iltis, I.; Cozzone, P.J.; Bernard, M. Myocardial blood flow mapping in mice using high-resolution spin labeling magnetic resonance imaging: Influence of ketamine/xylazine and isoflurane anesthesia. Magn. Reson. Med. 2005, 53, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiao, H.; Frank, R.; Eucker, S.; Lu, M.; Huang, B.; Armstead, W.; Margulies, S.; Ferrari, V.; Epstein, J.; et al. Endothelial Progenitor Cells Mediated Improvements in Post-Infarct Left Ventricular Myocardial Blood Flow Estimated by Spin Label CMR. Circulation 2010, 122, A20415. [Google Scholar]
- Kress, G.J.; Liao, F.; Dimitry, J.; Cedeno, M.R.; FitzGerald, G.A.; Holtzman, D.M.; Musiek, E.S. Regulation of amyloid-β dynamics and pathology by the circadian clock. J. Exp. Med. 2018, 215, 1059–1068. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Grant, G.R.; Paschos, G.; Song, W.L.; Musiek, E.S.; Lee, V.; McLoughlin, S.C.; Grosser, T.; Cotsarelis, G.; et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 2016, 8, 324ra316. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, J.K.; Ramachandran, D.; He, Y.; Palmer, C.; Jurczak, M.J.; Li, B.; Friedline, R.H.; Kim, J.K.; Ramsey, J.J.; Lantier, L.; et al. A big-data approach to understanding metabolic rate and response to obesity in laboratory mice. eLife 2020, 9, e53560. [Google Scholar] [CrossRef] [PubMed]
- Preclinical Small Animal Imaging Acquisition Context. Available online: https://www.dicomstandard.org/news/supplements/view/preclinical-small-animal-imaging-acquisition-context (accessed on 3 May 2023).
- Gammon, S.T.; Cohen, A.S.; Lehnert, A.L.; Sullivan, D.C.; Malyarenko, D.; Manning, H.C.; Hormuth, D.A.; Daldrup-Link, H.E.; An, H.; Quirk, J.D.; et al. An Online Repository for Pre-clinical Imaging Protocols (PIPs). Tomography 2023, 9, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Quantitative Imaging Biomarkers Alliance (QIBA). Available online: https://qibawiki.rsna.org/index.php/Profiles (accessed on 3 May 2023).
- Pre-Clinical Imaging Protocols. Available online: https://www.protocols.io/workspaces/pre-clinical-imaging-protocols (accessed on 3 May 2023).
- Malyarenko, D.; Amouzandeh, G.; Pickup, S.; Zhou, R.; Manning, H.C.; Gammon, S.T.; Shoghi, K.I.; Quirk, J.D.; Sriram, R.; Larson, P.; et al. Evaluation of ADC Repeatability and Reproducibility of Pre-Clinical MRIs Using Standardized Procedures and DWI Phantom. Tomography 2023, 9, 375–386. [Google Scholar] [CrossRef]
- Pydicom. Available online: https://pydicom.github.io/ (accessed on 3 May 2023).
- Pemmaraju, R.; Minahan, R.; Wang, E.; Schadl, K.; Daldrup-Link, H.; Habte, F. Web-Based Application for Biomedical Image Registry, Analysis, and Translation (BiRAT). Tomography 2022, 8, 1453–1462. [Google Scholar] [CrossRef]
- Kain, M.; Bodin, M.; Loury, S.; Chi, Y.; Louis, J.; Simon, M.; Lamy, J.; Barillot, C.; Dojat, M. Small Animal Shanoir (SAS) A Cloud-Based Solution for Managing Preclinical MR Brain Imaging Studies. Front. Neuroinform. 2020, 14, 20. [Google Scholar] [CrossRef]
- Persoon, L.; Hoof, S.V.; van der Kruijssen, F.; Granton, P.; Sanchez Rivero, A.; Beunk, H.; Dubois, L.; Doosje, J.W.; Verhaegen, F. A novel data management platform to improve image-guided precision preclinical biological research. Br. J. Radiol. 2019, 92, 20180455. [Google Scholar] [CrossRef]
- Zullino, S.; Paglialonga, A.; Dastru, W.; Longo, D.L.; Aime, S. XNAT-PIC: Extending XNAT to Preclinical Imaging Centers. J. Digit. Imaging 2022, 35, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.S.; Olsen, T.R.; Ramaratnam, M.; Buckner, R.L. The Extensible Neuroimaging Archive Toolkit: An informatics platform for managing, exploring, and sharing neuroimaging data. Neuroinformatics 2007, 5, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Herrick, R.; Horton, W.; Olsen, T.; McKay, M.; Archie, K.A.; Marcus, D.S. XNAT Central: Open sourcing imaging research data. Neuroimage 2016, 124, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Longabaugh, W.J.R.; Pot, D.; Clunie, D.A.; Pieper, S.; Aerts, H.; Homeyer, A.; Lewis, R.; Akbarzadeh, A.; Bontempi, D.; et al. NCI Imaging Data Commons. Cancer Res. 2021, 81, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Chen, B.; Holmes, D., III; Duan, X.; Yu, Z.; Yu, L.; Leng, S.; Fletcher, J. Data from Low Dose CT Image and Projection Data [Data set]. Cancer Imaging Arch. 2020. Available online: https://wiki.cancerimagingarchive.net/download/attachments/52758026/DICOM-CT-PD%20User%20Manual_Version%203.pdf?version=1&modificationDate=1586187875073&api=v2 (accessed on 3 May 2023).

| Category | Survey Items | Responders |
|---|---|---|
| Animal Identification | 11 | 9 |
| Animal Model | 12 | 9 |
| Animal Feeding | 8 | 9 |
| Environmental/Housing | 52 | 9 |
| Protocol Items | 6 | 9 |
| Imaging Common | 7 | 14 |
| MR Imaging | 109 | 8 |
| PET Imaging | 98 | 4 |
| CT Imaging | 80 | 2 |
| Element | Assessment | DICOM Tag | Definition |
|---|---|---|---|
| Patient ID | Essential | 0010,0020 | Primary identifier for the subject. Note: In the case of imaging a group of small animals simultaneously, the single value of this identifier corresponds to the identification of the entire group. |
| Patient’s Sex | Essential | 0010,0040 | Sex of the named subject. |
| Patient’s Birth Date | Essential | 0010,0030 | Date of birth of the named subject. |
| Patient’s Age | Recommended | 0010,1010 | Age of the subject. The DICOM representation for this field includes units (days, weeks, months, and years), and can also be computed from the date of birth and any time point |
| Patient’s Weight | Essential | 0010,1030 | Weight of the subject, in kilograms. |
| Patient Species Description | Essential | 0010,2201 | The taxonomic rank value (e.g., genus, subgenus, species, and subspecies) of the patient |
| Strain Description | Essential | 0010,0212 | The strain of the subject. |
| Strain Nomenclature | Essential | 0010,0213 | The nomenclature used for the strain description (0010,0212) |
| Strain Source | Essential | 0010,0218 > 0010,0217 | Identification of the organization that is the source of the animal |
| Strain Stock Number | Essential | 0010,0214 | Strain stock ID at the source. |
| Mouse Strain for Humanized Immune System | Essential | Background of the mouse strain used for a humanized system (for example, NSG). | |
| Type of Humanization | Essential | Method of humanization—for example, introduction of human CD34+ or PBMC cells. | |
| Litter ID | Recommended | Identification of the mouse’s litter. | |
| Date Weaned | Recommended | Date on which the mouse pup was weaned. |
| Subcategory | Element | Assessment | Comments |
|---|---|---|---|
| PDX | PDX source | Essential | DICOM TID 8182 Exogenous Substance Administration → Brand Name |
| PDX | PDX ID/stock number | Essential | PDX stock number or identification (ID) at the source |
| PDX | Tumor (PDX) passage number | Essential | The passage number of the tumor implanted to generate PDX |
| PDX | Tumor (PDX) passage method | Essential | How tumors were passaged |
| PDX | PDX storage/retrieval/archive Information | Recommended | Method of PDX storage |
| PDX | Tumor implantation method | Essential | Cell suspension or tissue implantation |
| PDX | Number of cells injected if in suspension | Essential | DICOM TID 8182 Exogenous Substance Administration → Usage/Exposure Qualitative Concept |
| PDX | Implant date | Essential | DICOM TID 8182 Exogenous Substance Administration → DateTime Started |
| PDX | Implant site | Essential | DICOM TID 8182 Exogenous Substance Administration → Route of administration → Site of |
| Cell Line | Cell line source | Essential | DICOM TID 8182 Exogenous Substance Administration → Brand Name |
| Cell Line | Cell line ID/Stock number | Essential | Cell line stock number or identification (ID) at the source |
| Cell Line | Cell line name | Essential | DICOM TID 8182 Exogenous Substance Administration → Brand Name |
| Cell Line | Injected site | Essential | DICOM TID 8182 Exogenous Substance Administration → Route of administration → Site of |
| Cell Line | Number of cells injected | Essential | DICOM TID 8182 Exogenous Substance Administration → Usage/Exposure Qualitative Concept |
| GEMM | Mouse name | Essential | Based on International Committee on Standardized Genetic Nomenclature http://www.informatics.jax.org/mgihome/nomen/ (accessed on 3 May 2023) |
| GEMM | Mouse name at source (if different from standard nomenclature) | Essential | Mouse name or reference at source if different from standard nomenclature. |
| GEMM | Source/vendor | Essential | DICOM TID 8182 Exogenous Substance Administration → Brand Name |
| GEMM | Stock/ID number | Essential | Stock number of ID of GEMM at source |
| Element | Assessment | Definition |
|---|---|---|
| Animal Feed | Recommended | The DICOM Animal Feed Type (CID 607) defines 5 coded values with these definitions (codes omitted) or equivalents:
|
| Feed Source | Recommended | The DICOM Animal Feed Source (CID 608) defines 2 coded values with these definitions (codes omitted):
|
| Feed Manufacturer | Essential | Free text item in a DICOM structured report. |
| Feed Product Name | Essential | Free text item in a DICOM structured report. |
| Feed Product Code | Recommended | Free text item in a DICOM structured report:
|
| Feeding Method | Essential | The DICOM Animal Feeding Method (CID 609) defines 4 coded values with these definitions (codes omitted):
|
| Water Types | Recommended | The DICOM Animal Feeding Method (CID 610) defines coded values with these definitions (codes omitted):
|
| Water Delivery | Essential | The DICOM Animal Feeding Method (CID 609) defines 4 coded values with these definitions (codes omitted):
|
| Element | Assessment | Comments |
|---|---|---|
| Number of Animals Within Same Housing Unit | Recommended | Number |
| Sex of Animals Within Same Housing Unit | Recommended | Code |
| Environmental Temperature | Recommended | Number |
| Housing Humidity (%) | Recommended | Number |
| Heating Conditions | Recommended | DICOM can encode this general topic using the specific items below. |
| Procedure Phase | Essential | Coded values including, but not limited to: preoperative, intraoperative, and postoperative |
| Heating/Heating Method | Essential | The DICOM Heating Method (CID 635) defines 14 values, such as electric blanket, forced air heater, heat lamp, and unheated. |
| Feedback Temperature Regulation | Essential | Yes/No |
| Temperature Sensor Device Component | Recommended | The DICOM Temperature Sensor Device Component Type for Small Animal Procedure (CID 636) defines 3 coded values with these definitions (codes omitted):
|
| Element | Assessment | Comments |
|---|---|---|
| Drug treatment, if relevant | Essential | DICOM Drugs/Contrast Administered (TID 3106) supports the recording of drugs administered in general, including the drug, dose, and route of administration. Medication, substance, and environmental exposure (TID 9002) are more specific to the context of image acquisition. |
| Treatment protocol | Essential | |
| Fasting; fasting duration | Essential | Included in DICOM Imaging Agent Administration Patient Characteristics (TID 10024). Not specific to small animal imaging. |
| Anesthesia used | Essential | DICOM Medication for Small Animal Anesthesia (CID 623) defines 43 coded values. |
| Route of anesthesia administration | Essential | DICOM Anesthesia Induction Code Type for Small Animal Anesthesia (CID 613) defines 5 coded values with these definitions:
|
| Chronobiology | Essential | DICOM Circadian Effects (TID 8150) include 3 distinct concepts to express this information:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, S.M.; Quirk, J.D.; Lassiter, A.W.; Laforest, R.; Ayers, G.D.; Badea, C.T.; Fedorov, A.Y.; Kinahan, P.E.; Holbrook, M.; Larson, P.E.Z.; et al. Co-Clinical Imaging Metadata Information (CIMI) for Cancer Research to Promote Open Science, Standardization, and Reproducibility in Preclinical Imaging. Tomography 2023, 9, 995-1009. https://doi.org/10.3390/tomography9030081
Moore SM, Quirk JD, Lassiter AW, Laforest R, Ayers GD, Badea CT, Fedorov AY, Kinahan PE, Holbrook M, Larson PEZ, et al. Co-Clinical Imaging Metadata Information (CIMI) for Cancer Research to Promote Open Science, Standardization, and Reproducibility in Preclinical Imaging. Tomography. 2023; 9(3):995-1009. https://doi.org/10.3390/tomography9030081
Chicago/Turabian StyleMoore, Stephen M., James D. Quirk, Andrew W. Lassiter, Richard Laforest, Gregory D. Ayers, Cristian T. Badea, Andriy Y. Fedorov, Paul E. Kinahan, Matthew Holbrook, Peder E. Z. Larson, and et al. 2023. "Co-Clinical Imaging Metadata Information (CIMI) for Cancer Research to Promote Open Science, Standardization, and Reproducibility in Preclinical Imaging" Tomography 9, no. 3: 995-1009. https://doi.org/10.3390/tomography9030081
APA StyleMoore, S. M., Quirk, J. D., Lassiter, A. W., Laforest, R., Ayers, G. D., Badea, C. T., Fedorov, A. Y., Kinahan, P. E., Holbrook, M., Larson, P. E. Z., Sriram, R., Chenevert, T. L., Malyarenko, D., Kurhanewicz, J., Houghton, A. M., Ross, B. D., Pickup, S., Gee, J. C., Zhou, R., ... Shoghi, K. I. (2023). Co-Clinical Imaging Metadata Information (CIMI) for Cancer Research to Promote Open Science, Standardization, and Reproducibility in Preclinical Imaging. Tomography, 9(3), 995-1009. https://doi.org/10.3390/tomography9030081









