Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes
Abstract
:1. Introduction
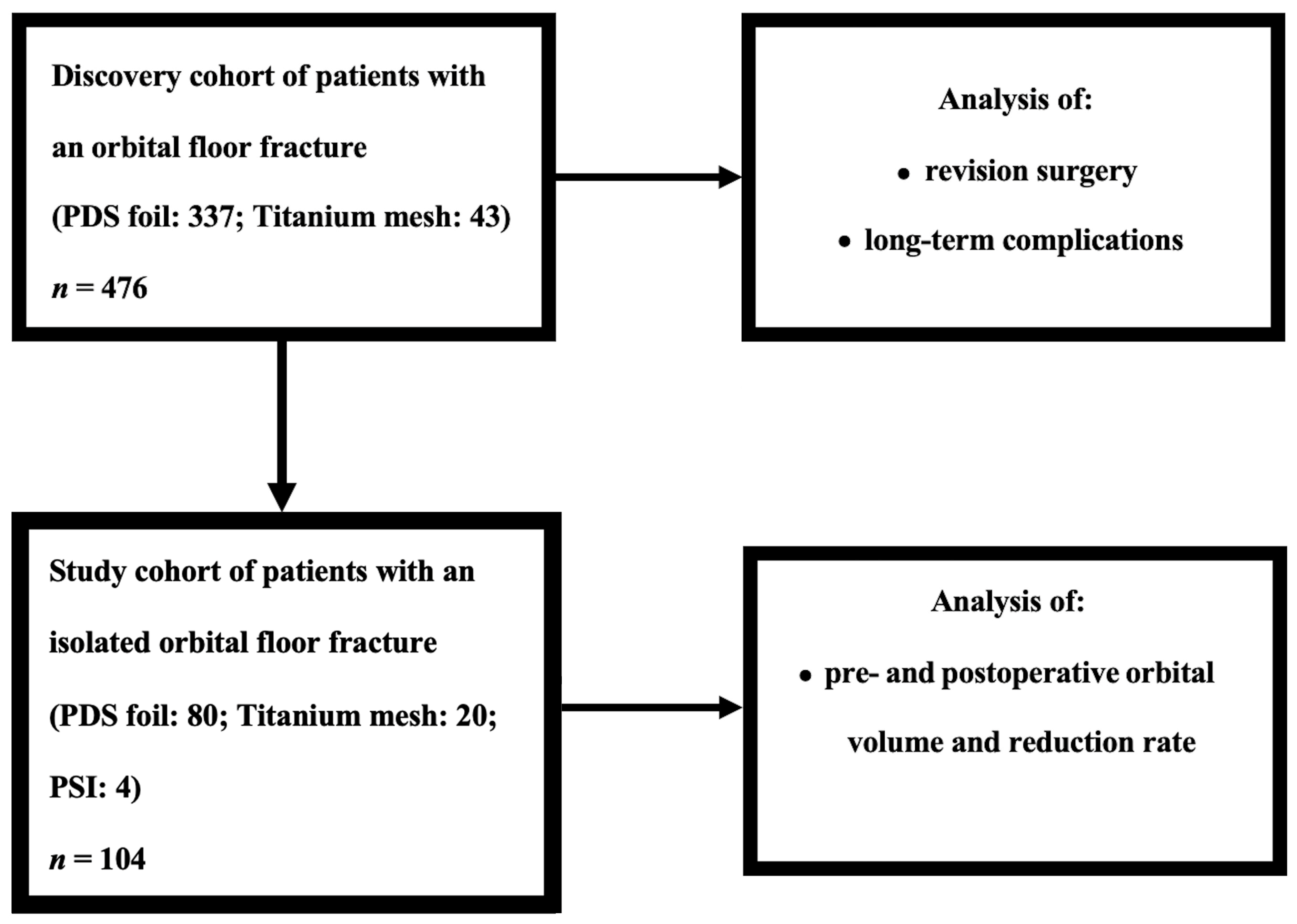
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. 3D Model Preparation
2.3. Orbital Volume Measurement
2.4. Statistical Analysis
3. Results
3.1. Characterization of the Patient Cohorts
3.2. Clinical Outcome Parameters in Relation to Distinct Reconstructive Materials within the Discovery Cohort
3.3. Orbital Volume Analysis in the Study Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, L.; Hatton, M.P.; Rubin, P.A. When is enophthalmos “significant”? Ophthalmic Plast. Reconstr. Surg. 2006, 22, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Burnstine, M.A. Clinical recommendations for repair of isolated orbital floor fractures: An evidence-based analysis. Ophthalmology 2002, 109, 1207–1210; discussion 1210-1; quiz 1212-3. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Shokri, T.; Ziai, K.; Lighthall, J.G. Controversies and Contemporary Management of Orbital Floor Fractures. Craniomaxillofac Trauma Reconstr. 2022, 15, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Bourry, M.; Hardouin, J.B.; Fauvel, F.; Corre, P.; Lebranchu, P.; Bertin, H. Clinical evaluation of the efficacy of materials used for primary reconstruction of orbital floor defects: Meta-analysis. Head Neck 2021, 43, 679–690. [Google Scholar] [CrossRef]
- Choi, J.; Lorenz, H.P.; Spain, D.A. Review of facial trauma management. J. Trauma Acute Care Surg. 2020, 88, e124–e130. [Google Scholar] [CrossRef]
- Gart, M.S.; Gosain, A.K. Evidence-based medicine: Orbital floor fractures. Plast. Reconstr. Surg. 2014, 134, 1345–1355. [Google Scholar] [CrossRef]
- Whitehouse, R.W.; Batterbury, M.; Jackson, A.; Noble, J.L. Prediction of enophthalmos by computed tomography after ‘blow out’ orbital fracture. Br. J. Ophthalmol. 1994, 78, 618–620. [Google Scholar] [CrossRef]
- Fan, X.; Li, J.; Zhu, J.; Li, H.; Zhang, D. Computer-assisted orbital volume measurement in the surgical correction of late enophthalmos caused by blowout fractures. Ophthalmic Plast. Reconstr. Surg. 2003, 19, 207–211. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Kalantar Motamedi, M.H.; Rasouli, H.R.; Naghdi, N. Enophthalmos and Orbital Volume Changes in Zygomaticomaxillary Complex Fractures: Is There a Correlation Between Them? J. Oral Maxillofac. Surg. 2019, 77, 134.e1–134.e9. [Google Scholar] [CrossRef]
- Holtmann, H.; Eren, H.; Sander, K.; Kübler, N.R.; Handschel, J. Orbital floor fractures--short- and intermediate-term complications depending on treatment procedures. Head Face Med. 2016, 12, 1. [Google Scholar] [CrossRef]
- Totir, M.; Ciuluvica, R.; Dinu, I.; Careba, I.; Gradinaru, S. Biomaterials for orbital fractures repair. J. Med. Life 2015, 8, 41–43. [Google Scholar] [PubMed]
- Mok, D.; Lessard, L.; Cordoba, C.; Harris, P.G.; Nikolis, A. A review of materials currently used in orbital floor reconstruction. Can. J. Plast. Surg. 2004, 12, 134–140. [Google Scholar] [CrossRef]
- Potter, J.K.; Malmquist, M.; Ellis, E., 3rd. Biomaterials for reconstruction of the internal orbit. Oral Maxillofac. Surg. Clin. 2012, 24, 609–627. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Tan, Y. Assessment of internal orbital reconstructions for pure blowout fractures: Cranial bone grafts versus titanium mesh. J. Oral Maxillofac. Surg. 2003, 61, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Messo, E. Use of nonresorbable alloplastic implants for internal orbital reconstruction. J. Oral Maxillofac. Surg. 2004, 62, 873–881. [Google Scholar] [CrossRef]
- Seifert, L.B.; Mainka, T.; Herrera-Vizcaino, C.; Verboket, R.; Sader, R. Orbital floor fractures: Epidemiology and outcomes of 1594 reconstructions. Eur. J. Trauma. Emerg. Surg. 2022, 48, 1427–1436. [Google Scholar] [CrossRef]
- Radović, P.; Janković, S.; Papović, M.; Dimitrijević, M.L.; Krasić, D. Comparison of the Fractured and Non-Fractured Orbit Before and After Surgery Using a Titanium Implant or a Resorbable Poly-d,l-lactic Acid (PDLLA) Implant: A Study from a Single Center in Niš, Serbia of 58 Patients with Unilateral Orbital Floor Fracture Using Volumetric Measurement. Med. Sci. Monit. 2023, 29, e939144. [Google Scholar] [CrossRef]
- Taxis, J.; Ungerboeck, L.; Gehrking, M.R.; Motel, C.; Wurm, M.; Eckert, A.W.; Spanier, G.; Nieberle, F.; Platz Batista da Silva, N.; Ludwig, N.; et al. Two-Dimensional Post-Traumatic Measurements of Orbital Floor Blowout Fractures Underestimate Defect Sizes Compared to Three-Dimensional Approaches. Tomography 2023, 9, 579–588. [Google Scholar] [CrossRef]
- Jaquiéry, C.; Aeppli, C.; Cornelius, P.; Palmowsky, A.; Kunz, C.; Hammer, B. Reconstruction of orbital wall defects: Critical review of 72 patients. Int. J. Oral Maxillofac. Surg. 2007, 36, 193–199. [Google Scholar] [CrossRef]
- Schönegg, D.; Wagner, M.; Schumann, P.; Essig, H.; Seifert, B.; Rücker, M.; Gander, T. Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall. J. Cranio-Maxillofac. Surg. 2018, 46, 1544–1549. [Google Scholar] [CrossRef]
- Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Avashia, Y.J.; Sastry, A.; Fan, K.L.; Mir, H.S.; Thaller, S.R. Materials used for reconstruction after orbital floor fracture. J. Craniofac. Surg. 2012, 23, 1991–1997. [Google Scholar] [CrossRef]
- Dietz, A.; Ziegler, C.M.; Dacho, A.; Althof, F.; Conradt, C.; Kolling, G.; von Boehmer, H.; Steffen, H. Effectiveness of a new perforated 0.15 mm poly-p-dioxanon-foil versus titanium-dynamic mesh in reconstruction of the orbital floor. J. Cranio-Maxillofac. Surg. 2001, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Essig, H.; Dressel, L.; Rana, M.; Rana, M.; Kokemueller, H.; Ruecker, M.; Gellrich, N.C. Precision of posttraumatic primary orbital reconstruction using individually bent titanium mesh with and without navigation: A retrospective study. Head Face Med. 2013, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.I.; Kim, H.K.; Kim, W.S.; Bae, T.H. Comparison of Absorbable Mesh Plate versus Titanium-Dynamic Mesh Plate in Reconstruction of Blow-Out Fracture: An Analysis of Long-Term Outcomes. Arch. Plast. Surg. 2014, 41, 355–361. [Google Scholar] [CrossRef]




| Category | Study Cohort |
|---|---|
| Total (n = 104) | |
| Gender: | |
| Female | 32 (30.8%) |
| Male | 72 (69.2%) |
| Age (MV in years) | 47.79 (14 to 86) |
| Cause of fracture: | |
| Rough offense | 20 (19.2%) |
| Fall | 31 (29.8%) |
| Sports accident | 11 (10.6%) |
| Traffic accident | 20 (19.2%) |
| Horse kick | 3 (2.9%) |
| Other | 19 (18.3%) |
| Surgery after fracture (MV in days) | 4.47 (0 to 22) |
| Surgery duration (MV in minutes) | 104.91 (23 to 376) |
| Classification according to Jaquiéry et al.: | |
| Class I | 1 (0.9%) |
| Class II | 68 (65.4%) |
| Class III | 35 (33.7%) |
| Supply type: | |
| PDS foil | 80 (76.9%) |
| Titanium mesh | 20 (19.2%) |
| PSI | 4 (3.8%) |
| Monocortical iliac crest | - |
| Maxillary sinus balloon | - |
| Only reduction | - |
| Untreated or refused supply | - |
| PDS foil thickness (mm): | |
| 0.15 | 39 (48.8%) |
| 0.25 | 41 (51.2%) |
| Inpatient stay (MV in days) | 11.13 (3 to 61) |
| n = 104 | PDS Foil | Titanium Mesh | ||||
|---|---|---|---|---|---|---|
| Volume (cm3) | Fold Change in Preoperative Values | Volume (cm3) | Fold Change in Preoperative Values | |||
| Preoperative | Postoperative | Preoperative | Postoperative | |||
| Mean | 30.60 | 30.09 | 0.98 | 32.65 | 29.69 | 0.91 |
| Median | 30.57 | 29.83 | 0.98 | 34.37 | 28.37 | 0.83 |
| SD | 3.75 | 4.07 | 1.14 | 4.47 | 3.76 | 0.84 |
| Minimum | 21.66 | 22.87 | 1.06 | 23.38 | 24.59 | 1.05 |
| Maximum | 44.27 | 45.16 | 1.02 | 37.99 | 37.65 | 0.99 |
| p-value | 0.0422 | 0.0056 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxis, J.; Ungerboeck, L.; Motel, C.; Eckert, A.W.; Platz Batista da Silva, N.; Nieberle, F.; Ludwig, N.; Meier, J.K.; Ettl, T.; Reichert, T.E.; et al. Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes. Tomography 2023, 9, 1515-1525. https://doi.org/10.3390/tomography9040121
Taxis J, Ungerboeck L, Motel C, Eckert AW, Platz Batista da Silva N, Nieberle F, Ludwig N, Meier JK, Ettl T, Reichert TE, et al. Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes. Tomography. 2023; 9(4):1515-1525. https://doi.org/10.3390/tomography9040121
Chicago/Turabian StyleTaxis, Juergen, Lena Ungerboeck, Constantin Motel, Alexander W. Eckert, Natascha Platz Batista da Silva, Felix Nieberle, Nils Ludwig, Johannes K. Meier, Tobias Ettl, Torsten E. Reichert, and et al. 2023. "Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes" Tomography 9, no. 4: 1515-1525. https://doi.org/10.3390/tomography9040121
APA StyleTaxis, J., Ungerboeck, L., Motel, C., Eckert, A. W., Platz Batista da Silva, N., Nieberle, F., Ludwig, N., Meier, J. K., Ettl, T., Reichert, T. E., & Spoerl, S. (2023). Thin PDS Foils Represent an Equally Favorable Restorative Material for Orbital Floor Fractures Compared to Titanium Meshes. Tomography, 9(4), 1515-1525. https://doi.org/10.3390/tomography9040121









