Measurement of Thickness at the Inferior Border of the Mandible Using Computed Tomography Images: A Retrospective Study including 300 Japanese Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
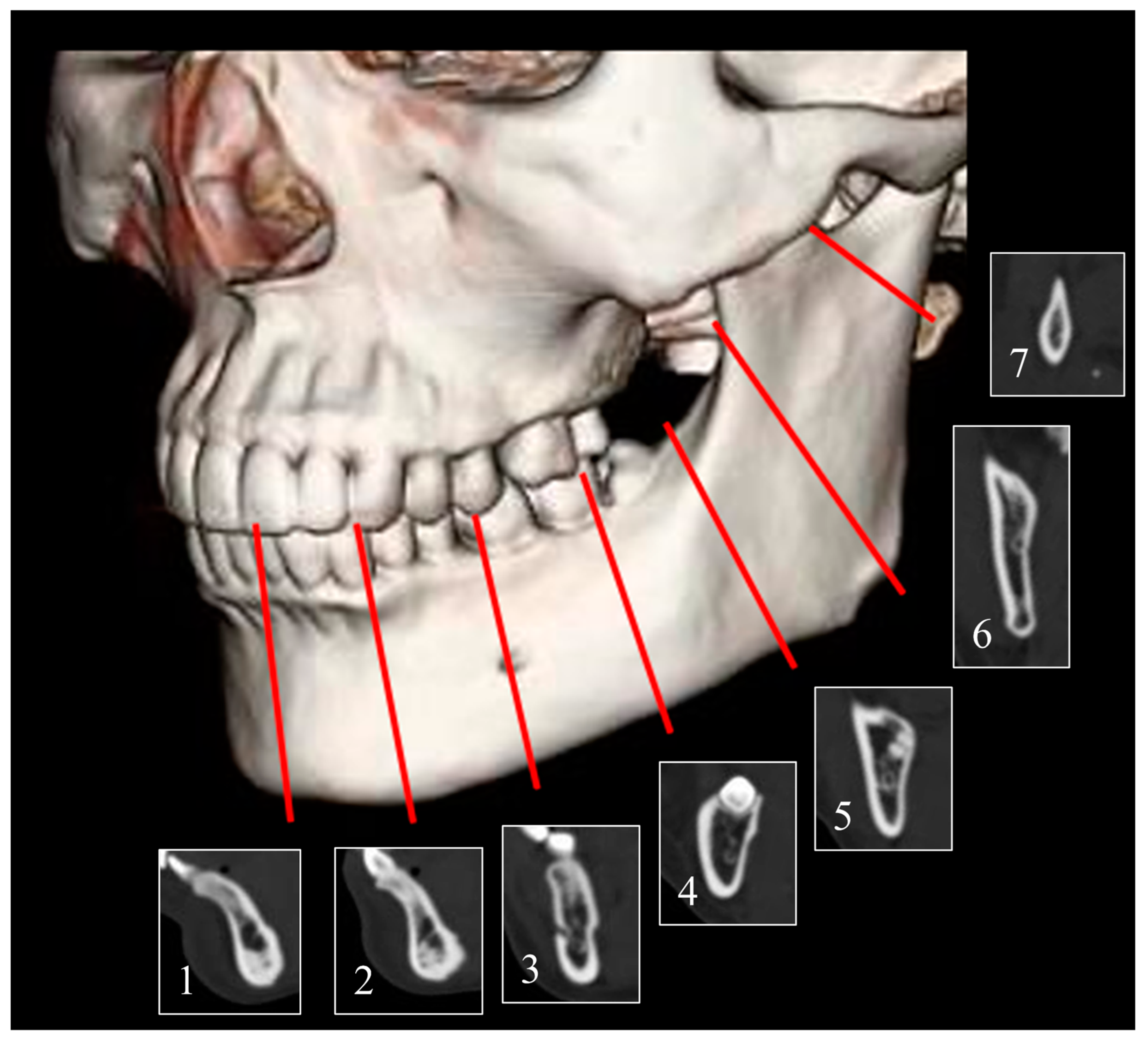
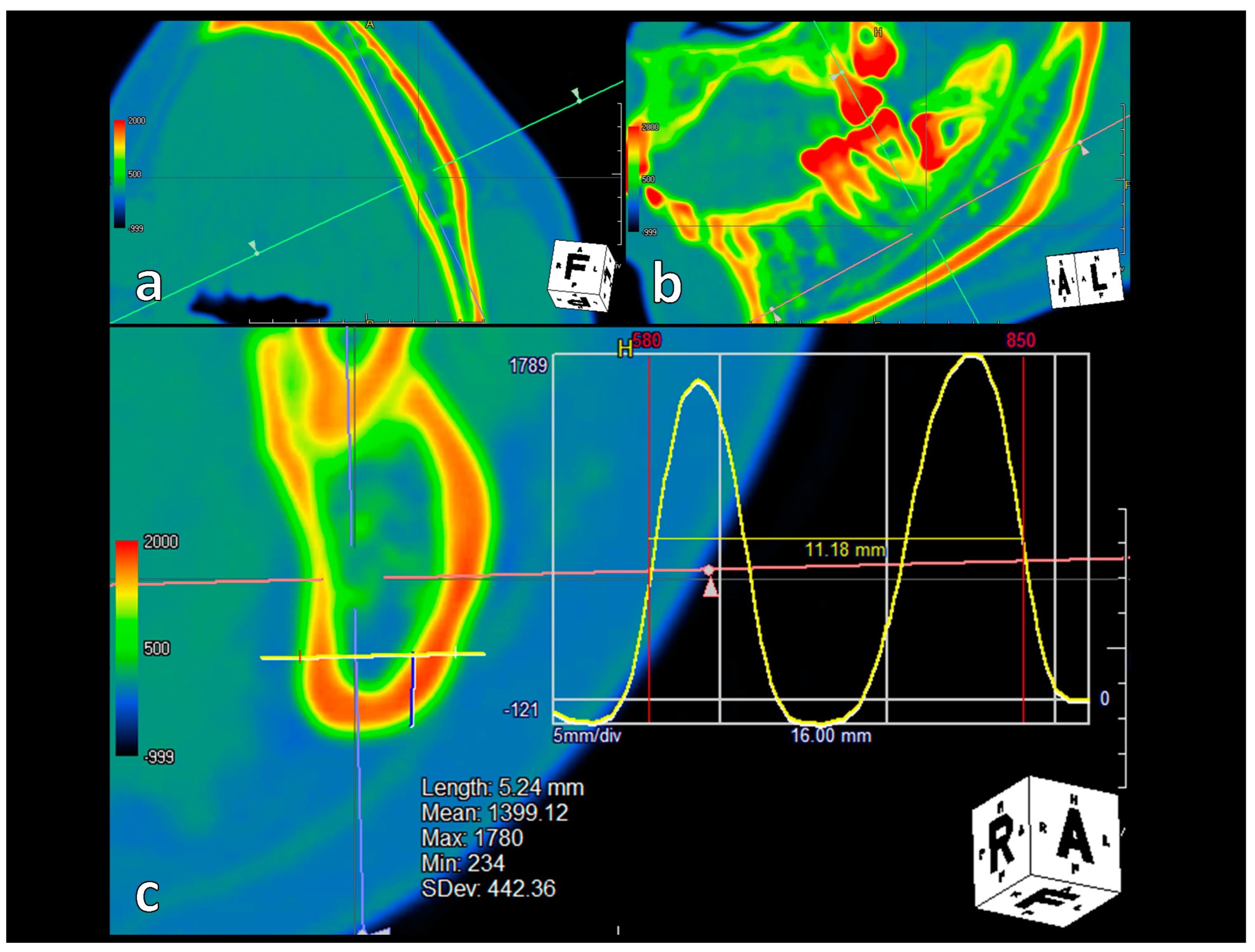
2.2. Variables
2.3. Ethical Approval
2.4. Statistical Analyses and Validation
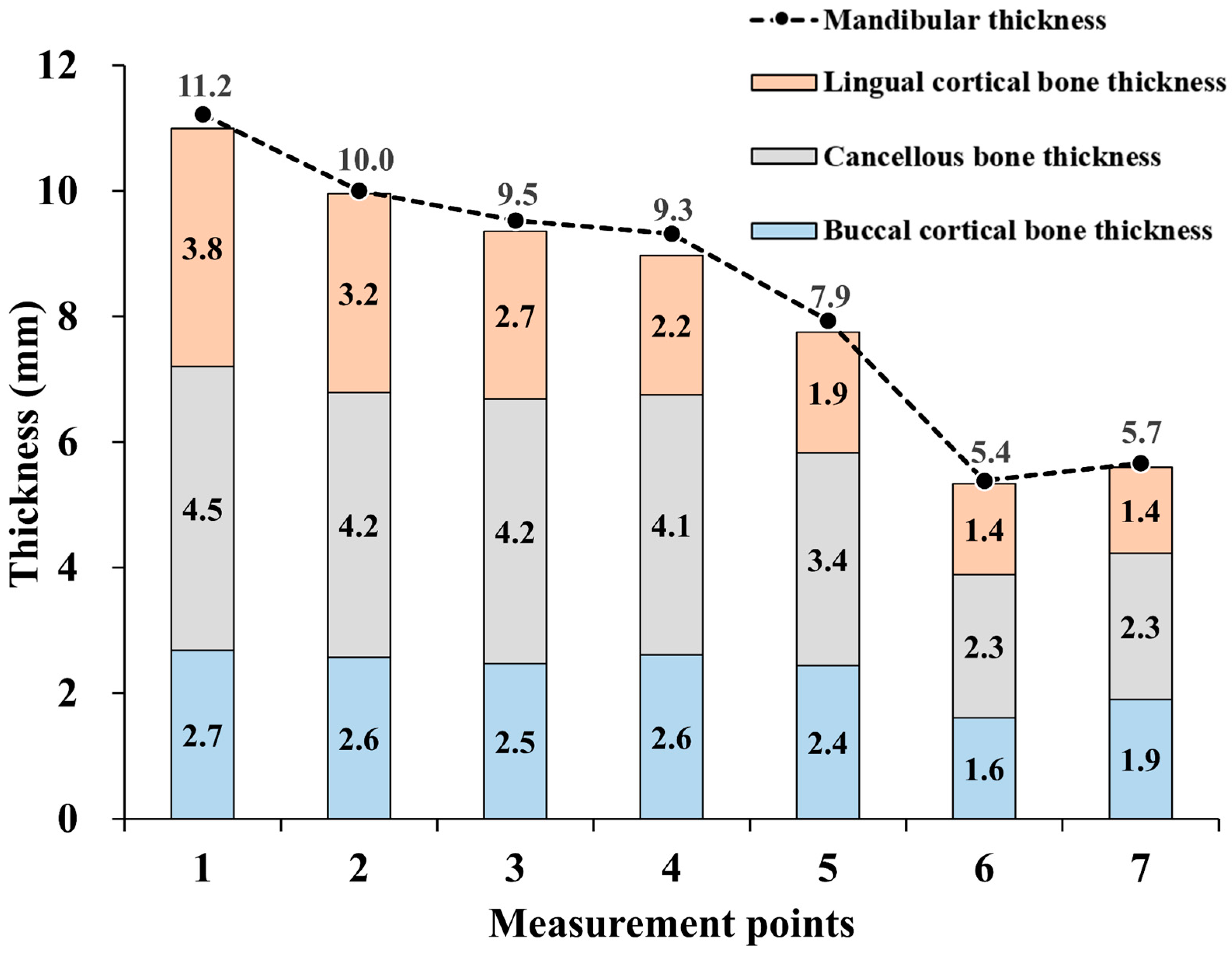
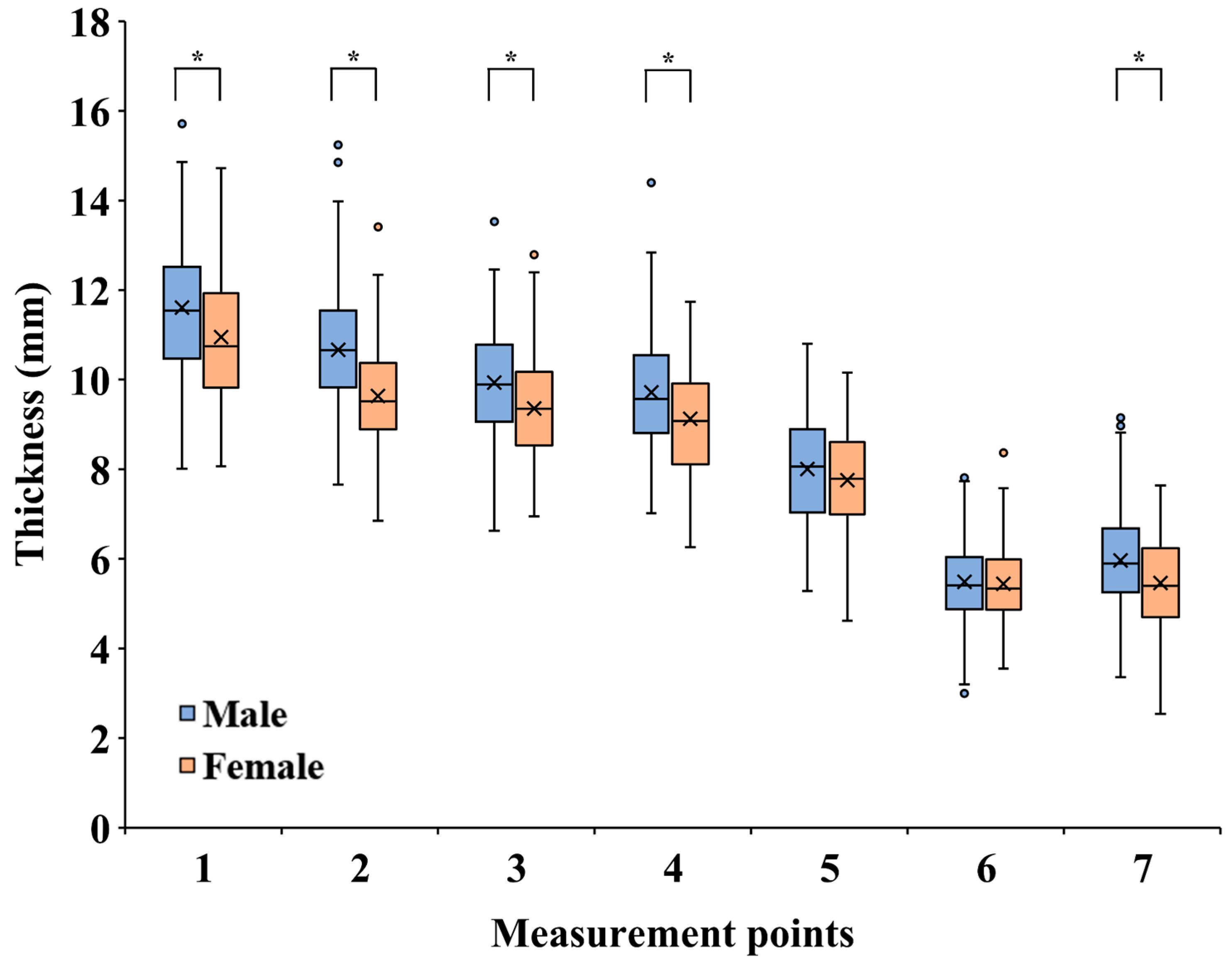
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular reconstruction: Overview. J. Maxillofac. Oral Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef]
- Pang, S.L.; Cheng, Y.T.; Choi, W.S. Immediate free flap reconstruction following the resection of benign jaw lesions: A 15-year perspective. J. Oral Maxillofac. Surg. Med. Pathol. 2023, 35, 129–134. [Google Scholar] [CrossRef]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef]
- Ueda, N.; Nakashima, C.; Aoki, K.; Shimotsuji, H.; Nakaue, K.; Yoshioka, H.; Kurokawa, S.; Imai, Y.; Kirita, T. Does inflammatory dental disease affect the development of medication-related osteonecrosis of the jaw in patients using high-dose bone-modifying agents? Clin. Oral Investig. 2021, 25, 3087–3093. [Google Scholar] [CrossRef]
- Ueda, N.; Aoki, K.; Shimotsuji, H.; Nakashima, C.; Kawakami, M.; Imai, Y.; Kirita, T. Oral risk factors associated with medication-related osteonecrosis of the jaw in patients with cancer. J. Bone Miner. Metab. 2021, 39, 623–630. [Google Scholar] [CrossRef]
- Sacco, R.; Umar, G.; Guerra, R.C.; Akintola, O. Evaluation of segmental mandibular resection without microvascular reconstruction in patients affected by medication-related osteonecrosis of the jaw: A systematic review. Br. J. Oral Maxillofac. Surg. 2021, 59, 648–660. [Google Scholar] [CrossRef]
- Oh, H.; Kwon, D.; Ahn, J.; Paeng, J.Y. Reconstruction of mandibular defects in osteoradionecrosis and medication-related osteonecrosis of the jaw using fibula free flap and management of postoperative wound infections. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 37. [Google Scholar] [CrossRef]
- Han, J.J.; Sodnom-Ish, B.; Eo, M.Y.; Kim, Y.J.; Oh, J.H.; Yang, H.J.; Kim, S.M. Accurate mandible reconstruction by mixed reality, 3D printing, and robotic-assisted navigation integration. J. Craniofacial Surg. 2022, 33, e701–e706. [Google Scholar] [CrossRef]
- Yodrabum, N.; Rudeejaroonrung, K.; Viriya, N.; Chaikangwan, I.; Kongkunnavat, N.; Tianrungroj, J.; Ongsiriporn, M.; Piyaman, P.; Puncreobutr, C. The precision of different types of plates fabricated with a computer-aided design and manufacturing system in mandibular reconstruction with fibular-free flaps. J. Craniofacial Surg. 2023, 34, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Robey, A.B.; Spann, M.L.; McAuliff, T.M.; Meza, J.L.; Hollins, R.R.; Johnson, P.J. Comparison of miniplates and reconstruction plates in fibular flap reconstruction of the mandible. Plast. Reconstr. Surg. 2008, 122, 1733–1738. [Google Scholar] [CrossRef]
- Rendenbach, C.; Steffen, C.; Hanken, H.; Schluermann, K.; Henningsen, A.; Beck-Broichsitter, B.; Kreutzer, K.; Heiland, M.; Precht, C. Complication rates and clinical outcomes of osseous free flaps: A retrospective comparison of CAD/CAM versus conventional fixation in 128 patients. Int. J. Oral Maxillofac. Surg. 2019, 48, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Swendseid, B.; Kumar, A.; Sweeny, L.; Zhan, T.; Goldman, R.A.; Krein, H.; Heffelfinger, R.N.; Luginbuhl, A.J.; Curry, J.M. Natural history and consequences of nonunion in mandibular and maxillary free flaps. Otolaryngol. Head Neck Surg. 2020, 163, 956–962. [Google Scholar] [CrossRef]
- Sobti, N.; Ahmed, K.S.; Polanco, T.; Chilov, M.; Cohen, M.A.; Boyle, J.; Shahzad, F.; Matros, E.; Nelson, J.A.; Allen, R.J., Jr. Mini-plate versus reconstruction bar fixation for oncologic mandibular reconstruction with free fibula flaps: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2691–2701. [Google Scholar] [CrossRef]
- Steffen, C.; Fischer, H.; Sauerbrey, M.; Heintzelmann, T.; Voss, J.O.; Koerdt, S.; Checa, S.; Kreutzer, K.; Heiland, M.; Rendenbach, C. Increased rate of pseudarthrosis in the anterior intersegmental gap after mandibular reconstruction with fibula free flaps: A volumetric analysis. Dentomaxillofacial Radiol. 2022, 51, 20220131. [Google Scholar] [CrossRef]
- Murakami, K.; Sugiura, T.; Yamamoto, K.; Kawakami, M.; Kang, Y.B.; Tsutsumi, S.; Kirita, T. Biomechanical analysis of the strength of the mandible after marginal resection. J. Oral Maxillofac. Surg. 2011, 69, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Taschieri, S.; Corbella, S.; Silnovic, A.; Francetti, L.; Messina, C.; Sconfienza, L.M.; Albano, D. Frequency and anatomic variability of the mandibular lingual foramina: A cone-beam CT study. BMC Med. Imaging 2022, 22, 12. [Google Scholar] [CrossRef]
- Huang, L.; Tang, S.; Yan, J.; Liu, Y.; Piao, Z. Three-dimensional analysis of mandible ramus morphology and transverse stability after intraoral vertical ramus osteotomy. Surg. Radiol. Anat. 2022, 44, 551–558. [Google Scholar] [CrossRef]
- Nithin 1; Ahmed, J.; Sujir, N.; Shenoy, N.; Binnal, A.; Ongole, R. Morphological assessment of TMJ spaces, mandibular condyle, and glenoid fossa using cone beam computed tomography (CBCT): A retrospective analysis. Indian J. Radiol. Imaging 2021, 31, 78–85. [Google Scholar] [CrossRef]
- Al-Kalaly, A.A.; Wong, R.W.K.; Cheung, L.K.; Purkayastha, S.K.; Schätzle, M.; Rabie, A.B.M. Evaluation of bone thickness around the mental foramen for potential fixation of a bone-borne functional appliance: A computer tomography scan study. Clin. Oral Implants Res. 2010, 21, 1288–1293. [Google Scholar] [CrossRef]
- Xie, L.; Li, T.; Chen, J.; Yin, D.; Wang, W.; Xie, Z. Cone-beam CT assessment of implant-related anatomy landmarks of the anterior mandible in a Chinese population. Surg. Radiol. Anat. 2019, 41, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, A.; Arshed, M.M.; Bagadia, R.K.; Ramanathan, M.; Tangutur, S.P. Correlation of transverse mandibular dimension with naso-pharyngeal and oro-pharyngeal airway using computed tomographic analysis—A retrospective observational study. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Shen, E.C.; Fu, E.; Peng, M.; Hsieh, Y.D.; Tu, H.P.; Fu, M.W. Bifid mandibular canals and their cortex thicknesses: A comparison study on images obtained from cone-beam and multislice computed tomography. J. Dent. Sci. 2016, 11, 170–174. [Google Scholar] [CrossRef]
- Jung, S.; Yun, H.; Chung, C.H.; Kim, K.; Chang, Y. A computed tomography-based analysis of the structure of the mandible according to age and sex. Arch. Craniofacial Surg. 2022, 23, 103–110. [Google Scholar] [CrossRef]
- Matsuda, S.; Yoshimura, H. Lingual bone thickness in the apical region of the horizontal mandibular third molar: A cross-sectional study in young Japanese. PLoS ONE 2022, 17, e0263094. [Google Scholar] [CrossRef]
- Firdoose Chintamani Subhan, N.; Awadalla AlSaleh, M.M.; Begum Syed, G.; Khair, S.U. Clinical anatomy of coronoid foramina of mandible and review of its implications in maxillofacial surgery. Surg. Radiol. Anat. 2023, 45, 445–452. [Google Scholar] [CrossRef]
- Kronseder, K.; Runte, C.; Kleinheinz, J.; Jung, S.; Dirksen, D. Distribution of bone thickness in the human mandibular ramus—A CBCT-based study. Head Face Med. 2020, 16, 13. [Google Scholar] [CrossRef]
- Beaty, N.B.; Le, T.T. Mandibular thickness measurements in young dentate adults. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 920–923. [Google Scholar] [CrossRef]
- Schwartz-Dabney, C.L.; Dechow, P.C. Variations in cortical material properties throughout the human dentate mandible. Am. J. Phys. Anthropol. 2003, 120, 252–277. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Tsai, M.T.; Fuh, L.J.; Tsai, M.J.; Wang, X.H.; Huang, H.L.; Hsu, J.T. Association between age of menopause and thickness of crestal cortical bone at dental implant site: A cross-sectional observational study. Int. J. Environ. Res. Public Health. 2020, 17, 5868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Zhao, Y.N.; Zhang, Y.Q.; Zhang, Y.; Liu, D.G. Morphologic analysis of alveolar bone in maxillary and mandibular incisors on sagittal views. Surg. Radiol. Anat. 2021, 43, 1009–1018. [Google Scholar] [CrossRef]
- Li, P.; Tang, Y.; Li, J.; Shen, L.; Tian, W.; Tang, W. Establishment of sequential software processing for a biomechanical model of mandibular reconstruction with custom-made plate. Comput. Methods Programs Biomed. 2013, 111, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Koper, D.C.; Leung, C.A.W.; Smeets, L.C.P.; Laeven, P.F.J.; Tuijthof, G.J.M.; Kessler, P.A.W.H. Topology optimization of a mandibular reconstruction plate and biomechanical validation. J. Mech. Behav. Biomed. Mater. 2021, 113, 104157. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Sun, Y.; Yang, S.; Van Dessel, J.; Lübbers, H.T.; Zhong, S.; Gu, Y.; Bila, M.; Politis, C. Preclinical study of additive manufactured plates with shortened lengths for complete mandible reconstruction: Design, biomechanics simulation, and fixation stability assessment. Comput. Biol. Med. 2021, 139, 105008. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Sellenschloh, K.; Gerbig, L.; Morlock, M.M.; Beck-Broichsitter, B.; Smeets, R.; Heiland, M.; Huber, G.; Hanken, H. CAD-CAM plates versus conventional fixation plates for primary mandibular reconstruction: A biomechanical in vitro analysis. J. Craniomaxillofac. Surg. 2017, 45, 1878–1883. [Google Scholar] [CrossRef]
- Knitschke, M.; Sonnabend, S.; Roller, F.C.; Pons-Kühnemann, J.; Schmermund, D.; Attia, S.; Streckbein, P.; Howaldt, H.P.; Böttger, S. Osseous Union after mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. patient-Specific Implants: A Retrospective Analysis of 89 Patients. Curr. Oncol. 2022, 29, 3375–3392. [Google Scholar] [CrossRef]
- Knitschke, M.; Yonan, M.; Roller, F.C.; Pons-Kühnemann, J.; Attia, S.; Howaldt, H.P.; Streckbein, P.; Böttger, S. Osseous Union after Jaw Reconstruction with Fibula-Free Flap: Conventional vs. CAD/CAM Patient-Specific Implants. Cancers 2022, 14, 5774. [Google Scholar] [CrossRef]
| Factors | Category | N = 300 |
|---|---|---|
| Sex | Male | 135 |
| Female | 165 | |
| Age (years) | 20–39 | 60 |
| 40–59 | 77 | |
| 60–79 | 125 | |
| ≥80 | 38 | |
| Number of remaining teeth in the mandible | <5 | 14 |
| 5–9 | 34 | |
| 10–13 | 96 | |
| ≥14 | 156 | |
| Height (cm) | <150 | 22 |
| 150–159 | 126 | |
| 160–169 | 94 | |
| 170–179 | 50 | |
| ≥180 | 8 | |
| Body weight (kg) | <50 | 69 |
| 50–59 | 86 | |
| 60–69 | 89 | |
| 70–79 | 40 | |
| ≥80 | 16 |
| Measurement Points | Age a | Number of Remaining Teeth a | Height b | Body Weight b | ||||
|---|---|---|---|---|---|---|---|---|
| R | p Value | R | p Value | R | p Value | R | p Value | |
| 1 | 0.011 | 0.86 | 0.102 | 0.078 | 0.235 * | <0.001 ** | 0.125 | 0.030 ** |
| 2 | –0.018 | 0.76 | 0.109 | 0.059 | 0.392 * | <0.001 ** | 0.274 * | <0.001 ** |
| 3 | 0.060 | 0.30 | 0.065 | 0.26 | 0.300 * | <0.001 ** | 0.171 | 0.003 ** |
| 4 | 0.113 | 0.051 | 0.005 | 0.93 | 0.226 * | <0.001 ** | 0.122 | 0.035 ** |
| 5 | –0.102 | 0.078 | 0.053 | 0.36 | 0.133 | 0.021 ** | 0.137 | 0.018 ** |
| 6 | 0.034 | 0.56 | −0.045 | 0.44 | 0.028 | 0.63 | 0.046 | 0.42 |
| 7 | 0.051 | 0.38 | 0.043 | 0.46 | 0.148 | 0.010 ** | 0.153 | 0.008 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, N.; Zaizen, M.; Imai, Y.; Kirita, T. Measurement of Thickness at the Inferior Border of the Mandible Using Computed Tomography Images: A Retrospective Study including 300 Japanese Cases. Tomography 2023, 9, 1236-1245. https://doi.org/10.3390/tomography9040098
Ueda N, Zaizen M, Imai Y, Kirita T. Measurement of Thickness at the Inferior Border of the Mandible Using Computed Tomography Images: A Retrospective Study including 300 Japanese Cases. Tomography. 2023; 9(4):1236-1245. https://doi.org/10.3390/tomography9040098
Chicago/Turabian StyleUeda, Nobuhiro, Miki Zaizen, Yuichiro Imai, and Tadaaki Kirita. 2023. "Measurement of Thickness at the Inferior Border of the Mandible Using Computed Tomography Images: A Retrospective Study including 300 Japanese Cases" Tomography 9, no. 4: 1236-1245. https://doi.org/10.3390/tomography9040098
APA StyleUeda, N., Zaizen, M., Imai, Y., & Kirita, T. (2023). Measurement of Thickness at the Inferior Border of the Mandible Using Computed Tomography Images: A Retrospective Study including 300 Japanese Cases. Tomography, 9(4), 1236-1245. https://doi.org/10.3390/tomography9040098






