The Effect of Cooling Fluid Composition on Ablation Size in Hepatic Laser Ablation: A Comparative Study in an Ex Vivo Bovine Setting
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Schmiegel, W.; Buchberger, B.; Follmann, M.; Graeven, U.; Heinemann, V.; Langer, T.; Nothacker, M.; Porschen, R.; Rödel, C.; Rösch, T.; et al. S3-Leitlinie—Kolorektales Karzinom. Z. Gastroenterol. 2017, 55, 1344–1498. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 10-1097. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yoshida, M.; Furuse, J.; Sano, K.; Ohtsuka, M.; Yamashita, S.; Beppu, T.; Iwashita, Y.; Wada, K.; Nakajima, T.E.; et al. Clinical practice guidelines for the management of liver metastases from extrahepatic primary cancers 2021. J. Hepatobiliary Pancreat Sci. 2021, 28, 1–25. [Google Scholar] [CrossRef]
- van Amerongen, M.J.; van der Stok, E.P.; Fütterer, J.J.; Jenniskens, S.F.; Moelker, A.; Grünhagen, D.J.; Verhoef, C.; de Wilt, J.H. Short term and long term results of patients with colorectal liver metastases undergoing surgery with or without radiofrequency ablation. Eur. J. Surg. Oncol. 2016, 42, 523–530. [Google Scholar] [CrossRef]
- Sasaki, K.; Margonis, G.A.; Andreatos, N.; Kim, Y.; Wilson, A.; Gani, F.; Amini, N.; Pawlik, T.M. Combined resection and RFA in colorectal liver metastases: Stratification of long-term outcomes. J. Surg. Res. 2016, 206, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Agcaoglu, O.; Aliyev, S.; Karabulut, K.; El-Gazzaz, G.; Aucejo, F.; Pelley, R.; Siperstein, A.E.; Berber, E. Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: An analysis of 395 patients. World J. Surg. 2013, 37, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Paulet, E.; Aubé, C.; Pessaux, P.; Lebigot, J.; Lhermitte, E.; Oberti, F.; Ponthieux, A.; Calès, P.; Ridereau-Zins, C.; Pereira, P.L. Factors limiting complete tumor ablation by radiofrequency ablation. Cardiovasc. Interv. Radiol. 2008, 31, 107–115. [Google Scholar] [CrossRef][Green Version]
- Bale, R.; Widmann, G.; Stoffner, D.I.R. Stereotaxy: Breaking the limits of current radiofrequency ablation techniques. Eur. J. Radiol. 2010, 75, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.D.; Frank, M.S.; Aribandi, M.; Chopra, S.; Chintapalli, K.N. Radiofrequency thermal ablation: Computer analysis of the size of the thermal injury created by overlapping ablations. AJR Am. J. Roentgenol. 2001, 177, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.C.; Bochnakova, T.; Koethe, Y.; Park, B.; Farsad, K. Percutaneous Therapies for Hepatocellular Carcinoma: Evolution of Liver Directed Therapies. J. Hepatocell. Carcinoma 2021, 8, 1181–1193. [Google Scholar] [CrossRef]
- Rhim, H.; Goldberg, S.N.; Dodd, G.D.; Solbiati, L.; Lim, H.K.; Tonolini, M.; Cho, O.K. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. RadioGraphics 2001, 21, S17–S35; discussion S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Georgiades, C. Radiofrequency ablation: Mechanism of action and devices. J. Vasc. Interv. Radiol. 2010, 21, S179–S186. [Google Scholar] [CrossRef] [PubMed]
- Germer, C.T.; Roggan, A.; Ritz, J.P.; Isbert, C.; Albrecht, D.; Müller, G.; Buhr, H.J. Optical properties of native and coagulated human liver tissue and liver metastases in the near infrared range. Lasers Surg. Med. 1998, 23, 194–203. [Google Scholar] [CrossRef]
- Jin, X.; Feng, Y.; Zhu, R.; Qian, L.; Yang, Y.; Yu, Q.; Zou, Z.; Li, W.; Liu, Y.; Qian, Z. Temperature control and intermittent time-set protocol optimization for minimizing tissue carbonization in microwave ablation. Int. J. Hyperth. 2022, 39, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.N.; Hahn, P.F.; Tanabe, K.K.; Mueller, P.R.; Schima, W.; Athanasoulis, C.A.; Compton, C.C.; Solbiati, L.; Gazelle, G.S. Percutaneous radiofrequency tissue ablation: Does perfusion-mediated tissue cooling limit coagulation necrosis? J. Vasc. Interv. Radiol. 1998, 9, 101–111. [Google Scholar] [CrossRef]
- An, C.; Li, W.Z.; Huang, Z.M.; Yu, X.L.; Han, Y.Z.; Liu, F.Y.; Wu, S.S.; Yu, J.; Liang, P.; Huang, J. Small single perivascular hepatocellular carcinoma: Comparisons of radiofrequency ablation and microwave ablation by using propensity score analysis. Eur. Radiol. 2021, 31, 4764–4773. [Google Scholar] [CrossRef]
- Lesurtel, M.; Lehmann, K.; de Rougemont, O.; Clavien, P.A. Clamping techniques and protecting strategies in liver surgery. Clamping techniques and protecting strategies in liver surgery. HPB 2009, 11, 290–295. [Google Scholar] [CrossRef][Green Version]
- Chouillard, E.K.; Gumbs, A.A.; Cherqui, D. Vascular clamping in liver surgery: Physiology, indications and techniques. Ann. Surg. Innov. Res. 2010, 4, 2. [Google Scholar] [CrossRef]
- Ercolani, G.; Ravaioli, M.; Grazi, G.L.; Cescon, M.; Del Gaudio, M.; Vetrone, G.; Zanello, M.; Pinna, A.D. Use of vascular clamping in hepatic surgery: Lessons learned from 1260 liver resections. Arch Surg. 2008, 143, 380–387; discussion 388. [Google Scholar] [CrossRef][Green Version]
- Vogl, T.J.; Mack, M.G.; Roggan, A.; Straub, R.; Eichler, K.C.; Müller, P.K.; Knappe, V.; Felix, R. Internally cooled power laser for MR-guided interstitial laser-induced thermotherapy of liver lesions: Initial clinical results. Radiology 1998, 209, 381–385. [Google Scholar] [CrossRef]
- Tschabrunn, C.M.; Pothineni, N.V.K.; Sauer, W.H.; Doynow, D.; Salas, J.; Liao, T.E.; Santangeli, P.; Arkles, J.; Hyman, M.C.; Frankel, D.S.; et al. Evaluation of Radiofrequency Ablation Irrigation Type: In Vivo Comparison of Normal Versus Half-Normal Saline Lesion Characteristics. JACC Clin. Electrophysiol. 2020, 6, 684–692. [Google Scholar] [CrossRef]
- Hosten, N.; Stier, A.; Weigel, C.; Kirsch, M.; Puls, R.; Nerger, U.; Jahn, D.; Stroszczynski, C.; Heidecke, C.D.; Speck, U. Laser-induzierte Thermotherapie (LITT) von Lungenmetastasen: Beschreibung eines miniaturisierten Applikators, Optimierung und erste Patientenbehandlungen. Rofo 2003, 175, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, S.A.; Ahmed, M.; Liu, Z.; Hines-Peralta, A.U.; Goldberg, S.N. High-power generator for radiofrequency ablation: Larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings. Radiology 2007, 242, 743–750. [Google Scholar] [CrossRef]
- Sonntag, P.D.; Hinshaw, J.L.; Lubner, M.G.; Brace, C.L.; Lee, F.T. Thermal ablation of lung tumors. Surg. Oncol. Clin. N. Am. 2011, 20, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Gallinghouse, G.J.; Al-Ahmad, A.; Horton, R.P.; Bailey, S.M.; Burkhardt, J.D.; Bassiouny, M.A.; MacDonald, B.C.; Quintero Mayedo, A.; Della Rocca, D.G.; et al. Half-normal saline versus normal saline for irrigation of open-irrigated radiofrequency catheters in atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2021, 32, 973–981. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kubota, T.; Horigome, R.; Kimura, N.; Honda, H.; Iwanaga, A.; Seki, K.; Honma, T.; Yoshida, T. Radiofrequency ablation during continuous saline infusion can extend ablation margins. World J. Gastroenterol. 2013, 19, 1278–1282. [Google Scholar] [CrossRef]
- Bruners, P.; Müller, H.; Günther, R.W.; Schmitz-Rode, T.; Mahnken, A.H. Fluid-modulated bipolar radiofrequency ablation: An ex-vivo evaluation study. Acta Radiol. 2008, 49, 258–266. [Google Scholar] [CrossRef]
- Mankertz, F.; Gemeinhardt, O.; Felbor, U.; Hadlich, S.; Hosten, N. Spacer-Supported Thermal Ablation to Prevent Carbonisation and Improve Ablation Size: A Proof of Concept Study. Biomedicines 2023, 11, 575. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Freichel, J.; Gruber-Rouh, T.; Nour-Eldin Abdelrehim, N.E.; Bechstein, W.O.; Zeuzem, S.; Naguib, N.N.N.; Stefenelli, U. Interventional oncological treatment of hepatocellular carcinoma (HCC)—A single-center long-term evaluation of thermoablation techniques like LITT, MWA, and TACE in a multimodal application over 26 years. Heliyon 2023, 9, e14646. [Google Scholar] [CrossRef]
- Nielsen, S.H.; Skjøth-Rasmussen, J.; Moldrup, S.D.; Engelmann, C.M.; Jespersen, B.; Rasmussen, R. Awake Laser Ablation with Continuous Neuropsychological Testing During Treatment of Brain Tumors and Epilepsy. Neurosurg. Clin. N. Am. 2023, 34, 239–245. [Google Scholar] [CrossRef]
- Hoffmann, C.O.; Rosenberg, C.; Linder, A.; Hosten, N. Residual tumor after laser ablation of human non-small-cell lung cancer demonstrated by ex vivo staining: Correlation with invasive temperature measurements. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 63–74. [Google Scholar] [CrossRef]
- Rempp, H.; Clasen, S.; Pereira, P.L. Image-based monitoring of magnetic resonance-guided thermoablative therapies for liver tumors. Cardiovasc. Interv. Radiol. 2012, 35, 1281–1294. [Google Scholar] [CrossRef]
- Kägebein, U.; Speck, O.; Wacker, F.; Hensen, B. Motion Correction in Proton Resonance Frequency-based Thermometry in the Liver. Top. Magn. Reson. Imaging 2018, 27, 53–61. [Google Scholar] [CrossRef]
- Bouda, D.; Lagadec, M.; Alba, C.G.; Barrau, V.; Dioguardi Burgio, M.; Moussa, N.; Vilgrain, V.; Ronot, M. Imaging review of hepatocellular carcinoma after thermal ablation: The good, the bad, and the ugly. J. Magn. Reson. Imaging 2016, 44, 1070–1090. [Google Scholar] [CrossRef]
- Ozkavukcu, E.; Haliloğlu, N.; Erden, A. Post-treatment MRI findings of hepatocellular carcinoma. Diagn. Interv. Radiol. 2009, 15, 111–120. [Google Scholar]
- UCLA Advanced Research Computing: Statistical Methods and Data Analysis. Choosing the Correct Statistical Test in SAS, STATA, SPSS and R. Los Angeles. Available online: Stats.oarc.ucla.edu/other/mult-pkg/whatstat/ (accessed on 27 April 2023).
- Wei, J. The adoption of repeated measurement of variance analysis and Shapiro-Wilk test. Front. Med. 2022, 16, 659–660. [Google Scholar] [CrossRef]
- Field, A. (Ed.) Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage: Los Angeles, CA, USA, 2013; 915p. [Google Scholar]
- Cheng, Z.; Xiao, Q.; Wang, Y.; Sun, Y.; Lu, T.; Liang, P. 915MHz microwave ablation with implanted internal cooled-shaft antenna: Initial experimental study in in vivo porcine livers. Eur. J. Radiol. 2011, 79, 131–135. [Google Scholar] [CrossRef]
- Francica, G.; Marone, G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a ‘cooled-tip needle’. A preliminary clinical experience. Eur. J. Ultrasound. 1999, 9, 145–153. [Google Scholar] [CrossRef]
- Huang, L.; Yang, S.; Bai, M.; Lin, Y.; Chen, X.; Li, G.; Cui, L.G.; Wang, X. Thermal shielding performance of self-healing hydrogel in tumor thermal ablation. Colloids Surf. B Biointerfaces 2022, 213, 112382. [Google Scholar] [CrossRef] [PubMed]
- Kho, A.S.; Ooi, E.H.; Foo, J.J.; Ooi, E.T. Role of saline concentration during saline-infused radiofrequency ablation: Observation of secondary Joule heating along the saline-tissue interface. Comput. Biol. Med. 2021, 128, 104112. [Google Scholar] [CrossRef] [PubMed]
- Laeseke, P.F.; Sampson, L.A.; Brace, C.L.; Winter, T.C.; Fine, J.P.; Lee, F.T. Unintended thermal injuries from radiofrequency ablation: Protection with 5% dextrose in water. AJR Am. J. Roentgenol. 2006, 186, S249–S254. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.; Vietze, A.; Laskowski, U.; Ritter, C.; Linder, A.; Hosten, N. Ex-vivo human lung tumor model: Use for temperature measurements during thermal ablation of NSCLC. Rofo 2011, 183, 251–259. [Google Scholar] [CrossRef] [PubMed]

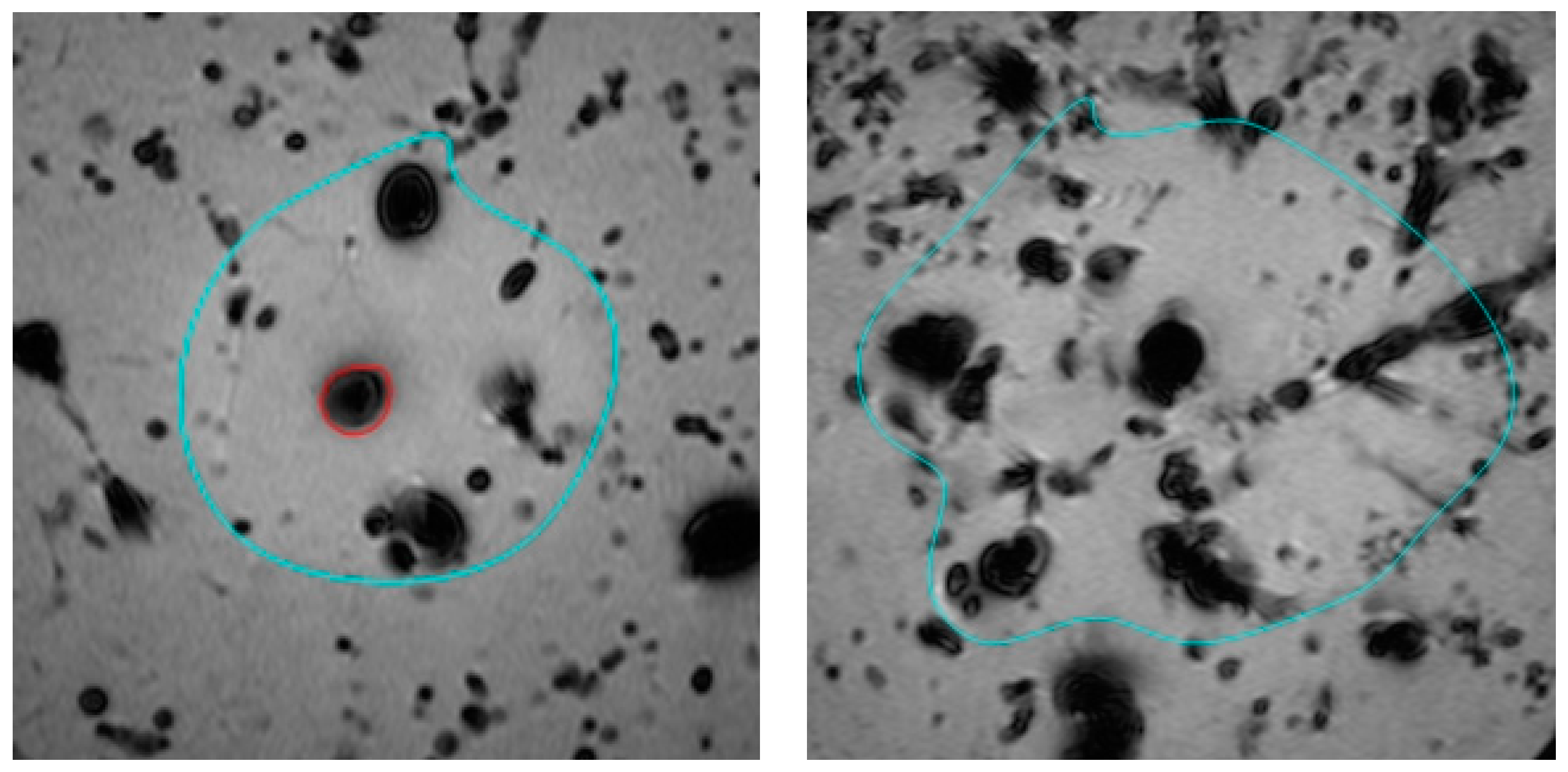
| Fluid Composition | Ablation Volume [cm3] | Std. Error | |
|---|---|---|---|
| Distilled Water | 95% CI Lower Bound | 20.41 | 0.24 |
| 95% CI Upper Bound | 21.43 | ||
| Median | 21.06 | ||
| Minimum | 19.24 | ||
| Maximum | 22.07 | ||
| Mean | 20.92 | ||
| Std. Deviation | 0.92 | ||
| 0.9% Saline | 95% CI Lower Bound | 20.47 | 0.29 |
| 95% CI Upper Bound | 21.69 | ||
| Median | 20.91 | ||
| Minimum | 19.23 | ||
| Maximum | 22.93 | ||
| Mean | 21.08 | ||
| Std. Deviation | 1.11 | ||
| Saline-Water Mixture | 95% CI Lower Bound | 22.09 | 0.26 |
| 95% CI Upper Bound | 23.19 | ||
| Median | 22.96 | ||
| Minimum | 21.09 | ||
| Maximum | 23.89 | ||
| Mean | 22.64 | ||
| Std. Deviation | 0.99 |
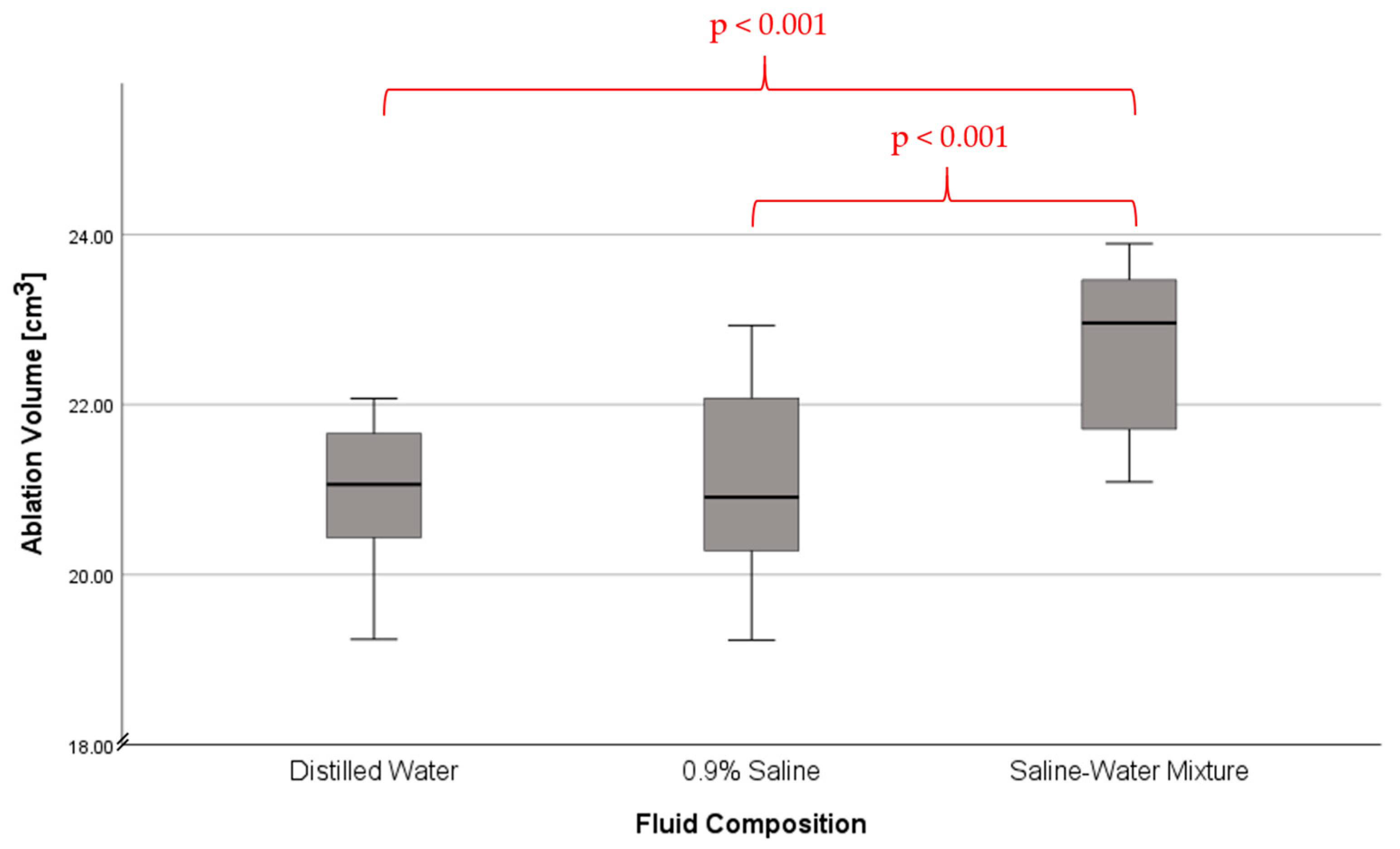
| (IA) Fluid Composition | (JA) Fluid Composition | Mean Difference (IA − JA) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Distilled Water | 0.9% Saline | −0.15 | 0.37 | 0.908 | −1.05 | 0.74 |
| Saline-Water Mixture | −1.71 | 0.37 | <0.001 * | −2.61 | −0.82 | |
| 0.9% Saline | Distilled Water | 0.16 | 0.37 | 0.908 | −0.74 | 1.05 |
| Saline-Water Mixture | −1.56 | 0.37 | <0.001 * | −2.46 | −0.67 | |
| Saline-Water Mixture | Distilled Water | 1.72 | 0.37 | <0.001 * | 0.82 | 2.61 |
| 0.9% Saline | 1.56 | 0.37 | <0.001 * | 0.67 | 2.46 | |
| (IB) Carbonisation | (JB) Carbonisation | Mean Difference (IB − JB) | Std. Error | Sig. | 95% Confidence Interval | |
| Lower Bound | Upper Bound | |||||
| Distilled Water | 0.9% Saline | −0.73 | 0.135 | <0.001 * | −1.06 | −0.41 |
| Saline-Water Mixture | −0.13 | 0.135 | 0.588 | −0.46 | 0.19 | |
| 0.9% Saline | Distilled Water | 0.73 | 0.135 | <0.001 * | 0.41 | 1.06 |
| Saline-Water Mixture | 0.60 | 0.135 | <0.001 * | 0.27 | 0.93 | |
| Saline-Water Mixture | Distilled Water | 0.13 | 0.135 | 0.588 | −0.19 | 0.46 |
| 0.9% Saline | −0.60 | 0.135 | <0.001 * | −0.93 | −0.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mankertz, F.; Bayerl, N.; Gemeinhardt, O.; Hosten, N.; Kromrey, M.-L. The Effect of Cooling Fluid Composition on Ablation Size in Hepatic Laser Ablation: A Comparative Study in an Ex Vivo Bovine Setting. Tomography 2023, 9, 1638-1648. https://doi.org/10.3390/tomography9050131
Mankertz F, Bayerl N, Gemeinhardt O, Hosten N, Kromrey M-L. The Effect of Cooling Fluid Composition on Ablation Size in Hepatic Laser Ablation: A Comparative Study in an Ex Vivo Bovine Setting. Tomography. 2023; 9(5):1638-1648. https://doi.org/10.3390/tomography9050131
Chicago/Turabian StyleMankertz, Fiona, Nadine Bayerl, Ole Gemeinhardt, Norbert Hosten, and Marie-Luise Kromrey. 2023. "The Effect of Cooling Fluid Composition on Ablation Size in Hepatic Laser Ablation: A Comparative Study in an Ex Vivo Bovine Setting" Tomography 9, no. 5: 1638-1648. https://doi.org/10.3390/tomography9050131
APA StyleMankertz, F., Bayerl, N., Gemeinhardt, O., Hosten, N., & Kromrey, M.-L. (2023). The Effect of Cooling Fluid Composition on Ablation Size in Hepatic Laser Ablation: A Comparative Study in an Ex Vivo Bovine Setting. Tomography, 9(5), 1638-1648. https://doi.org/10.3390/tomography9050131







