Transcatheter Arterial Embolization for Bleeding Related to Pelvic Trauma: Comparison of Technical and Clinical Results between Hemodynamically Stable and Unstable Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment
- Patients with hemodynamic instability who underwent temporary pelvic stabilization, the start of aggressive volume resuscitation, and exclusion of extra-pelvic blood loss [5];
- Patients who already underwent pelvic AG with or without AE, with persistent signs of ongoing bleeding and exclusion of extra-pelvic blood loss [5];
- Hemodynamically stable patients with stable/unstable pelvic fractures or unremarkable CT scans but still clinical signs of significant ongoing bleeding. The rationale was to differentiate venous or bone bleeding from arterial bleeding that may be absent or unidentifiable at the time of CT. The clinical significance of the ongoing bleeding may justify the continuation of the imaging workup with AG, as an absence of blush at CT does not always exclude active pelvic bleeding at AG [4,32,33]. It is worth noting that sacroiliac joint disruption and female gender were proven to be reliable predictors of patients who would benefit from pelvic AG [34].
2.3. Outcomes and Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rhee, P.; Joseph, B.; Pandit, V.; Aziz, H.; Vercruysse, G.; Kulvatunyou, N.; Friese, R.S. Increasing trauma deaths in the United States. Ann. Surg. 2014, 260, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Subcommittee, A.; Tchorz, K.M. ATLS Subcommittee, American College of Surgeons’ Committee on Trauma, International ATLS working group. Advanced trauma life support (ATLS®): The ninth edition. J. Trauma Acute Care Surg. 2013, 74, 1363–1366. [Google Scholar] [CrossRef]
- Burkhardt, M.; Nienaber, U.; Pizanis, A.; Maegele, M.; Culemann, U.; Bouillon, B.; Flohé, S.; Pohlemann, T.; Paffrath, T.; the TraumaRegister DGU and the German Pelvic Injury Register of the Deutsche Gesellschaft für Unfallchirurgie. Acute management and outcome of multiple trauma patients with pelvic disruptions. Crit. Care 2012, 16, R163. [Google Scholar] [CrossRef]
- Renzulli, M.; Ierardi, A.M.; Brandi, N.; Battisti, S.; Giampalma, E.; Marasco, G.; Spinelli, D.; Principi, T.; Catena, F.; Khan, M.; et al. Proposal of standardization of every step of angiographic procedure in bleeding patients from pelvic trauma. Eur. J. Med. Res. 2021, 26, 123. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Stahel, P.F.; Montori, G.; Biffl, W.; Horer, T.M.; Catena, F.; Kluger, Y.; Moore, E.E.; Peitzman, A.B.; Ivatury, R.; et al. Pelvic trauma: WSES classification and guidelines. World J. Emerg. Surg. 2017, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Papakostidis, C.; Kanakaris, N.; Dimitriou, R.; Giannoudis, P.V. The role of arterial embolization in controlling pelvic fracture haemorrhage: A systematic review of the literature. Eur. J. Radiol. 2012, 81, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, E.K. Transcatheter embolization in the treatment of hemorrhage in pelvic trauma. Semin. Interv. Radiol. 2008, 25, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, D.J.; Verbeek, D.O.; Ponsen, K.J.; Carel Goslings, J.; van Delden, O.M. Imaging and Endovascular Treatment of Bleeding Pelvic Fractures: Review Article. Cardiovasc. Interv. Radiol. 2019, 42, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, D.; Karaiskakis, M.; Toutouzas, K.; Alo, K.; Velmahos, G.; Chan, L. Pelvic fractures: Epidemiology and predictors of associated abdominal injuries and outcomes. J. Am. Coll. Surg. 2002, 195, 1–10. [Google Scholar] [CrossRef]
- Verbeek, D.O.F.; Zijlstra, I.A.J.; Van Der Leij, C.; Ponsen, K.J.; Van Delden, O.M.; Goslings, J.C. Predicting the need for abdominal hemorrhage control in major pelvic fracture patients: The importance of quantifying the amount of free fluid. J. Trauma Acute Care Surg. 2014, 76, 1259–1263. [Google Scholar] [CrossRef]
- Hagiwara, A.; Minakawa, K.; Fukushima, H.; Murata, A.; Masuda, H.; Shimazaki, S. Predictors of death in patients with life-threatening pelvic hemorrhage after successful transcatheter arterial embolization. J. Trauma 2003, 55, 696–703. [Google Scholar] [CrossRef]
- Awwad, A.; Dhillon, P.S.; Ramjas, G.; Habib, S.B.; Al-Obaydi, W. Trans-arterial embolisation (TAE) in haemorrhagic pelvic injury: Review of management and mid-term outcome of a major trauma centre. CVIR Endovasc. 2018, 1, 32. [Google Scholar] [CrossRef]
- Athanasoulis, C.A.; Duffield, R.; Shapiro, J.H. Angiography to assess pelvic vascular injury. N. Engl. J. Med. 1971, 284, 1329. [Google Scholar] [CrossRef]
- Margolies, M.N.; Ring, E.J.; Waltman, A.C.; Kerr, W.S.; Baum, S. Arteriography in the management of hemorrhage from pelvic fractures. N. Engl. J. Med. 1972, 287, 317–321. [Google Scholar] [CrossRef]
- Weir, A.; Kennedy, P.; Joyce, S.; Ryan, D.; Spence, L.; McEntee, M.; Maher, M.; O’connor, O. Endovascular management of pelvic trauma. Ann. Transl. Med. 2021, 9, 1196. [Google Scholar] [CrossRef]
- Aukerman, W.; Simunich, T.; Boer, J.; Dumire, R. Limited Resources at a Community Based Level 1 Trauma Center: Does This Affect Pelvic Angioembolization Times During Daylight Hours versus after Hours, Weekends, and Holidays? Am. Surg. 2023, 89, 3626–3628. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Medina, M.; Cotton, B.A.; Rahbar, E.; Wade, C.E.; Cohen, A.M.; Beeler, A.M.B.; Burgess, A.R.; Holcomb, J.B. Are we delivering two standards of care for pelvic trauma? Availability of angioembolization after hours and on weekends increases time to therapeutic intervention. J. Trauma Acute Care Surg. 2014, 76, 134–139. [Google Scholar] [CrossRef]
- Magnone, S.; Coccolini, F.; Manfredi, R.; Piazzalunga, D.; Agazzi, R.; Arici, C.; Barozzi, M.; Bellanova, G.; Belluati, A.; Berlot, G.; et al. Management of hemodynamically unstable pelvic trauma: Results of the first Italian consensus conference (cooperative guidelines of the Italian Society of Surgery, the Italian Association of Hospital Surgeons, the Multi-specialist Italian Society of Young Surgeons, the Italian Society of Emergency Surgery and Trauma, the Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care, the Italian Society of Orthopaedics and Traumatology, the Italian Society of Emergency Medicine, the Italian Society of Medical Radiology-Section of Vascular and Interventional Radiology- and the World Society of Emergency Surgery). World J. Emerg. Surg. 2014, 9, 18. [Google Scholar] [CrossRef]
- Vanheer, R.; De Wever, L.; Maleux, G. Posttraumatic hemorrhagic bladder rupture managed with transurethral foley catheter placement and bilateral transcatheter vesical artery embolization. Acta Chir. Belg. 2023, 123, 427–429. [Google Scholar] [CrossRef]
- Mejía-Quiñones, V.; Álvarez-Saa, T.; Gallo, J.E.G.; Holguín-Holguín, A.J.; Toro-Gutiérrez, J.S. Successful retrograde embolization through the arch of cavernous arteries of a bilateral post-traumatic arterio-cavernous fistula: Case report. Radiol. Case Rep. 2023, 18, 2602–2606. [Google Scholar] [CrossRef]
- Bokenkamp, M.; Gallastegi, A.D.; Brown, T.; Hwabejire, J.O.; Fawley, J.; Mendoza, A.E.; Saillant, N.N.; Fagenholz, P.J.; Kaafarani, H.M.; Velmahos, G.C.; et al. Angioembolization in Severe Pelvic Trauma is Associated with Venous Thromboembolism. J. Surg. Res. 2023, 283, 540–549. [Google Scholar] [CrossRef]
- Aoki, R.; Nakajima, K.; Kobayashi, Y.; Sakai, Y.; Kamide, H.; Yamamoto, T.; Furugori, S.; Sawamura, S.; Terauchi, M.; Kamiyama, K.; et al. Common and uncommon vascular injuries and endovascular treatment associated with pelvic blunt trauma: A real-world experience. JPN J. Radiol. 2023, 41, 258–265. [Google Scholar] [CrossRef]
- Al-Thani, H.; Abdelrahman, H.; Barah, A.; Asim, M.; El-Menyar, A. Utility of Angioembolization in Patients with Abdominal and Pelvic Traumatic Bleeding: Descriptive Observational Analysis from a Level 1 Trauma Center. Ther. Clin. Risk Manag. 2021, 17, 333–343. [Google Scholar] [CrossRef]
- Marmor, M.; El Naga, A.N.; Barker, J.; Matz, J.; Stergiadou, S.; Miclau, T. Management of Pelvic Ring Injury Patients With Hemodynamic Instability. Front. Surg. 2020, 7, 588845. [Google Scholar] [CrossRef]
- Vaidya, R.; Waldron, J.; Scott, A.; Nasr, K. Angiography and Embolization in the Management of Bleeding Pelvic Fractures. JAAOS J. Am. Acad. Orthop. Surg. 2018, 26, e68–e76. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.J.; Rahim, S.; Davidson, J.C.; Hanks, S.E.; Tam, A.L.; Walker, T.G.; Wilkins, L.R.; Sarode, R.; Weinberg, I. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions—Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2019, 30, 1168–1184.e1. [Google Scholar] [CrossRef]
- Klein, E.N.; Kirton, O.C. Angioembolization: Indications, Approach and Optimal Use. Curr. Trauma Rep. 2015, 1, 26–34. [Google Scholar] [CrossRef]
- Verbeek, D.O.F.; Zijlstra, I.A.J.; van der Leij, C.; Ponsen, K.J.; van Delden, O.M.; Goslings, J.C. Management of pelvic ring fracture patients with a pelvic “blush” on early computed tomography. J. Trauma Acute Care Surg. 2014, 76, 374–379. [Google Scholar] [CrossRef]
- Brun, J.; Guillot, S.; Bouzat, P.; Broux, C.; Thony, F.; Genty, C.; Heylbroeck, C.; Albaladejo, P.; Arvieux, C.; Tonetti, J.; et al. Detecting active pelvic arterial haemorrhage on admission following serious pelvic fracture in multiple trauma patients. Injury 2014, 45, 101–106. [Google Scholar] [CrossRef]
- Brasel, K.J.; Pham, K.; Yang, H.; Christensen, R.; Weigelt, J.A. Significance of contrast extravasation in patients with pelvic fracture. J. Trauma 2007, 62, 1149–1152. [Google Scholar] [CrossRef]
- Verbeek, D.O.; Ponsen, K.J.; van Delden, O.M.; Goslings, J.C. The need for pelvic angiographic embolisation in stable pelvic fracture patients with a “blush” on computed tomography. Injury 2014, 45, 2111. [Google Scholar] [CrossRef]
- Cullinane, D.C.; Schiller, H.J.; Zielinski, M.D.; Bilaniuk, J.W.; Collier, B.R.; Como, J.; Holevar, M.; Sabater, E.A.; Sems, S.A.; Vassy, W.M.; et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture—Update and systematic review. J. Trauma 2011, 71, 1850–1868. [Google Scholar] [CrossRef]
- Marzi, I.; Lustenberger, T. Management of Bleeding Pelvic Fractures. Scand J. Surg. 2014, 103, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Teixeira, P.G.; DuBose, J.; Ottochian, M.; Inaba, K.; Margulies, D.R.; Demetriades, D. Predictors of positive angiography in pelvic fractures: A prospective study. J. Am. Coll. Surg. 2008, 207, 656–662. [Google Scholar] [CrossRef]
- Burgess, A.R.; Eastridge, B.J.; Young, J.W.; Ellison, T.S.; Ellison, P.S.; Poka, A.; Bathon, G.H.; Brumback, R.J. Pelvic ring disruptions: Effective classification system and treatment protocols. J. Trauma 1990, 30, 848–856. [Google Scholar] [CrossRef]
- Mohseni, S.; Talving, P.; Kobayashi, L.; Lam, L.; Inaba, K.; Branco, B.C.; Oliver, M.; Demetriades, D. The Diagnostic Accuracy of 64-Slice Computed Tomography in Detecting Clinically Significant Arterial Bleeding after Pelvic Fractures. Am. Surg. 2011, 77, 1176–1182. [Google Scholar] [CrossRef]
- Lee, H.-J.; No, H.-K.; Choi, N.-J.; Sun, H.-W.; Lee, J.-S.; Jung, Y.-J.; Hong, S.-K. The size of pelvic hematoma can be a predictive factor for angioembolization in hemodynamically unstable pelvic trauma. Ann. Surg. Treat. Res. 2020, 98, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Agolini, S.F.; Shah, K.; Jaffe, J.; Newcomb, J.; Rhodes, M.; Reed, J.F. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J. Trauma 1997, 43, 395–399. [Google Scholar] [CrossRef]
- Hauschild, O.; Aghayev, E.; von Heyden, J.; Strohm, P.C.; Culemann, U.; Pohlemann, T.; Suedkamp, N.P.; Schmal, H. Angioembolization for pelvic hemorrhage control: Results from the German pelvic injury register. J. Trauma Acute Care Surg. 2012, 73, 679–684. [Google Scholar] [CrossRef]
- Cerva, D.S.; Mirvis, S.E.; Shanmuganathan, K.; Kelly, I.M.; Pais, S.O. Detection of bleeding in patients with major pelvic fractures: Value of contrast-enhanced CT. AJR Am. J. Roentgenol. 1996, 166, 131–135. [Google Scholar] [CrossRef]
- Pereira, S.J.; O’Brien, D.P.; Luchette, F.A.; Choe, K.; Lim, E.; Davis, J.K.; Hurst, J.M.; Johannigman, J.A.; Frame, S.B. Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery 2000, 128, 678–685. [Google Scholar] [CrossRef]
- Loffroy, R.; Guiu, B.; D’Athis, P.; Mezzetta, L.; Gagnaire, A.; Jouve, J.; Ortega–Deballon, P.; Cheynel, N.; Cercueil, J.; Krausé, D. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: Predictors of early rebleeding. Clin. Gastroenterol. Hepatol. 2009, 7, 515–523. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dariushnia, S.R.; Redstone, E.A.; Heran, M.K.; Cramer, H.R.; Ganguli, S.; Gomes, A.S.; Hogan, M.J.; Himes, E.A.; Patel, S.; Schiro, B.J.; et al. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Transcatheter Embolization. J. Vasc. Interv. Radiol. 2021, 32, e1–e476. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology Clinical Practice Guidelines. J. Vasc. Interv. Radiol. 2003, 14, S199–S202. [Google Scholar] [CrossRef]
- Filippiadis, D.K.; Binkert, C.; Pellerin, O.; Hoffmann, R.T.; Krajina, A.; Pereira, P.L. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc. Interv. Radiol. 2017, 40, 1141–1146. [Google Scholar] [CrossRef]
- Minici, R.; Ammendola, M.; Manti, F.; Siciliano, M.A.; Minici, M.; Komaei, I.; Currò, G.; Laganà, D. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization (DSM-TACE) in the Downstaging of Intermediate-Stage Hepatocellular Carcinoma (HCC) in Patients With a Child-Pugh Score of 8–9. Front. Pharmacol. 2021, 12, 634087. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Ammendola, M.; Manti, F.; Siciliano, M.A.; Giglio, E.; Minici, M.; Melina, M.; Currò, G.; Laganà, D. Safety and Efficacy of Degradable Starch Microspheres Transcatheter Arterial Chemoembolization as a Bridging Therapy in Patients with Early Stage Hepatocellular Carcinoma and Child-Pugh Stage B Eligible for Liver Transplant. Front. Pharmacol. 2021, 12, 634084. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Ielapi, N.; Minici, R.; Peluso, A.; Bracale, U.M.; Andreucci, M.; Serra, R. Risk Factors for Bleeding Varicose Veins in Patients with Chronic Venous Disease. Medicina 2023, 59, 1034. [Google Scholar] [CrossRef]
- Minici, R.; Mercurio, M.; Iannò, B.; Galasso, O.; Gasparini, G.; Laganà, D. Advantages of the Use of Axial Traction Magnetic Resonance Imaging (MRI) of the Shoulder in Patients with Suspected Rota-Tor Cuff Tears: An Exploratory Pilot Study. Healthcare 2023, 11, 724. [Google Scholar] [CrossRef]
- Ammendola, M.; Filice, F.; Battaglia, C.; Romano, R.; Manti, F.; Minici, R.; De’Angelis, N.; Memeo, R.; Laganà, D.; Navarra, G.; et al. Left hemicolectomy and low anterior resection in colorectal cancer patients: Knight-griffen vs. transanal purse-string suture anastomosis with no-coil placement. Front. Surg. 2023, 10, 1093347. [Google Scholar] [CrossRef]
- Rossi, R.; Talarico, M.; Schepis, F.; Coppi, F.; Sgura, F.A.; Monopoli, D.E.; Minici, R.; Boriani, G. Effects of sildenafil on right ventricle remodelling in Portopulmonary hypertension. Pulm. Pharmacol. Ther. 2021, 70, 102071. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Talarico, M.; Pascale, A.; Pascale, V.; Minici, R.; Boriani, G. Low Levels of Vitamin D and Silent Myocardial Ischemia in Type 2 Diabetes: Clinical Correlations and Prognostic Significance. Diagnostics 2022, 12, 2572. [Google Scholar] [CrossRef]
- Cernigliaro, M.; Stanca, C.; Galbiati, A.; Spinetta, M.; Coda, C.; Negroni, D.; Laganà, D.; Minici, R.; Airoldi, C.; Carriero, A.; et al. Innovation in Acute Ischemic Stroke Patients over 80 y/o—A Retrospective Monocentric Study on Mechanical Thrombectomy of Consecutive Patients: Is Age an Adequate Selection Criterion? J. Clin. Med. 2023, 12, 3688. [Google Scholar] [CrossRef]
- Cook, R.E.; Keating, J.F.; Gillespie, I. The role of angiography in the management of haemorrhage from major fractures of the pelvis. J. Bone Joint Surg. Br. 2002, 84, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Velmahos, G.C.; Toutouzas, K.G.; Vassiliu, P.; Sarkisyan, G.; Chan, L.S.; Hanks, S.H.; Berne, T.V.; Demetriades, D. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J. Trauma 2002, 53, 303–308; discussion 308. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.; McDonald, A.A.; Knight, D.; Johannigman, J.A.; Cuschieri, J. The role of repeat angiography in the management of pelvic fractures. J. Trauma 2005, 58, 227–231. [Google Scholar] [CrossRef]
- Barentsz, M.W.; Vonken, E.P.A.; van Herwaarden, J.A.; Leenen, L.P.H.; Mali, W.P.T.M.; van den Bosch, M.A.A.J. Clinical outcome of intra-arterial embolization for treatment of patients with pelvic trauma. Radiol. Res. Pract. 2011, 2011, 935484. [Google Scholar] [CrossRef]
- Velmahos, G.C.; Chahwan, S.; Falabella, A.; Hanks, S.E.; Demetriades, D. Angiographic embolization for intraperitoneal and retroperitoneal injuries. World J. Surg. 2000, 24, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Venturini, M.; Fontana, F.; Guzzardi, G.; Pingitore, A.; Piacentino, F.; Serra, R.; Coppola, A.; Santoro, R.; Laganà, D. Efficacy and Safety of Ethylene-Vinyl Alcohol (EVOH) Copolymer-Based Non-Adhesive Liquid Embolic Agents (NALEAs) in Transcatheter Arterial Embolization (TAE) of Acute Non-Neurovascular Bleeding: A Multicenter Retrospective Cohort Study. Medicina 2023, 59, 710. [Google Scholar] [CrossRef]
- Lucatelli, P.; Corona, M.; Teodoli, L.; Nardis, P.; Cannavale, A.; Rocco, B.; Trobiani, C.; Cipollari, S.; de Gyurgyokai, S.Z.; Bezzi, M.; et al. Use of Phil Embolic Agent for Bleeding in Non-Neurological Interventions. J. Clin. Med. 2021, 10, 701. [Google Scholar] [CrossRef]
- Minici, R.; Fontana, F.; Venturini, M.; Guzzardi, G.; Siciliano, A.; Piacentino, F.; Serra, R.; Coppola, A.; Guerriero, P.; Apollonio, B.; et al. Transcatheter Arterial Embolization (TAE) in the Management of Bleeding in the COVID-19 Patient. Medicina 2023, 59, 1062. [Google Scholar] [CrossRef]
- Loffroy, R.; Guiu, B. Role of transcatheter arterial embolization for massive bleeding from gastroduodenal ulcers. World J. Gastroenterol. 2009, 15, 5889–5897. [Google Scholar] [CrossRef]
- Evers, B.M.; Cryer, H.M.; Miller, F.B. Pelvic fracture hemorrhage. Priorities in management. Arch. Surg. 1989, 124, 422–424. [Google Scholar] [CrossRef]
- Metsemakers, W.-J.; Vanderschot, P.; Jennes, E.; Nijs, S.; Heye, S.; Maleux, G. Transcatheter embolotherapy after external surgical stabilization is a valuable treatment algorithm for patients with persistent haemorrhage from unstable pelvic fractures: Outcomes of a single centre experience. Injury 2013, 44, 964–968. [Google Scholar] [CrossRef]
- Osborn, P.M.; Smith, W.R.; Moore, E.E.; Cothren, C.C.; Morgan, S.J.; Williams, A.E.; Stahel, P.F. Direct retroperitoneal pelvic packing versus pelvic angiography: A comparison of two management protocols for haemodynamically unstable pelvic fractures. Injury 2009, 40, 54–60. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Grotz, M.R.W.; Tzioupis, C.; Dinopoulos, H.; Wells, G.E.; Bouamra, O.; Lecky, F. Prevalence of Pelvic Fractures, Associated Injuries, and Mortality: The United Kingdom Perspective. J. Trauma Acute Care Surg. 2007, 63, 875–883. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Mirvis, S.E.; Sover, E.R. Value of contrast-enhanced CT in detecting active hemorrhage in patients with blunt abdominal or pelvic trauma. AJR Am. J. Roentgenol. 1993, 161, 65–69. [Google Scholar] [CrossRef]
- Michailidou, M.; Velmahos, G.C.; van der Wilden, G.M.; Alam, H.B.; de Moya, M.; Chang, Y. “Blush” on trauma computed tomography: Not as bad as we think! J. Trauma Acute Care Surg. 2012, 73, 580–584; discussion 584–586. [Google Scholar] [CrossRef]
- Diamond, I.R.; Hamilton, P.A.; Garber, A.B.; Tien, H.C.; Chughtai, T.; Rizoli, S.B.; Tremblay, L.N.; Brenneman, F.D. Extravasation of intravenous computed tomography scan contrast in blunt abdominal and pelvic trauma. J. Trauma 2009, 66, 1102–1107. [Google Scholar] [CrossRef]
- Padia, S.A.; Ingraham, C.R.; Moriarty, J.M.; Wilkins, L.R.; Bream, P.R.; Tam, A.L.; Patel, S.; McIntyre, L.; Wolinsky, P.R.; Hanks, S.E. Society of Interventional Radiology Position Statement on Endovascular Intervention for Trauma. J. Vasc. Interv. Radiol. 2020, 31, 363–369.e2. [Google Scholar] [CrossRef]
- Dietrich, H.H.; Dacey, R.G. Molecular keys to the problems of cerebral vasospasm. Neurosurgery 2000, 46, 517–530. [Google Scholar] [CrossRef]
- Hallinan, J.T.P.D.; Tan, C.H.; Pua, U. Emergency computed tomography for acute pelvic trauma: Where is the bleeder? Clin. Radiol. 2014, 69, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Wu, Y.-T.; Fu, C.-Y.; Liao, C.-H.; Wang, S.-Y.; Bajani, F.; Hsieh, C.-H. Hemostasis as soon as possible? The role of the time to angioembolization in the management of pelvic fracture. World J. Emerg. Surg. 2019, 14, 28. [Google Scholar] [CrossRef]
- Tanizaki, S.; Maeda, S.; Matano, H.; Sera, M.; Nagai, H.; Ishida, H. Time to pelvic embolization for hemodynamically unstable pelvic fractures may affect the survival for delays up to 60 min. Injury 2014, 45, 738–741. [Google Scholar] [CrossRef]
- Park, J.Y.; Yim, N.Y.; Kim, J.K.; Kim, H.O.; Kang, Y.J.; Jung, H.D.; Kim, S.K.; Yoon, W. Embolization of Trauma-Associated Pelvic Hemorrhage: Feasibility of Super-Selective Catheterization in Heavily Injured Patients as a Damage Control for Life-Threatening Pelvic Bleeding. J. Korean Soc. Radiol. 2016, 74, 236–244. [Google Scholar] [CrossRef]
- Sheridan, M.K.; Blackmore, C.C.; Linnau, K.F.; Hoffer, E.K.; Lomoschitz, F.; Jurkovich, G.J. Can CT predict the source of arterial hemorrhage in patients with pelvic fractures? Emerg. Radiol. 2002, 9, 188–194. [Google Scholar] [CrossRef]
- Travis, T.; Monsky, W.L.; London, J.; Danielson, M.; Brock, J.; Wegelin, J.; Link, D.P. Evaluation of short-term and long-term complications after emergent internal iliac artery embolization in patients with pelvic trauma. J. Vasc. Interv. Radiol. 2008, 19, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Matityahu, A.; Marmor, M.; Elson, J.K.; Lieber, C.; Rogalski, G.; Lin, C.; Belaye, T.; Miclau, T.; Kandemir, U. Acute complications of patients with pelvic fractures after pelvic angiographic embolization. Clin. Orthop. Relat. Res. 2013, 471, 2906–2911. [Google Scholar] [CrossRef]
- Takahira, N.; Shindo, M.; Tanaka, K.; Nishimaki, H.; Ohwada, T.; Itoman, M. Gluteal muscle necrosis following transcatheter angiographic embolisation for retroperitoneal haemorrhage associated with pelvic fracture. Injury 2001, 32, 27–32. [Google Scholar] [CrossRef]
- El-Haj, M.; Bloom, A.; Mosheiff, R.; Liebergall, M.; Weil, Y.A. Outcome of angiographic embolisation for unstable pelvic ring injuries: Factors predicting success. Injury 2013, 44, 1750–1755. [Google Scholar] [CrossRef]
- Lindahl, J.; Handolin, L.; Söderlund, T.; Porras, M.; Hirvensalo, E. Angiographic embolization in the treatment of arterial pelvic hemorrhage: Evaluation of prognostic mortality-related factors. Eur. J. Trauma Emerg. Surg. 2013, 39, 57–63. [Google Scholar] [CrossRef]
- Wong, Y.C.; Wang, L.J.; Ng, C.J.; Tseng, I.C.; See, L.C. Mortality after successful transcatheter arterial embolization in patients with unstable pelvic fractures: Rate of blood transfusion as a predictive factor. J. Trauma 2000, 49, 71–75. [Google Scholar] [CrossRef]
- Perez, J.V.; Hughes, T.M.; Bowers, K. Angiographic embolisation in pelvic fracture. Injury 1998, 29, 187–191. [Google Scholar] [CrossRef] [PubMed]
- van der Vlies, C.H.; Saltzherr, T.P.; Reekers, J.A.; Ponsen, K.J.; van Delden, O.M.; Goslings, J.C. Failure rate and complications of angiography and embolization for abdominal and pelvic trauma. J. Trauma Acute Care Surg. 2012, 73, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Sapoval, M.; Vidal, V.; Déan, C.; Del Giudice, C.; Tradi, F.; Chevallier, O.; Charles-Nelson, A.; Pellerin, O.; Loffroy, R. Safety and Efficacy of Peripheral Embolization with EASYX Liquid Embolic Agent: A Multicenter Prospective Study. J. Vasc. Interv. Radiol. 2021, 32, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Ammendola, M.; Talarico, M.; Luposella, M.; Minici, M.; Ciranni, S.; Guzzardi, G.; Laganà, D. Endovascular recanalization of chronic total occlusions of the native superficial femoral artery after failed femoropopliteal bypass in patients with critical limb ischemia. CVIR Endovasc. 2021, 4, 68. [Google Scholar] [CrossRef]
- Kim, P.H.; Tsauo, J.; Shin, J.H.; Yun, S.-C. Transcatheter Arterial Embolization of Gastrointestinal Bleeding with N-Butyl Cyanoacrylate: A Systematic Review and Meta-Analysis of Safety and Efficacy. J. Vasc. Interv. Radiol. 2017, 28, 522–531.e5. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; Ierardi, A.M.; Petullà, M.; Bracale, U.M.; Carrafiello, G.; Laganà, D. Thoracic endovascular repair for blunt traumatic thoracic aortic injury: Long-term results. Vascular 2022. [Google Scholar] [CrossRef]
- Né, R.; Chevallier, O.; Falvo, N.; Facy, O.; Berthod, P.-E.; Galland, C.; Gehin, S.; Midulla, M.; Loffroy, R. Embolization with ethylene vinyl alcohol copolymer (Onyx®) for peripheral hemostatic and non-hemostatic applications: A feasibility and safety study. Quant. Imaging Med. Surg. 2018, 8, 280–290. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; Giurdanella, M.; Talarico, M.; Siciliano, M.A.; Carrafiello, G.; Laganà, D. Efficacy and Safety of Distal Radial Access for Transcatheter Arterial Chemoembolization (TACE) of the Liver. J. Pers. Med. 2023, 13, 640. [Google Scholar] [CrossRef]
- Bracale, U.M.; Peluso, A.; Panagrosso, M.; Cecere, F.; DEL Guercio, L.; Minici, R.; Giannotta, N.; Ielapi, N.; Licastro, N.; Serraino, G.F.; et al. Ankle-Brachial Index evaluation in totally percutaneous approach vs. femoral artery cutdown for endovascular aortic repair of abdominal aortic aneurysms. Chirurgia 2022, 35, 349–354. [Google Scholar] [CrossRef]
- Minici, R.; Paone, S.; Talarico, M.; Zappia, L.; Abdalla, K.; Petullà, M.; Laganà, D. Percutaneous treatment of vascular access-site complications: A ten years’ experience in two centres. CVIR Endovasc. 2020, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Regine, R.; Palmieri, F.; Siero, M.; Rescigno, A.; Sica, V.; Cantarela, R.; Villari, V. Embolization of traumatic and non-traumatic peripheral vascular lesions with Onyx. Interv. Med. Appl. Sci. 2015, 7, 22–29. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; Maglia, C.; Guzzardi, G.; Spinetta, M.; Fontana, F.; Venturini, M.; Laganà, D. Efficacy and Safety of Axiostat® Hemostatic Dressing in Aiding Manual Compression Closure of the Femoral Arterial Access Site in Patients Undergoing Endovascular Treatments: A Preliminary Clinical Experience in Two Centers. J. Pers. Med. 2023, 13, 812. [Google Scholar] [CrossRef]
- Minici, R.; Serra, R.; De Rosi, N.; Ciranni, S.; Talarico, M.; Petullà, M.; Guzzardi, G.; Fontana, F.; Laganà, D. Endovascular treatment of femoro-popliteal occlusions with retrograde tibial access after failure of the antegrade approach. Catheter Cardiovasc. Interv. 2023, 101, 1108–1119. [Google Scholar] [CrossRef]
- Becce, F.; Richarme, D.; Omoumi, P.; Djahangiri, A.; Farron, A.; Meuli, R.; Theumann, N. Direct MR arthrography of the shoulder under axial traction: Feasibility study to evaluate the superior labrum-biceps tendon complex and articular cartilage. J. Magn. Reson. Imaging 2013, 37, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Minici, R.; Fontana, F.; Venturini, M.; Guzzardi, G.; Piacentino, F.; Spinetta, M.; Bertucci, B.; Serra, R.; Costa, D.; Ielapi, N.; et al. A Multicenter Retrospective Cohort Study Evaluating the Clinical Outcomes of Patients with Coagulopathy Undergoing Transcatheter Arterial Embolization (TAE) for Acute Non-Neurovascular Bleeding. Medicina 2023, 59, 1333. [Google Scholar] [CrossRef]
- Minici, R.; Guzzardi, G.; Venturini, M.; Fontana, F.; Coppola, A.; Spinetta, M.; Piacentino, F.; Pingitore, A.; Serra, R.; Costa, D.; et al. Transcatheter Arterial Embolization (TAE) of Cancer-Related Bleeding. Medicina 2023, 59, 1323. [Google Scholar] [CrossRef]
- Salsamendi, J.; Quintana, D.; Kably, I.; Narayanan, G. Special Considerations for Embolization in Trauma Cases n.d. Endovasc. Today 2013, 2013, 42–50. [Google Scholar]
- Sandhu, J.; Abrahams, R.; Miller, Z.; Bhatia, S.; Zakrison, T.L.; Mohan, P. Pelvic Trauma: Factors predicting arterial hemorrhage and the role of Angiography and preperitoneal pelvic packing. Eur. Radiol. 2020, 30, 6376–6383. [Google Scholar] [CrossRef]
- Yonemitsu, T.; Kawai, N.; Sato, M.; Tanihata, H.; Takasaka, I.; Nakai, M.; Minamiguchi, H.; Sahara, S.; Iwasaki, Y.; Shima, Y.; et al. Evaluation of transcatheter arterial embolization with gelatin sponge particles, microcoils, and n-butyl cyanoacrylate for acute arterial bleeding in a coagulopathic condition. J. Vasc. Interv. Radiol. 2009, 20, 1176–1187. [Google Scholar] [CrossRef]
- Tipaldi, M.A.; Orgera, G.; Krokidis, M.; Rebonato, A.; Maiettini, D.; Vagnarelli, S.; Ambrogi, C.; Rossi, M. Trans Arterial Embolization of Non-variceal Upper Gastrointestinal Bleeding: Is the Use of Ethylene-Vinyl Alcohol Copolymer as Safe as Coils? Cardiovasc. Interv. Radiol. 2018, 41, 1340–1345. [Google Scholar] [CrossRef]
- Yonemitsu, T.; Kawai, N.; Sato, M.; Sonomura, T.; Takasaka, I.; Nakai, M.; Minamiguchi, H.; Sahara, S.; Iwasaki, Y.; Naka, T.; et al. Comparison of hemostatic durability between N-butyl cyanoacrylate and gelatin sponge particles in transcatheter arterial embolization for acute arterial hemorrhage in a coagulopathic condition in a swine model. Cardiovasc. Interv. Radiol. 2010, 33, 1192–1197. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chang, C.-C.; Liou, J.-M.; Jaw, F.-S.; Liu, K.-L. Transcatheter arterial embolization with N-butyl cyanoacrylate for nonvariceal upper gastrointestinal bleeding in hemodynamically unstable patients: Results and predictors of clinical outcomes. J. Vasc. Interv. Radiol. 2014, 25, 1850–1857. [Google Scholar] [CrossRef]
- Abdulmalak, G.; Chevallier, O.; Falvo, N.; Di Marco, L.; Bertaut, A.; Moulin, B.; Abi-Khalil, C.; Gehin, S.; Charles, P.-E.; Latournerie, M.; et al. Safety and efficacy of transcatheter embolization with Glubran®2 cyanoacrylate glue for acute arterial bleeding: A single-center experience with 104 patients. Abdom. Radiol. 2018, 43, 723–733. [Google Scholar] [CrossRef]
- Shi, Z.X.; Yang, J.; Liang, H.W.; Cai, Z.H.; Bai, B. Emergency transcatheter arterial embolization for massive gastrointestinal arterial hemorrhage. Medicine 2017, 96, e9437. [Google Scholar] [CrossRef] [PubMed]
- Aina, R.; Oliva, V.L.; Therasse, E.; Perreault, P.; Bui, B.T.; Dufresne, M.-P.; Soulez, G. Arterial embolotherapy for upper gastrointestinal hemorrhage: Outcome assessment. J. Vasc. Interv. Radiol. 2001, 12, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Nakano, S.; Kumada, T.; Takeda, I.; Sugiyama, K.; Osada, T.; Kiriyama, S. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J. Gastroenterol. Hepatol. 1996, 11, 252–258. [Google Scholar] [CrossRef]
- López-Martínez, L.; Molina-Nuevo, J.D.; Pedrosa-Jiménez, M.J.; Juliá-Mollá, E. Spontaneous Haematomas in Anticoagulated COVID-19 Patients: Diagnosis and Treatment by Embolization. Cardiovasc. Interv. Radiol. 2022, 45, 1001–1006. [Google Scholar] [CrossRef]
- Khalil, A.; Fartoukh, M.; Bazot, M.; Parrot, A.; Marsault, C.; Carette, M.-F. Systemic arterial embolization in patients with hemoptysis: Initial experience with ethylene vinyl alcohol copolymer in 15 cases. AJR Am. J. Roentgenol. 2010, 194, W104–W110. [Google Scholar] [CrossRef]
- Vaidya, S.; Tozer, K.R.; Chen, J. An overview of embolic agents. Semin Interv. Radiol. 2008, 25, 204–215. [Google Scholar] [CrossRef]
- Spies, J.B.; Cornell, C.; Worthington-Kirsch, R.; Lipman, J.C.; Benenati, J.F. Long-term outcome from uterine fibroid embolization with tris-acryl gelatin microspheres: Results of a multicenter study. J. Vasc. Interv. Radiol. 2007, 18, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.; Zhang, X.; Ye, C.; Wang, S.; An, X. Development of PVA-based microsphere as a potential embolization agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 135, 112677. [Google Scholar] [CrossRef]
- Comai, A.; Zatelli, M.; Haglmuller, T.; Bonatti, G. The Role of Transcatheter Arterial Embolization in Traumatic Pelvic Hemorrhage: Not Only Pelvic Fracture. Cureus 2016, 8, e722. [Google Scholar] [CrossRef]
- O’Neill, P.A.; Riina, J.; Sclafani, S.; Tornetta, P. Angiographic findings in pelvic fractures. Clin. Orthop. Relat. Res. 1996, 329, 60–67. [Google Scholar] [CrossRef]
- Gourlay, D.; Hoffer, E.; Routt, M.; Bulger, E. Pelvic angiography for recurrent traumatic pelvic arterial hemorrhage. J. Trauma 2005, 59, 1168–1173; discussion 1173–1174. [Google Scholar] [CrossRef]
- Fang, J.-F.; Shih, L.-Y.; Wong, Y.-C.; Lin, B.-C.; Hsu, Y.-P. Repeat transcatheter arterial embolization for the management of pelvic arterial hemorrhage. J. Trauma 2009, 66, 429–435. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Starr, A.; Minei, J.P.; O’Keefe, G.E.; Scalea, T.M. The importance of fracture pattern in guiding therapeutic decision-making in patients with hemorrhagic shock and pelvic ring disruptions. J. Trauma 2002, 53, 446–450; discussion 450–451. [Google Scholar] [CrossRef]

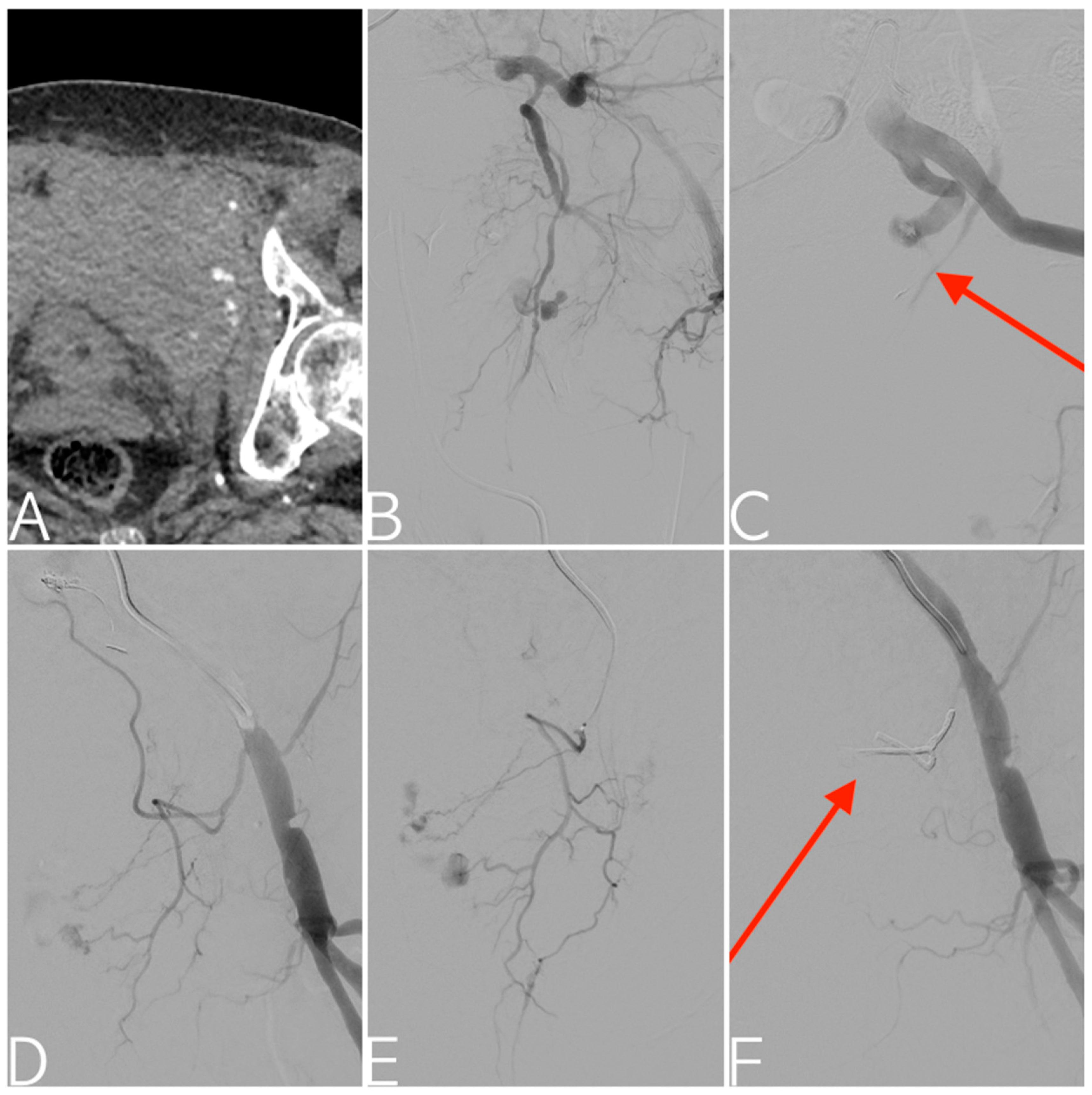
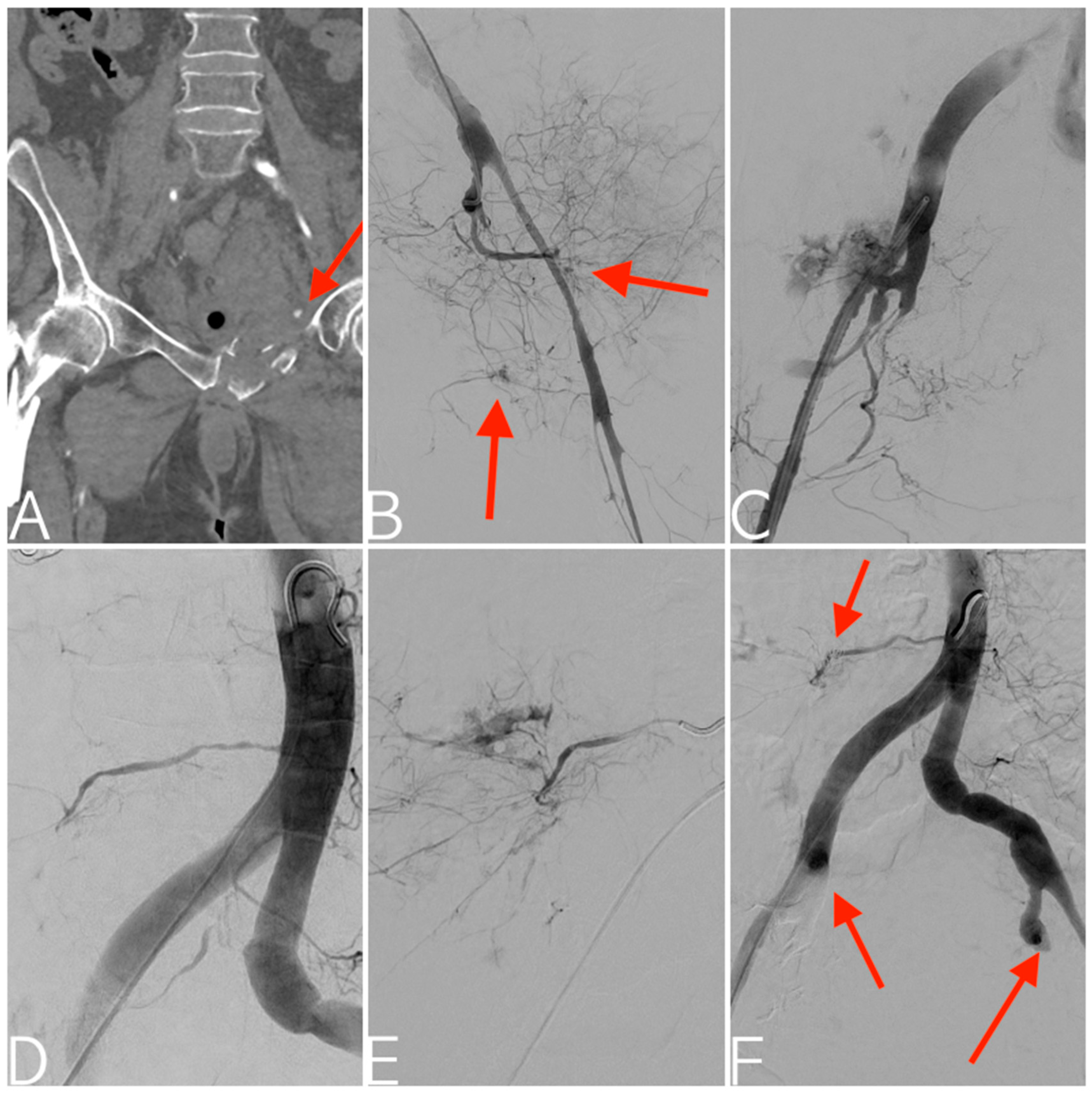

| Variables | All Patients (n = 116) |
|---|---|
| Age (years) | 56.5 (±23.4) |
| Sex (M/F) | 82 (70.7%)/34 (29.3%) |
| BMI | 26.1 (±4) |
| eGFR (mL/min) | 69.3 (±24.1) |
| INR | 1.3 (±0.3) |
| aPTT (s) | 41.1 (±5.9) |
| Platelet count (No. ×103/μL) | 325.1 (±109) |
| Coagulopathy (no/yes) | 80 (69.0%)/36 (31.0%) |
| - INR > 1.5 | 32 (27.6%) |
| - aPTT > 45 s | 32 (27.6%) |
| - PLT < 80,000/mm3 | 10 (8.6%) |
| Baseline hemoglobin (g/dL) | 7.9 (±0.9) |
| Antiplatelet therapy | 23 (19.8%) |
| Anticoagulant therapy | 35 (30.2%) |
| Antiplatelet AND anticoagulant therapy | 0 (0%) |
| Antiplatelet OR anticoagulant therapy | 58 (50.0%) |
| Mechanism of pelvic trauma | |
| - Blunt | 102 (87.9%) |
| - Penetrating | 14 (12.1%) |
| Hemodynamic stability/instability | 72 (62.1%)/44 (37.9%) |
| Young–Burgess classification of pelvic fracture | |
| - Stable | 36 (31.0%) |
| - Unstable | 80 (69.0%) |
| WSES classification pelvic ring injuries | |
| - Minor (grade I) | 24 (20.7%) |
| - Moderate (grade II/grade III) | 48 (41.4%)–(20.7%/20.7%) |
| - Severe (grade IV) | 44 (37.9%) |
| Injury Severity Score | 28.8 (±15) |
| Extra-pelvic injury | 78 (67.2%) |
| CT angiography execution | 98 (84.5%) |
| Bleeding on CT angiography | 86 (74.1%) |
| - Direct sign | 56 (65.1%) |
| - Indirect sign | 30 (34.9%) |
| Hematoma volume (mL) | 289.1 (±309) |
| Variables | All Patients (n = 116) |
|---|---|
| Bleeding on XA | |
| - No (blind embolization) | 6 (5.2%) |
| - Yes (targeted embolization) | 110 (94.8%) |
| Site of bleeding | |
| - Internal iliac artery (uni-/bi-lateral) | 104 (89.7%)–(62.1%/27.6%) |
| - External iliac artery | 6 (5.2%) |
| - Internal AND external iliac arteries | 6 (5.2%) |
| Main bleeding vessel | |
| - Superior gluteal | 36 (31%) |
| - Iliolumbar | 17 (14.6%) |
| - Lateral sacral | 5 (4.3%) |
| - Inferior gluteal | 6 (5.2%) |
| - Superior vesical | 5 (4.3%) |
| - Inferior vesical/vaginal | 4 (3.4%) |
| - Middle rectal | 6 (5.2%) |
| - Internal pudendal | 14 (12.1%) |
| - Obturator | 13 (11.2%) |
| - Others (e.g., uterine, ext. iliac branches, etc.) | 10 (8.7%) |
| Number of embolized vessels | 1.4 (±0.5) |
| Type of angioembolization | |
| - Prophylactic (uni-/bi-lateral) | 60 (51.7%) |
| - Distal | 56 (48.3%) |
| Main embolic agent | |
| - Temporary (gelatin sponge) | 66 (56.9%) |
| - Others | 50 (43.1%) |
| o Coils | 30 (25.9%) |
| o PVA particles or microspheres | 2 (1.7%) |
| o NBCA | 10 (8.6%) |
| o NALEAs (Onyx or Squid) | 8 (6.9%) |
| Intraoperative contrast medium (mL) | 36.8 (±15.4) |
| Volume of contrast to creatinine clearance ratio | 0.7 (±0.8) |
| Vascular access site | |
| - Femoral | 108 (93.1%) |
| - Radial | 4 (3.4%) |
| - Brachial | 4 (3.4%) |
| Sheath diameter, 4F/5F/6F/≥ 7F | 22 (19.0%)/76 (65.5%)/14 (12.1%)/4 (3.4%) |
| Door-to-groin puncture time (min) | 91.2 (±76.2) |
| Procedure time (min) | 30.2 (±10.9) |
| Time-to-embolization time (min) | 120.1 (±75.4) |
| Fluoroscopy time (min) | 9.5 (±4) |
| Cumulative air kerma (mGy) | 169.6 (±66.8) |
| Dose area product (DAP) (Gy/cm2) | 27.4 (±10.4) |
| Variables | All Patients (n = 116) |
|---|---|
| Technical success | 116 (100%) |
| Clinical success | 106 (91.4%) |
| Coagulopathy correction within 24 h of TAE | 36 (100%) |
| Vascular access site hemostasis | |
| - Manual compression | 50 (43.1%) |
| - Vascular closure device | 66 (56.9%) |
| Units of packed red blood cells transfused per patient | 1.5 (±1.5) |
| Rebleeding | 22 (19.0%) |
| Repeated XA | 22 (19.0%) |
| - Same bleeding site | 8 (36.4%) |
| - Different bleeding site | 14 (63.6%) |
| Non-target embolization | 0 (0%) |
| Trauma-induced coagulopathy (TIC) occurrence after TAE | 28 (24.1%) |
| Procedure-related complication rate | 14 (12.1%) |
| Vascular access site complication (VASC) rate | 4 (3.4%) |
| Procedure-related complications (SIR classification) | |
| - None | 102 (87.9%) |
| - Minor (grades 1-2) | 13 (11.2%) |
| - Major (grades 3-4-5) | 1 (0.9%) |
| Procedure-related complications (CIRSE classification) | |
| - None | 102 (87.9%) |
| - Grade 2 | 6 (5.2%) |
| - Grade 3 | 8 (6.9%) |
| Treatment required for complications | |
| - None | 6 (42.8%) |
| - Medical | 7 (50%) |
| - Interventional | 0 (0%) |
| - Surgical | 1 (7.2%) |
| 30-day bleeding-related mortality | 14 (12.1%) |
| 30-day mortality | 20 (17.2%) |
| Variables | Group 1 (n = 72) Hemodynamic Stability | Group 2 (n = 44) Hemodynamic Instability | p-Value |
|---|---|---|---|
| Age (years) | 57.5 (±23) | 54.8 (±24.3) | 0.3246 |
| BMI | 26.1 (±4.2) | 26.1 (±3.8) | 0.8798 |
| INR | 1.33 (±0.3) | 1.26 (±0.3) | 0.2446 |
| Coagulopathy | 22 (30.5%) | 14 (31.8%) | 1 |
| Baseline hemoglobin (g/dL) | 8 (±0.3) | 7.7 (±0.8) | 0.0869 |
| Young–Burgess classification of pelvic fracture (stable/unstable) | 24 (33.3%)/48 (66.7%) | 12 (27.3%)/32 (72.7%) | 0.5404 |
| Injury Severity Score | 27.4 (±15.4) | 31 (±14.1) | 0.2593 |
| Extra-pelvic injury | 46 (63.9%) | 32 (72.7%) | 0.4156 |
| Hematoma volume (mL) | 222.3 (±226.4) | 398.5 (±388.5) | 0.2704 |
| Prophylactic angioembolization | 20 (27%) | 40 (90.9%) | <0.0001 |
| Temporary embolic agent (gelatin sponge) | 28 (38.9%) | 38 (86.4%) | <0.0001 |
| Time-to-embolization time (min) | 139.2 (±75.6) | 64.6 (±64.6) | <0.0001 |
| Technical success | 72 (100%) | 44 (100%) | 1 |
| Clinical success | 66 (91.7%) | 40 (90.9%) | 1 |
| Coagulopathy correction within 24 h of TAE | 22 (100%) | 14 (100%) | 1 |
| Rebleeding | 14 (19.4%) | 8 (18.2%) | 1 |
| Trauma-induced coagulopathy (TIC) occurrence after TAE | 14 (19.4%) | 14 (31.8%) | 0.1794 |
| Procedure-related complication rate | 8 (11.1%) | 6 (13.6%) | 0.7717 |
| Vascular access site complication (VASC) Rate | 2 (2.8%) | 2 (4.5%) | 0.6335 |
| 30-day bleeding-related mortality | 8 (11.1%) | 6 (13.6%) | 0.7717 |
| 30-day mortality | 10 (13.9%) | 10 (22.7%) | 0.3108 |
| Variables | Prophylactic Angioembolization (n = 60) | Targeted Angioembolization (n = 56) | p-Value |
|---|---|---|---|
| Technical success | 60 (100%) | 56 (100%) | 1 |
| Clinical success | 60 (100%) | 46 (82.1%) | 0.001 |
| Rebleeding | 8 (13.3%) | 14 (25%) | 0.109 |
| Procedure-related Complication rate | 6 (10%) | 8 (14.3%) | 0.479 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minici, R.; Mercurio, M.; Guzzardi, G.; Venturini, M.; Fontana, F.; Brunese, L.; Guerriero, P.; Serra, R.; Piacentino, F.; Spinetta, M.; et al. Transcatheter Arterial Embolization for Bleeding Related to Pelvic Trauma: Comparison of Technical and Clinical Results between Hemodynamically Stable and Unstable Patients. Tomography 2023, 9, 1660-1682. https://doi.org/10.3390/tomography9050133
Minici R, Mercurio M, Guzzardi G, Venturini M, Fontana F, Brunese L, Guerriero P, Serra R, Piacentino F, Spinetta M, et al. Transcatheter Arterial Embolization for Bleeding Related to Pelvic Trauma: Comparison of Technical and Clinical Results between Hemodynamically Stable and Unstable Patients. Tomography. 2023; 9(5):1660-1682. https://doi.org/10.3390/tomography9050133
Chicago/Turabian StyleMinici, Roberto, Michele Mercurio, Giuseppe Guzzardi, Massimo Venturini, Federico Fontana, Luca Brunese, Pasquale Guerriero, Raffaele Serra, Filippo Piacentino, Marco Spinetta, and et al. 2023. "Transcatheter Arterial Embolization for Bleeding Related to Pelvic Trauma: Comparison of Technical and Clinical Results between Hemodynamically Stable and Unstable Patients" Tomography 9, no. 5: 1660-1682. https://doi.org/10.3390/tomography9050133
APA StyleMinici, R., Mercurio, M., Guzzardi, G., Venturini, M., Fontana, F., Brunese, L., Guerriero, P., Serra, R., Piacentino, F., Spinetta, M., Zappia, L., Costa, D., Coppola, A., MGJR Research Team, Galasso, O., & Laganà, D. (2023). Transcatheter Arterial Embolization for Bleeding Related to Pelvic Trauma: Comparison of Technical and Clinical Results between Hemodynamically Stable and Unstable Patients. Tomography, 9(5), 1660-1682. https://doi.org/10.3390/tomography9050133











