An Updated Review on Imaging and Staging of Anal Cancer—Not Just Rectal Cancer
Abstract
:1. Introduction
2. Clinical Features and Epidemiology
3. Histology
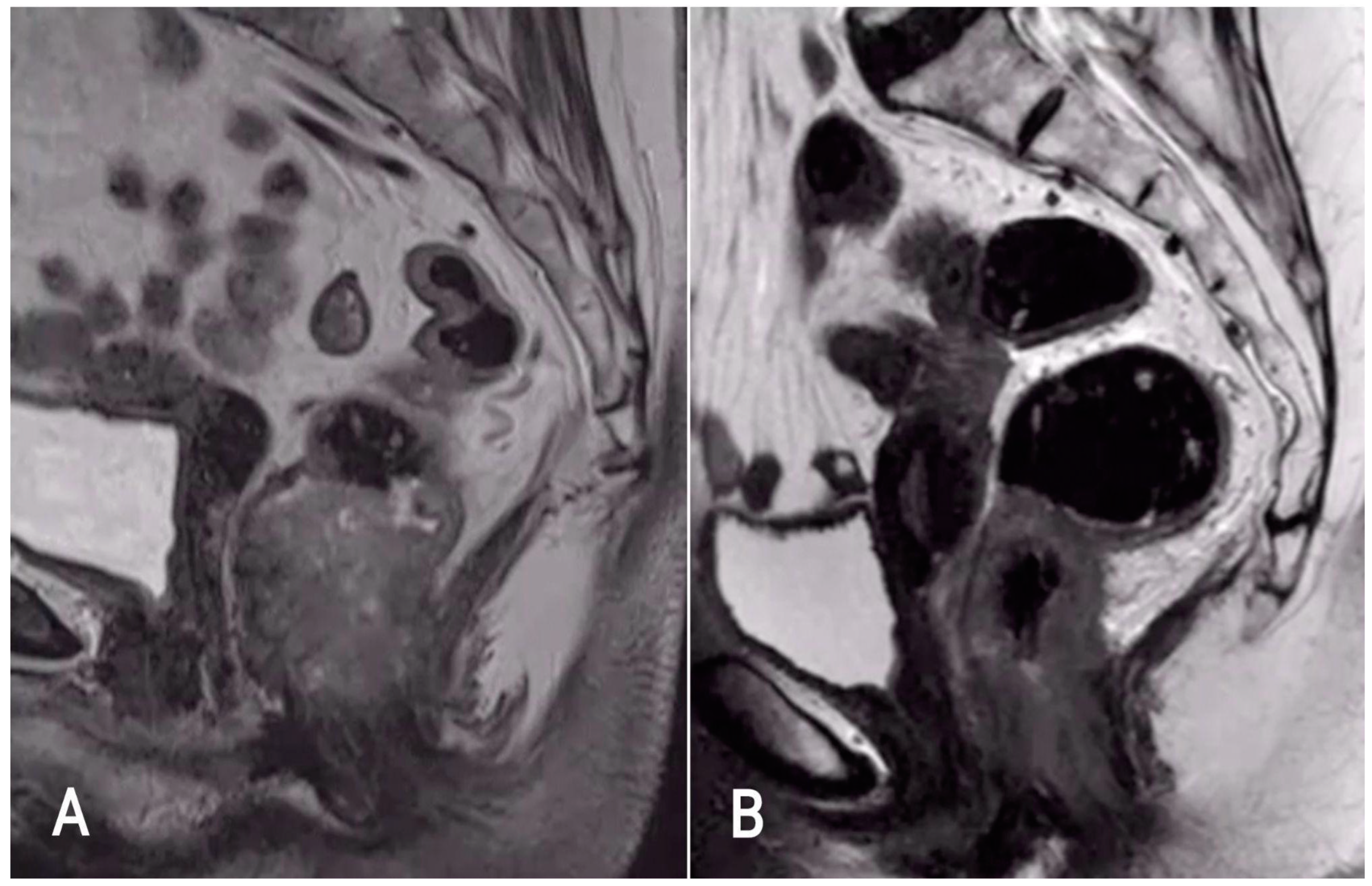
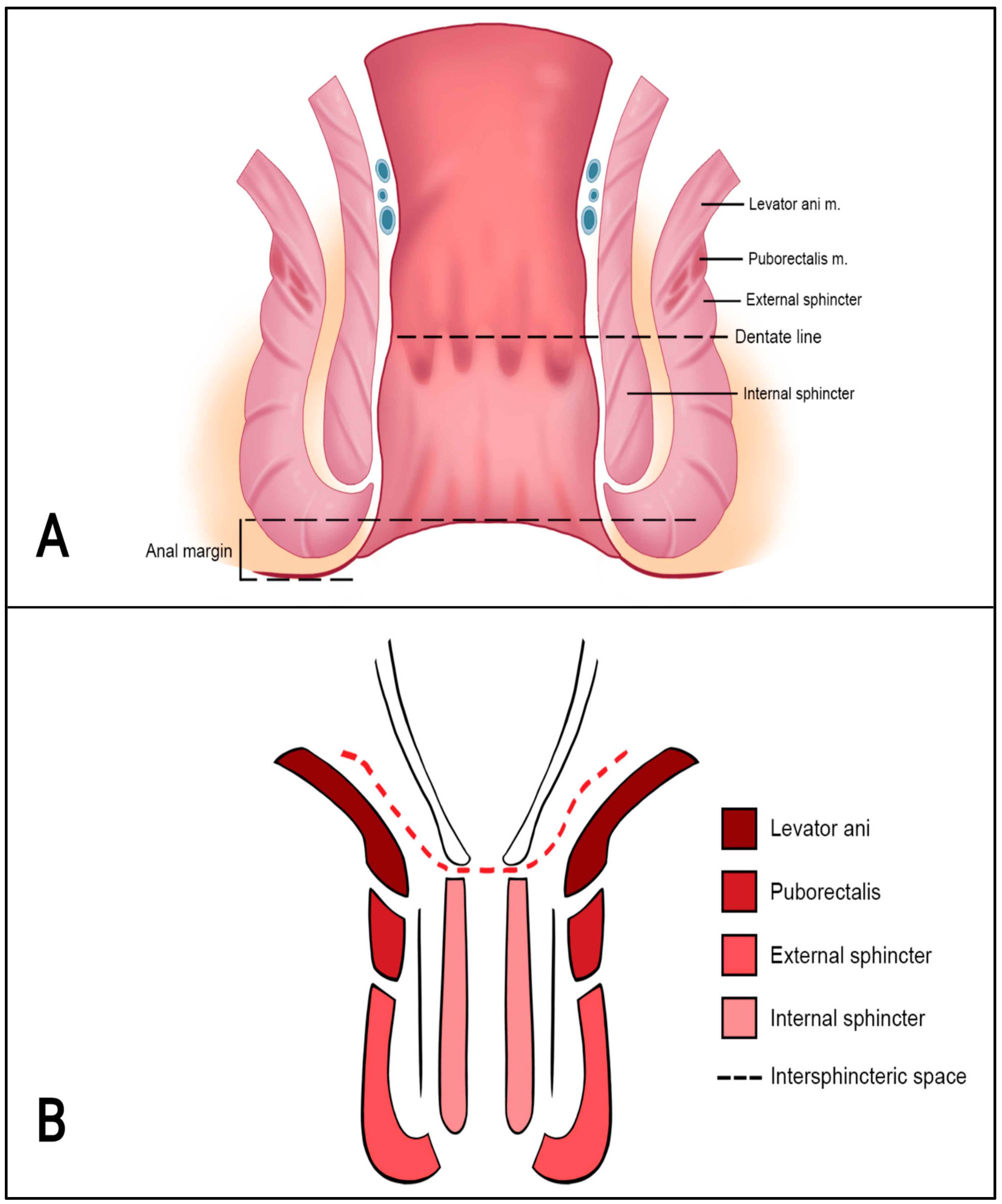
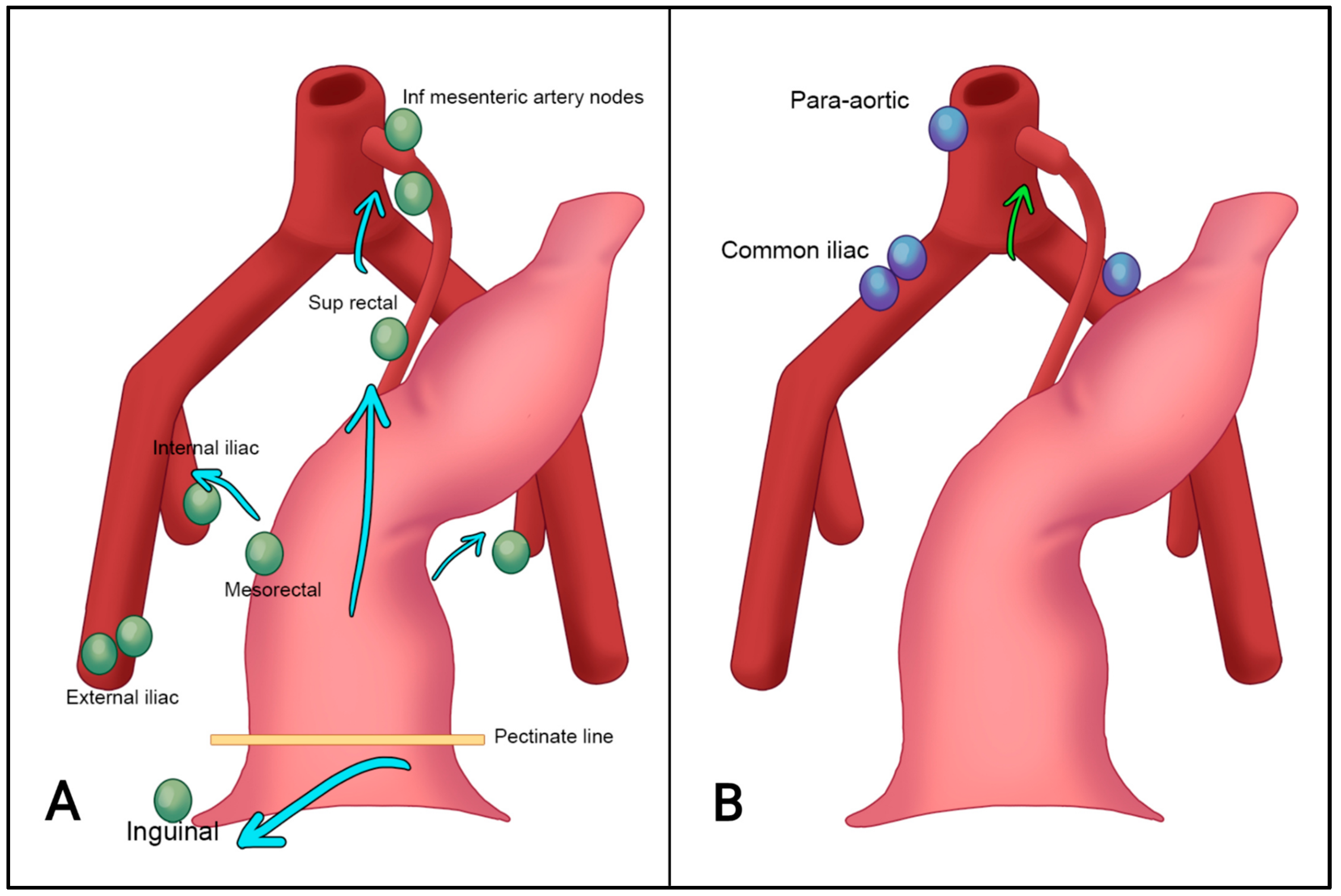
4. Anatomy of the Anal Canal Location
5. MR Technique
6. Diagnosis
7. Staging
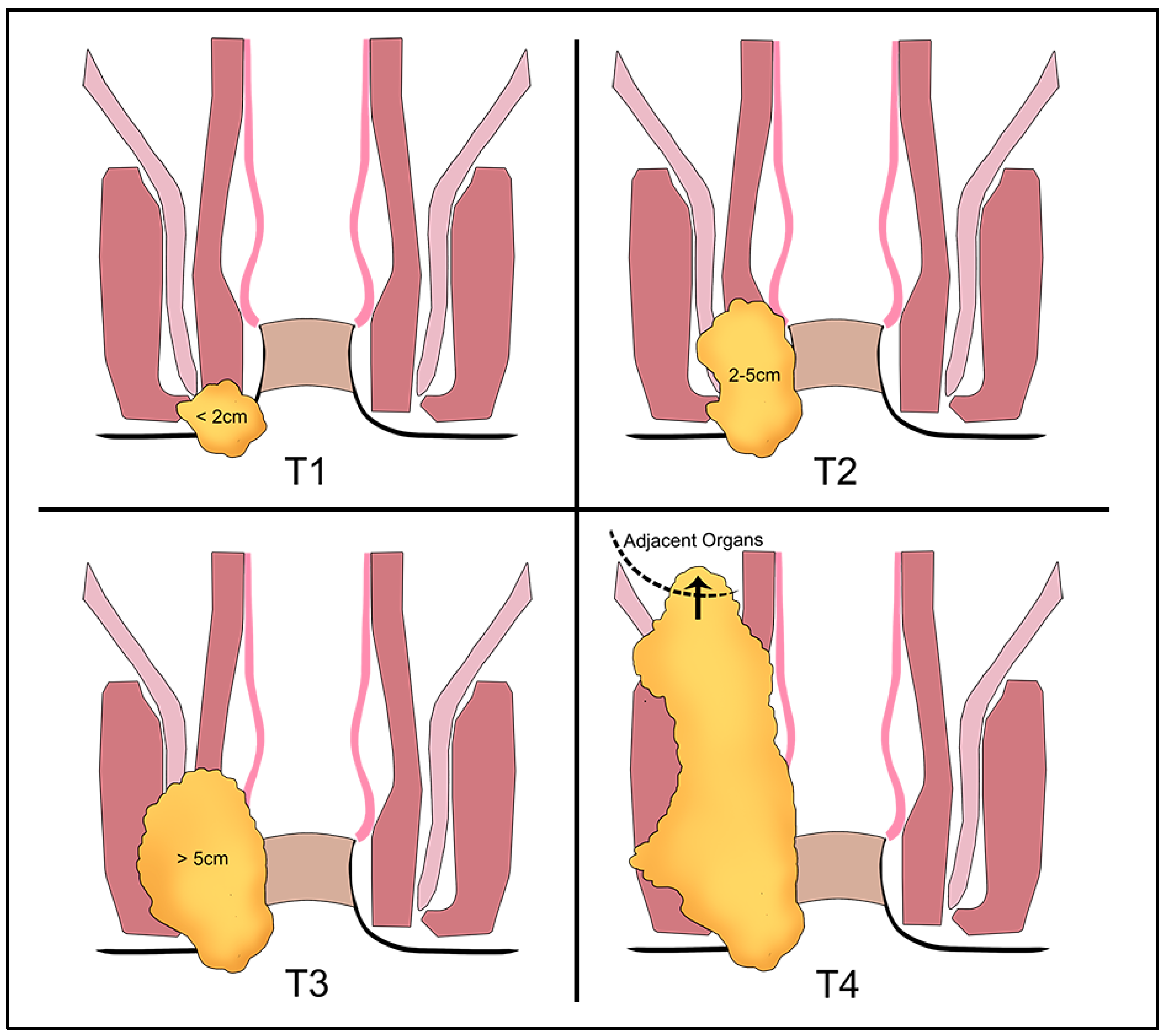
7.1. T-Staging
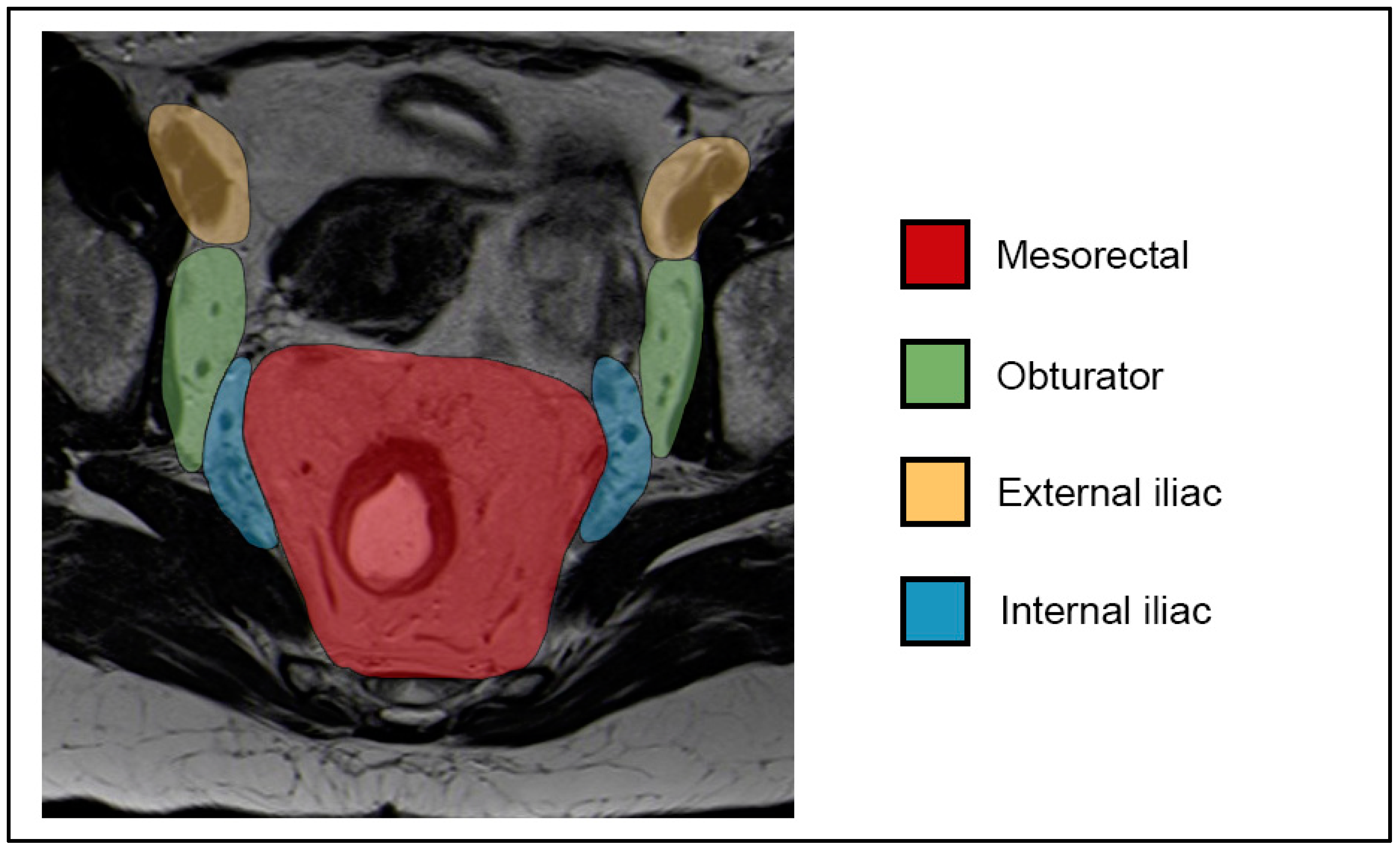
7.2. N-Staging
7.3. M-Staging
8. Treatment
Evaluation after Chemoradiation Therapy
9. Difference between Anal and Rectal Cancer TNM
- Short-axis diameter ≥ 9 mm;
- Short-axis diameter 5–8 mm AND ≥2 morphologically suspicious characteristics *;
- Short-axis diameter < 5 mm AND 3 morphologically suspicious characteristics *;
- Mucinous lymph nodes (any size).
- Round shape;
- Irregular border;
- Heterogeneous signal.
Difference in Staging in Anal Cancer from TNM7 to TNM8
10. Summary
11. Example Report for Baseline Staging of Anal Cancer [68]
- Perianal skin vs. anal canal (cranial or caudal half);
- Circumferential position + degree of sphincter involvement;
- Extension into the rectum.
- It is not possible to evaluate the primary tumor/no primary tumor can be identified (Tx/T0);
- ≤2 cm (T1);
- >2 and ≤5 cm (T2);
- >5 cm (T3).
- Inguinal, mesorectal, internal iliac/obturator (N1a);
- External iliac (N1b);
- Both N1a and N1b (N1c);
- Common iliac, para-aortic (M1).
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; Werner, R.N.; Koswig, S.; Gaskins, M.; Rödel, C.; Aigner, F. Anal cancer—Diagnosis, treatment and follow-up. Dtsch. Arztebl. Int. 2021, 118, 217–224. [Google Scholar] [PubMed]
- Cattapan, K.; Chulroek, T.; Wancharoenrung, D.; Kordbacheh, H.; Harisinghani, M. Can MR imaging be useful in differentiating low rectal cancer from anal cancer? Abdom. Radiol. 2019, 44, 438–445. [Google Scholar] [CrossRef]
- Nelson, R.A.; Levine, A.M.; Bernstein, L.; Smith, D.D.; Lai, L.L. Changing patterns of anal canal carcinoma in the United States. J. Clin. Oncol. 2013, 31, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Golia Pernicka, J.S.; Sheedy, S.P.; Ernst, R.D.; Minsky, B.D.; Ganeshan, D.; Rauch, G.M. MR staging of anal cancer: What the radiologist needs to know. Abdom. Radiol. 2019, 44, 3726–3739. [Google Scholar] [CrossRef]
- Hemachandran, N.; Goyal, A.; Bhattacharjee, H.K.; Sharma, R. Radiology of anal and lower rectal cancers. Clin. Radiol. 2021, 76, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Pessia, B.; Romano, L.; Giuliani, A.; Lazzarin, G.; Carlei, F.; Schietroma, M. Squamous cell anal cancer: Management and therapeutic options. Ann. Med. Surg. 2020, 55, 36–46. [Google Scholar] [CrossRef]
- Islami, F.; Ferlay, J.; Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 2017, 46, 924–938. [Google Scholar] [CrossRef]
- Young, A.N.; Jacob, E.; Willauer, P.; Smucker, L.; Monzon, R.; Oceguera, L. Anal Cancer. Surg. Clin. N. Am. 2020, 100, 629–634. [Google Scholar] [CrossRef]
- Mahmud, A.; Poon, R.; Jonker, D. PET imaging in anal canal cancer: A systematic review and meta-analysis. Br. J. Radiol. 2017, 90, 1080. [Google Scholar] [CrossRef]
- Durot, C.; Dohan, A.; Boudiaf, M.; Servois, V.; Soyer, P.; Hoeffel, C. Cancer of the anal canal: Diagnosis, staging and follow-up with MRI. Korean J. Radiol. 2017, 18, 946–956. [Google Scholar] [CrossRef]
- Ciombor, K.K.; Ernst, R.D.; Brown, G. Diagnosis and Diagnostic Imaging of Anal Canal Cancer. Surg. Oncol. Clin. N. Am. 2017, 26, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Erden, A. MRI of anal canal: Normal anatomy, imaging protocol, and perianal fistulas: Part 1. Abdom. Radiol. 2018, 43, 1334–1352. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, F.; Di Serafino, M.; Brillantino, A.; Mottola, A.; Del Giudice, S.; Stavolo, C.; Festa, P.; Patlas, M.N.; Scaglione, M.; Romano, L. Role of MRI in early follow-up of patients with solid organ injuries: How and why we do it? Radiol. Medica 2021, 126, 1328–1334. [Google Scholar] [CrossRef]
- Chiloiro, G.; Cusumano, D.; de Franco, P.; Lenkowicz, J.; Boldrini, L.; Carano, D.; Barbaro, B.; Corvari, B.; Dinapoli, N.; Giraffa, M.; et al. Does restaging MRI radiomics analysis improve pathological complete response prediction in rectal cancer patients? A prognostic model development. Radiol. Medica 2022, 127, 11–20. [Google Scholar] [CrossRef]
- Shannon, B.A.; Ahlawat, S.; Morris, C.D.; Levin, A.S.; Fayad, L.M. Do contrast-enhanced and advanced MRI sequences improve diagnostic accuracy for indeterminate lipomatous tumors? Radiol. Medica 2022, 127, 90–99. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R.; Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Medica 2021, 126, 1434–1450. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Stecco, A.; Micci, G.; Sconfienza, L.M.; Colagrande, S.; Reginelli, A.; Grassi, R.; Carriero, A.; Midiri, M.; Lagalla, R.; et al. Whole-body magnetic resonance imaging (WB-MRI) in oncology: An Italian survey. Radiol. Medica 2021, 126, 299–305. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Wang, A.; Chen, Y.; Wang, T.; Chen, D.; Zhang, J.; Brismar, T.B. Systematic review of machine learning-based radiomics approach for predicting microsatellite instability status in colorectal cancer. Radiol. Medica 2023, 128, 136–148. [Google Scholar] [CrossRef]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Medica 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- Di Serafino, M.; Vallone, G. The role of point of care ultrasound in radiology department: Update and prospective. A statement of Italian college ultrasound. Radiol. Medica 2021, 126, 636–641. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Nilsson, P.J.; Aschele, C.; Goh, V.; Peiffert, D.; Cervantes, A.; Arnold, D.; ESMO; ESSO; ESTRO. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother. Oncol. 2014, 111, 330–339. [Google Scholar] [CrossRef]
- Stewart, D.B.; Gaertner, W.B.; Glasgow, S.C.; Herzig, D.O.; Feingold, D.; Steele, S.R.; Prepared on Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American society of colon and rectal surgeons clinical practice guidelines for anal squamous cell cancers (Revised 2018). Dis. Colon Rectum 2018, 61, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Moureau-Zabotto, L.; Vendrely, V.; Abramowitz, L.; Borg, C.; Francois, E.; Goere, D.; Huguet, F.; Peiffert, D.; Siproudhis, L.; Ducreux, M.; et al. Anal cancer: French Intergroup Clinical Practice Guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SNFCP). Dig. Liver Dis. 2017, 49, 831–840. [Google Scholar] [CrossRef]
- Chulroek, T.; Kordbacheh, H.; Wangcharoenrung, D.; Cattapan, K.; Heidari, P.; Harisinghani, M.G. Comparative accuracy of qualitative and quantitative 18F-FDG PET/CT analysis in detection of lymph node metastasis from anal cancer. Abdom. Radiol. 2019, 44, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Medica 2022, 127, 21–29. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Medica 2022, 127, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Faggioni, L.; Regge, D.; Giovagnoni, A.; Golfieri, R.; Bibbolino, C.; Miele, V.; Neri, E.; Grassi, R. Artificial intelligence: Radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol. Medica 2021, 126, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Medica 2021, 126, 998–1006. [Google Scholar] [CrossRef]
- Zerunian, M.; Pucciarelli, F.; Caruso, D.; Polici, M.; Masci, B.; Guido, G.; De Santis, D.; Polverari, D.; Principessa, D.; Benvenga, A.; et al. Artificial intelligence based image quality enhancement in liver MRI: A quantitative and qualitative evaluation. Radiol. Medica 2022, 127, 1098–1105. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Medica 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Medica 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Flammia, F.; Innocenti, T.; Galluzzo, A.; Danti, G.; Chiti, G.; Grazzini, G.; Bettarini, S.; Tortoli, P.; Busoni, S.; Dragoni, G.; et al. Branch duct-intraductal papillary mucinous neoplasms (BD-IPMNs): An MRI-based radiomic model to determine the malignant degeneration potential. Radiol. Medica 2023, 128, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-L.; Zheng, H.-L.; Ding, F.-H.; Lu, J.; Chen, Q.-Y.; Xu, B.-B.; Xue, Z.; Lin, J.; Huang, C.-M.; Zheng, C.-H. Delta computed tomography radiomics features-based nomogram predicts long-term efficacy after neoadjuvant chemotherapy in advanced gastric cancer. Radiol. Medica 2023, 128, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Medica 2021, 126, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Palatresi, D.; Fedeli, F.; Danti, G.; Pasqualini, E.; Castiglione, F.; Messerini, L.; Massi, D.; Bettarini, S.; Tortoli, P.; Busoni, S.; et al. Correlation of CT radiomic features for GISTs with pathological classification and molecular subtypes: Preliminary and monocentric experience. Radiol. Medica 2022, 127, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol. Medica 2021, 126, 1388–1395. [Google Scholar] [CrossRef]
- Cozzi, D.; Bicci, E.; Cavigli, E.; Danti, G.; Bettarini, S.; Tortoli, P.; Mazzoni, L.N.; Busoni, S.; Pradella, S.; Miele, V. Radiomics in pulmonary neuroendocrine tumours (NETs). Radiol. Medica 2022, 127, 609–615. [Google Scholar] [CrossRef]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic features for prostate cancer grade detection through formal verification. Radiol. Medica 2021, 126, 688–697. [Google Scholar] [CrossRef]
- Xue, K.; Liu, L.; Liu, Y.; Guo, Y.; Zhu, Y.; Zhang, M. Radiomics model based on multi-sequence MR images for predicting preoperative immunoscore in rectal cancer. Radiol. Medica 2022, 127, 702–713. [Google Scholar] [CrossRef]
- Maas, M.; Tielbeek, J.A.W.; Stoker, J. Staging of Anal Cancer: Role of MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2020, 28, 127–140. [Google Scholar] [CrossRef]
- Matalon, S.; Mamon, H.; Rosenthal, M. Anorectal cancer: Critical anatomic and staging distinctions that affect use of radiation therapy. Radiographics 2015, 35, 2090–2107. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Caravatta, L.; Di Tommaso, M.; Fasciolo, D.; Gasparini, L.; Di Guglielmo, F.C.; Augurio, A.; Vinciguerra, A.; Vecchi, C.; Genovesi, D. Cone-beam computed tomography for organ motion evaluation in locally advanced rectal cancer patients. Radiol. Medica 2021, 126, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Touboul, E.; Schlienger, M.; Buffat, L.; Lefkopoulos, D.; Pène, F.; Parc, R.; Tiret, E.; Gallot, D.; Malafosse, M.; Laugier, A. Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer 1994, 73, 1569–1579. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Anal Carcinoma, version 2.2018 clinical practice guidelines in Oncology. JNCCN J. Natl. Compr. Cancer Netw. 2018, 16, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Hruby, G.; Solomon, M.; Rutherford, N.; Martin, J. The Role of FDG-PET in the Initial Staging and Response Assessment of Anal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2015, 22, 3574–3581. [Google Scholar] [CrossRef]
- Sandach, P.; Kasper-Virchow, S.; Rischpler, C.; Herrmann, K. Molecular Imaging and Therapy of Colorectal and Anal Cancer. Semin. Nucl. Med. 2020, 50, 465–470. [Google Scholar] [CrossRef]
- Scialpi, M.; Moschini, T.O.; De Filippis, G. PET/contrast-enhanced CT in oncology: “to do, or not to do, that is the question”. Radiol. Medica 2022, 127, 925–927. [Google Scholar] [CrossRef]
- Parikh, J.; Shaw, A.; Grant, L.A.; Schizas, A.M.; Datta, V.; Williams, A.B.; Griffin, N. Anal carcinomas: The role of endoanal ultrasound and magnetic resonance imaging in staging, response evaluation and follow-up. Eur. Radiol. 2011, 21, 776–785. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Masci, G.M.; Ciccarelli, F.; Mattei, F.I.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol. Medica 2022, 127, 251–258. [Google Scholar] [CrossRef]
- Rampado, O.; Depaoli, A.; Marchisio, F.; Gatti, M.; Racine, D.; Ruggeri, V.; Ruggirello, I.; Darvizeh, F.; Fonio, P.; Ropolo, R. Effects of different levels of CT iterative reconstruction on low-contrast detectability and radiation dose in patients of different sizes: An anthropomorphic phantom study. Radiol. Medica 2021, 126, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Niyoteka, S.; Seban, R.D.; Rouhi, R.; Scarsbrook, A.; Genestie, C.; Classe, M.; Carré, A.; Sun, R.; La Greca Saint-Esteven, A.; Chargari, C.; et al. A common [18F]-FDG PET radiomic signature to predict survival in patients with HPV-induced cancers. Eur. J. Nucl. Med. Mol. Imaging, 2023; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Medica 2022, 127, 763–772. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Medica 2022, 127, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Meijer, G.; Lenkowicz, J.; Chiloiro, G.; Boldrini, L.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Casà, C.; Damiani, A.; et al. A field strength independent MR radiomics model to predict pathological complete response in locally advanced rectal cancer. Radiol. Medica 2021, 126, 421–429. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Medica 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Wright, J.L.; Wright, J.L.; Patil, S.M.; Temple, L.K.; Minsky, B.D.; Saltz, L.B.; Goodman, K.A. Squamous cell carcinoma of the anal canal: Patterns and predictors of failure and implications for intensity-modulated radiation treatment planning. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1064–1072. [Google Scholar] [CrossRef]
- Gourtsoyianni, S.; Goh, V. MRI of anal cancer: Assessing response to definitive chemoradiotherapy. Abdom. Imaging 2014, 39, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.M.; Dzik-Jurasz, A.; O’Neill, B.; Tait, D.; Husband, J.E.; Brown, G. Pelvic phased-array MR imaging of anal carcinoma before and after chemoradiation. Br. J. Radiol. 2014, 81, 91–98. [Google Scholar] [CrossRef]
- Pozzessere, C.; Boudiaf, M.; Cirigliano, A.; Dohan, A.; Mazzei, M.A.; Barat, M.; Volterrani, L.; Soyer, P. MR-enterography: Role in the assessment of suspected anastomotic recurrence of Crohn disease after ileocolic resection. Radiol. Medica 2022, 127, 238–250. [Google Scholar] [CrossRef]
- Cicero, G.; Alibrandi, A.; Blandino, A.; Ascenti, V.; Fries, W.; Viola, A.; Mazziotti, S. DWI ratios: New indexes for Crohn’s disease activity at magnetic resonance enterography? Radiol. Medica 2022, 128, 16–26. [Google Scholar] [CrossRef]
- Gitto, S.; Bologna, M.; Corino, V.D.A.; Emili, I.; Albano, D.; Messina, C.; Armiraglio, E.; Parafioriti, A.; Luzzati, A.; Mainardi, L.; et al. Diffusion-weighted MRI radiomics of spine bone tumors: Feature stability and machine learning-based classification performance. Radiol. Medica 2022, 127, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, R.; Renehan, A.G.; Mullan, D.; Chakrabarty, B.; Saunders, M.P.; Carrington, B.M. The assessment of local response using magnetic resonance imaging at 3- and 6-month post chemoradiotherapy in patients with anal cancer. Eur. Radiol. 2017, 27, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Rocha CC, T.; Oliveira, B.C.; Petkovska, I.; Gollub, M.J. MRI of rectal cancer: Tumor staging, imaging techniques, and management. Radiographics 2019, 39, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef]
- Zhao, L.; Liang, M.; Wu, P.Y.; Yang, Y.; Zhang, H.; Zhao, X. A preliminary study of synthetic magnetic resonance imaging in rectal cancer: Imaging quality and preoperative assessment. Insights Imaging 2021, 12, 120. [Google Scholar] [CrossRef]
- Goffredo, P.; Garancini, M.; Robinson, T.J.; Frakes, J.; Hoshi, H.; Hassan, I. A National-Level Validation of the New American Joint Committee on Cancer 8th Edition Subclassification of Stage IIA and B Anal Squamous Cell Cancer. Ann. Surg. Oncol. 2018, 25, 1654–1660. [Google Scholar] [CrossRef]
- Kassam, Z.; Lang, R.; Arya, S.; Bates, D.D.B.; Chang, K.J.; Fraum, T.J.; Friedman, K.A.; Golia Pernicka, J.S.; Gollub, M.J.; Harisinghani, M.; et al. Update to the structured MRI report for primary staging of rectal cancer: Perspective from the SAR Disease Focused Panel on Rectal and Anal Cancer. Abdom. Radiol. 2022, 47, 3364–3374. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Congedo, A.; Mallardi, D.; Danti, G.; De Muzio, F.; Granata, V.; Miele, V. An Updated Review on Imaging and Staging of Anal Cancer—Not Just Rectal Cancer. Tomography 2023, 9, 1694-1710. https://doi.org/10.3390/tomography9050135
Congedo A, Mallardi D, Danti G, De Muzio F, Granata V, Miele V. An Updated Review on Imaging and Staging of Anal Cancer—Not Just Rectal Cancer. Tomography. 2023; 9(5):1694-1710. https://doi.org/10.3390/tomography9050135
Chicago/Turabian StyleCongedo, Alessio, Davide Mallardi, Ginevra Danti, Federica De Muzio, Vincenza Granata, and Vittorio Miele. 2023. "An Updated Review on Imaging and Staging of Anal Cancer—Not Just Rectal Cancer" Tomography 9, no. 5: 1694-1710. https://doi.org/10.3390/tomography9050135
APA StyleCongedo, A., Mallardi, D., Danti, G., De Muzio, F., Granata, V., & Miele, V. (2023). An Updated Review on Imaging and Staging of Anal Cancer—Not Just Rectal Cancer. Tomography, 9(5), 1694-1710. https://doi.org/10.3390/tomography9050135





