Our Journey from Individual Efforts to Nationwide Support: Implementing Newborn Screening for Spinal Muscular Atrophy in Serbia
Abstract
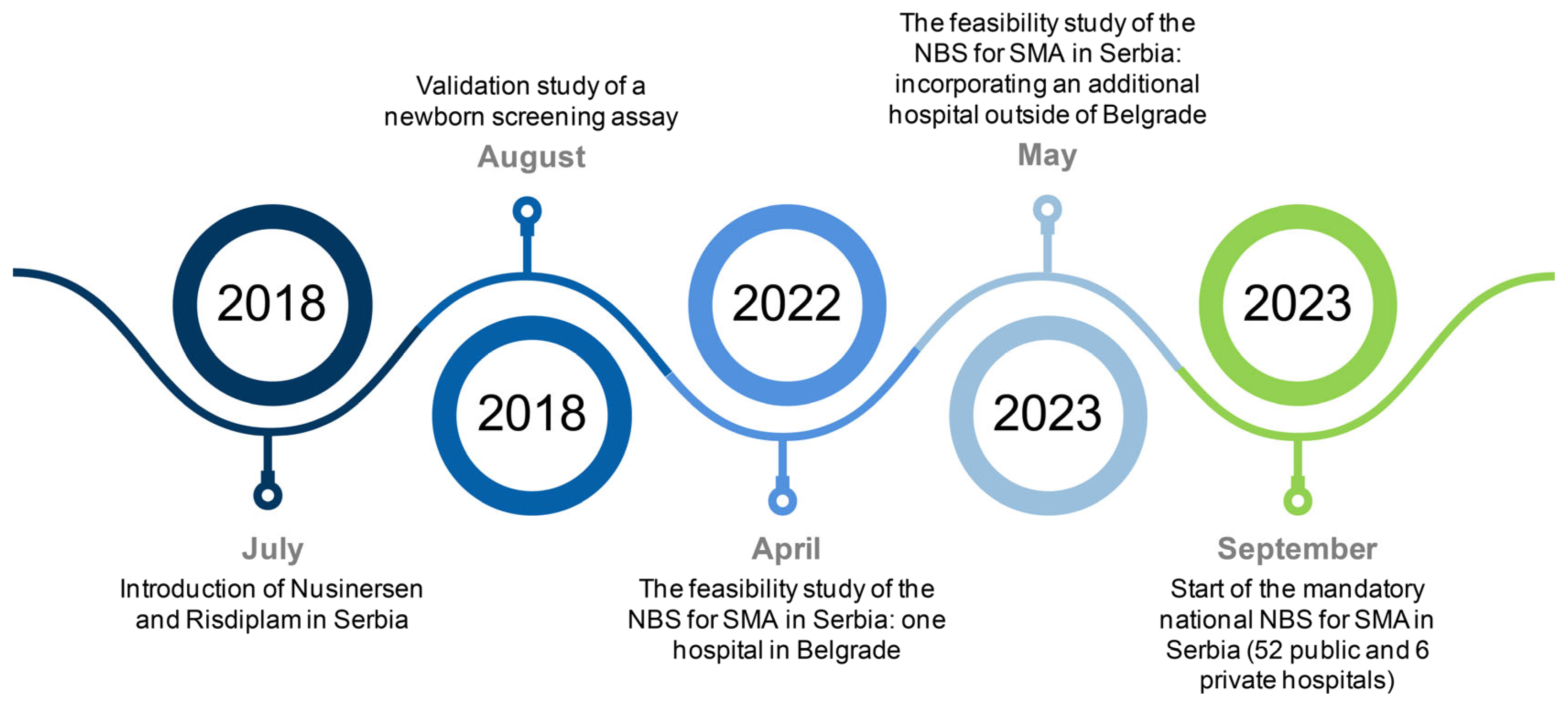
1. Introduction
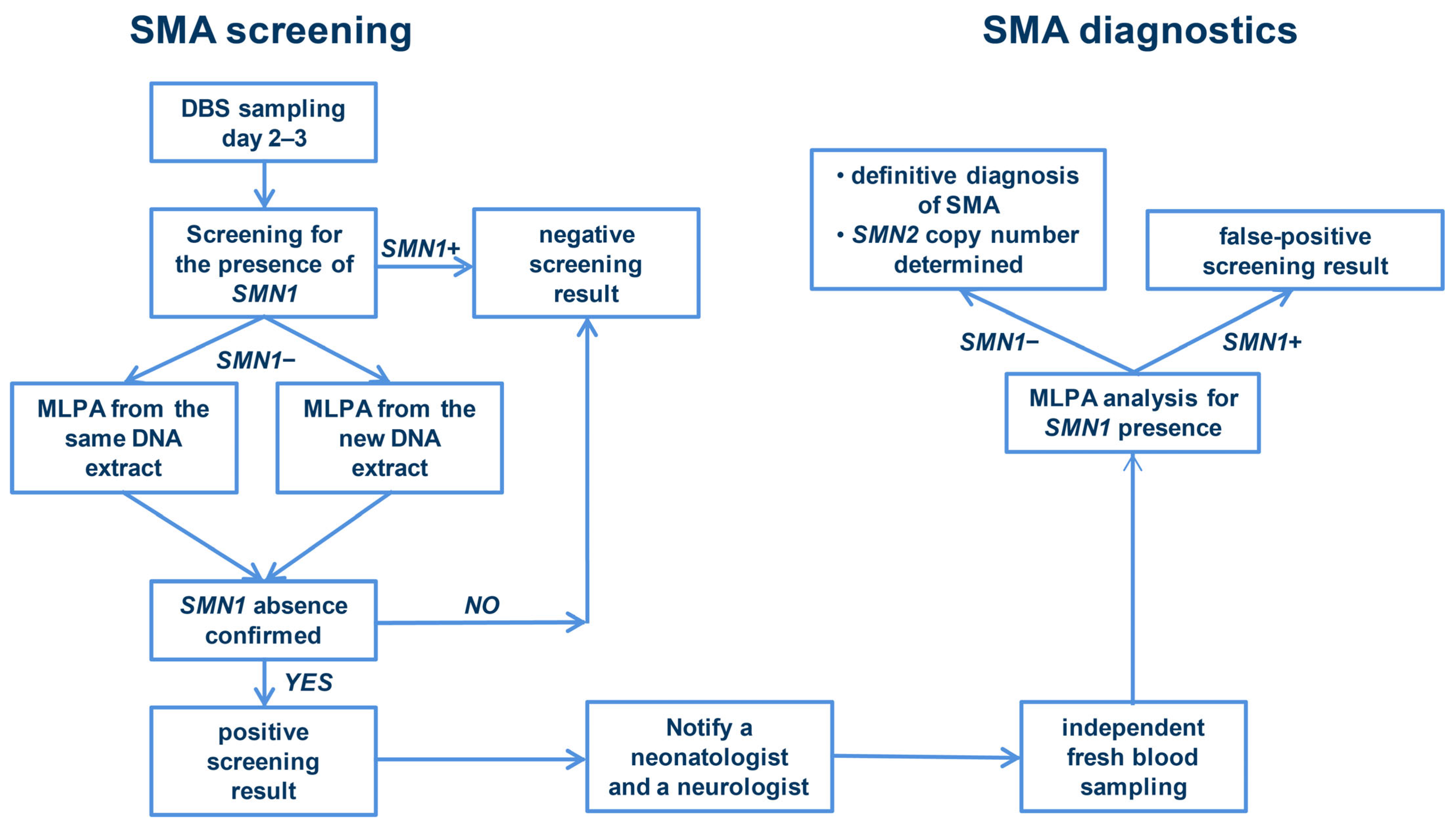
2. Materials and Methods
2.1. Validation Study of a Newborn Screening Assay
2.2. Collaborative Efforts Leading to Feasibility Study of the Newborn Screening for SMA
2.3. The Feasibility Study of the NBS for SMA in Serbia
3. Results
3.1. Results of the Validation Study of a NBS Assay
3.2. Results of the Feasibility Study of the NBS for SMA in Serbia
3.3. Key Findings of the National NBS for SMA in Serbia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brzustowicz, L.M.; Lehner, T.; Castilla, L.H.; Penchaszadeh, G.K.; Wilhelmsen, K.C.; Daniels, R.; Davies, K.E.; Leppert, M.; Ziter, F.; Wood, D.; et al. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2–13.3. Nature 1990, 344, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: Fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Brichta, L.; Schrank, B.; Lochmüller, H.; Blick, S.; Baasner, A.; Heller, R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006, 119, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Brkušanin, M.; Kosać, A.; Jovanović, V.; Pešović, J.; Brajušković, G.; Dimitrijević, N.; Todorović, S.; Romac, S.; Milić Rašić, V.; Savić-Pavićević, D. Joint effect of the SMN2 and SERF1A genes on childhood-onset types of spinal muscular atrophy in Serbian patients. J. Hum. Genet. 2015, 60, 723–728. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-spinal-muscular-atrophy (accessed on 26 June 2024).
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-oral-treatment-spinal-muscular-atrophy (accessed on 26 June 2024).
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/vaccines-blood-biologics/zolgensma (accessed on 26 June 2024).
- Le, T.T.; McGovern, V.L.; Alwine, I.E.; Wang, X.; Massoni-Laporte, A.; Rich, M.M.; Burghes, A.H. Temporal requirement for high SMN expression in SMA mice. Hum. Mol. Genet. 2011, 20, 3578–3591. [Google Scholar] [CrossRef]
- Staropoli, J.F.; Li, H.; Chun, S.J.; Allaire, N.; Cullen, P.; Thai, A.; Fleet, C.M.; Hua, Y.; Bennett, C.F.; Krainer, A.R.; et al. Rescue of gene-expression changes in an induced mouse model of spinal muscular atrophy by an antisense oligonucleotide that promotes inclusion of SMN2 exon 7. Genomics 2015, 105, 220–228. [Google Scholar] [CrossRef]
- Motyl, A.A.L.; Gillingwater, T.H. Timing is everything: Clinical evidence supports pre-symptomatic treatment for spinal muscular atrophy. Cell Rep. Med. 2022, 3, 100725. [Google Scholar] [CrossRef]
- Pera, M.C.; Coratti, G.; Berti, B.; D’Amico, A.; Sframeli, M.; Albamonte, E.; De Sanctis, R.; Messina, S.; Catteruccia, M.; Brigati, G.; et al. Diagnostic journey in Spinal Muscular Atrophy: Is it still an odyssey? PLoS ONE 2020, 15, e0230677. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Kalb, S.J.; Yeh, W.S. Delay in Diagnosis of Spinal Muscular Atrophy: A Systematic Literature Review. Pediatr. Neurol. 2015, 53, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Strunk, A.; Abbes, A.; Stuitje, A.R.; Hettinga, C.; Sepers, E.M.; Snetselaar, R.; Schouten, J.; Asselman, F.L.; Cuppen, I.; Lemmink, H.; et al. Validation of a Fast, Robust, Inexpensive, Two-Tiered Neonatal Screening Test algorithm on Dried Blood Spots for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2019, 5, 21. [Google Scholar] [CrossRef]
- MRC-Holland. Available online: https://www.mrcholland.com/products/34597/Instructions%20for%20Use%20MC002-A%20SMA%20Newborn%20Screen-v09.pdf (accessed on 13 August 2024).
- Schrank, B.; Götz, R.; Gunnersen, J.M.; Ure, J.M.; Toyka, K.V.; Smith, A.G.; Sendtner, M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl. Acad. Sci. USA 1997, 94, 9920–9925. [Google Scholar] [CrossRef] [PubMed]
- Maretina, M.A.; Zheleznyakova, G.Y.; Lanko, K.M.; Egorova, A.A.; Baranov, V.S.; Kiselev, A.V. Molecular Factors Involved in Spinal Muscular Atrophy Pathways as Possible Disease-modifying Candidates. Curr. Genom. 2018, 19, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Official Gazette of the Republic of Serbia. Available online: http://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/vlada/drugiakt/2019/86/1/reg (accessed on 27 June 2024).
- Verhaart, I.E.C.; Robertson, A.; Leary, R.; McMacken, G.; König, K.; Kirschner, J.; Jones, C.C.; Cook, S.F.; Lochmüller, H. A multi-source approach to determine SMA incidence and research ready population. J. Neurol. 2017, 264, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Genentech. Available online: https://www.gene.com/media/press-releases/14955/2022-05-30/fda-approves-genentechs-evrysdi-risdipla (accessed on 27 June 2024).
- Statistical Office of the Republic of Serbia. Natural Changes of Population. Available online: https://data.stat.gov.rs/Home/Result/180304?languageCode=en-US&displayMode=table&guid=0d48411b-7810-422b-a497-b9d807331058 (accessed on 27 June 2024).
- European Alliance for Newborn Screening in SMA. Whitepaper—Spinal Muscular Atrophy: Screen at Birth, Save Lives (Version 2, 25 November 2021). Available online: https://www.sma-screening-alliance.org/resources (accessed on 27 June 2024).
- CureSMA. Available online: https://www.curesma.org/wp-content/uploads/2024/01/NBS_Maps_Screening_States_2024.pdf (accessed on 27 June 2024).
- SMA NBS Alliance Status Map. Available online: https://www.sma-screening-alliance.org/map (accessed on 21 June 2024).
- Eggermann, K.; Gläser, D.; Abicht, A.; Wirth, B. Spinal muscular atrophy (5qSMA): Best practice of diagnostics, newborn screening and therapy. Med. Genetik. 2020, 32, 263–272. [Google Scholar] [CrossRef]
- Wirth, B.; Herz, M.; Wetter, A.; Moskau, S.; Hahnen, E.; Rudnik-Schöneborn, S.; Wienker, T.; Zerres, K. Quantitative analysis of survival motor neuron copies: Identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am. J. Hum. Genet. 1999, 64, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Alías, L.; Barceló, M.J.; Bernal, S.; Martínez-Hernández, R.; Also-Rallo, E.; Vázquez, C.; Santana, A.; Millán, J.M.; Baiget, M.; Tizzano, E.F. Improving detection and genetic counseling in carriers of spinal muscular atrophy with two copies of the SMN1 gene. Clin. Genet. 2014, 85, 470–475. [Google Scholar] [CrossRef]
- Glascock, J.; Sampson, J.; Connolly, A.M.; Darras, B.T.; Day, J.W.; Finkel, R.; Howell, R.R.; Klinger, K.W.; Kuntz, N.; Prior, T.; et al. Revised Recommendations for the Treatment of Infants Diagnosed with Spinal Muscular Atrophy Via Newborn Screening Who Have 4 Copies of SMN2. J. Neuromuscul. Dis. 2020, 7, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Brkušanin, M.; Kosać, A.; Branković-Srećković, V.; Jovanović, K.; Perić, S.; Karanović, J.; Matijašević Joković, S.; Garai, N.; Pešović, J.; Nikolić, D.; et al. Phosphorylated neurofilament heavy chain in cerebrospinal fluid and plasma as a Nusinersen treatment response marker in childhood-onset SMA individuals from Serbia. Front. Neurol. 2024, 15, 1394001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brkušanin, M.; Garai, N.; Karanović, J.; Šljivančanin Jakovljević, T.; Dimitrijević, A.; Jovanović, K.; Mitrović, T.L.; Miković, Ž.; Brajušković, G.; Nikolić, D.M.; et al. Our Journey from Individual Efforts to Nationwide Support: Implementing Newborn Screening for Spinal Muscular Atrophy in Serbia. Int. J. Neonatal Screen. 2024, 10, 57. https://doi.org/10.3390/ijns10030057
Brkušanin M, Garai N, Karanović J, Šljivančanin Jakovljević T, Dimitrijević A, Jovanović K, Mitrović TL, Miković Ž, Brajušković G, Nikolić DM, et al. Our Journey from Individual Efforts to Nationwide Support: Implementing Newborn Screening for Spinal Muscular Atrophy in Serbia. International Journal of Neonatal Screening. 2024; 10(3):57. https://doi.org/10.3390/ijns10030057
Chicago/Turabian StyleBrkušanin, Miloš, Nemanja Garai, Jelena Karanović, Tamara Šljivančanin Jakovljević, Aleksandra Dimitrijević, Kristina Jovanović, Tanja Lazić Mitrović, Željko Miković, Goran Brajušković, Dimitrije Mihailo Nikolić, and et al. 2024. "Our Journey from Individual Efforts to Nationwide Support: Implementing Newborn Screening for Spinal Muscular Atrophy in Serbia" International Journal of Neonatal Screening 10, no. 3: 57. https://doi.org/10.3390/ijns10030057
APA StyleBrkušanin, M., Garai, N., Karanović, J., Šljivančanin Jakovljević, T., Dimitrijević, A., Jovanović, K., Mitrović, T. L., Miković, Ž., Brajušković, G., Nikolić, D. M., & Savić-Pavićević, D. (2024). Our Journey from Individual Efforts to Nationwide Support: Implementing Newborn Screening for Spinal Muscular Atrophy in Serbia. International Journal of Neonatal Screening, 10(3), 57. https://doi.org/10.3390/ijns10030057






