Newborn Screening for Spinal Muscular Atrophy: Variations in Practice and Early Management of Infants with Spinal Muscular Atrophy in the United States
Abstract
1. Introduction
2. Methods
2.1. Survey of State NBS Programs
2.2. Survey of Providers Caring for Newborns with SMA
3. Results
3.1. Survey of State Newborn Screening Programs
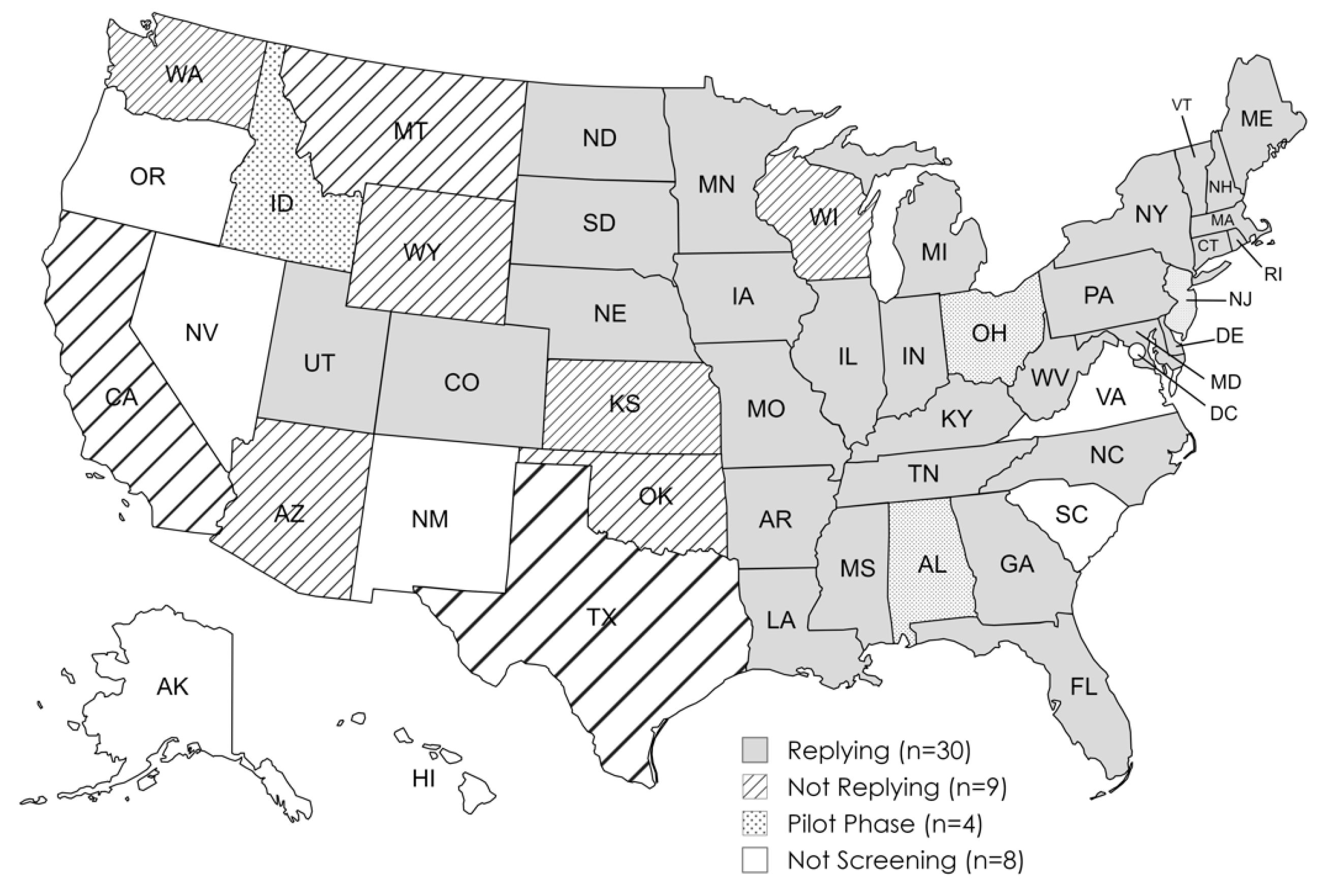
3.1.1. Participating States
3.1.2. NBS Results
3.1.3. Testing Methodology and Approach to Follow-Up
3.2. Survey of Providers Caring for Newborns with SMA
3.2.1. Respondent Characteristics
3.2.2. Respondent Practice Patterns
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef]
- Swoboda, K.J.; Prior, T.W.; Scott, C.B.; McNaught, T.P.; Wride, M.C.; Reyna, S.P.; Bromberg, M.B. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann. Neurol. 2005, 57, 704–712. [Google Scholar] [CrossRef]
- Lowes, L.P.; Alfano, L.N.; Arnold, W.D.; Shell, R.; Prior, T.W.; McColly, M.; Mendell, J. Impact of Age and Motor Function in a Phase 1/2A Study of Infants with SMA Type 1 Receiving Single-Dose Gene Replacement Therapy. Pediatr. Neurol. 2019, 98, 39–45. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Glascock, J.; Sampson, J.; Connolly, A.M.; Darras, B.T.; Day, J.W.; Finkel, R.; Howell, R.R.; Klinger, K.W.; Kuntz, N.; Prior, T.; et al. Revised Recommendations for the Treatment of Infants Diagnosed with Spinal Muscular Atrophy Via Newborn Screening Who Have 4 Copies of SMN2. J. Neuromuscul. Dis. 2020, 7, 97–100. [Google Scholar] [CrossRef]
- Hale, K.; Ojodu, J.; Singh, S. Landscape of Spinal Muscular Atrophy Newborn Screening in the United States: 2018–2021. Int. J. Neonatal Screen. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services CfDCaP, National Center for Environmental Health, Sciences DoL. Newborn Screening Quality Assurance Program. 2022 Annual Summary Report, Volume 40. Available online: https://www.cdc.gov/newborn-screening/media/pdfs/2024/05/NSQAP-Annual-Summary-2022-508_1.pdf (accessed on 1 May 2024).
- Curry, M.A.; Cruz, R.E.; Belter, L.T.; Schroth, M.K.; Jarecki, J. Assessment of Barriers to Referral and Appointment Wait Times for the Evaluation of Spinal Muscular Atrophy (SMA): Findings from a Web-Based Physician Survey. Neurol. Ther. 2024, 13, 583–598. [Google Scholar] [CrossRef]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—A literature review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
- Lee, B.H.; Deng, S.; Chiriboga, C.A.; Kay, D.M.; Irumudomon, O.; Laureta, E.; Delfiner, L.; Trfromer, S.O.; Anziska, Y.; Sakonju, A.; et al. Newborn Screening for Spinal Muscular Atrophy in New York State: Clinical Outcomes from the First 3 Years. Neurology 2022, 99, e1527–e1537. [Google Scholar] [CrossRef]
- Matteson, J.; Wu, C.H.; Mathur, D.; Tang, H.; Sciortino, S.; Feuchtbaum, L.; Bishop, T.; Sharma, S.C.; Neogi, P.; Fitzgibbon, I.; et al. California’s experience with SMA newborn screening: A successful path to early intervention. J. Neuromuscul. Dis. 2022, 9, 777–785. [Google Scholar] [CrossRef]
- Elkins, K.; Wittenauer, A.; Hagar, A.F.; Logan, R.; Sekul, E.; Xiang, Y.; Verma, S.; Wilcox, W.R. Georgia state spinal muscular atrophy newborn screening experience: Screening assay performance and early clinical outcomes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 187–196. [Google Scholar] [CrossRef]
- Hale, J.E.; Darras, B.T.; Swoboda, K.J.; Estrella, E.; Chen, J.Y.H.; Abbott, M.-A.; Hay, B.N.; Kumar, B.; Counihan, A.M.; Gerstel-Thompson, J.; et al. Massachusetts’ Findings from Statewide Newborn Screening for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2021, 7, 26. [Google Scholar] [CrossRef]
- Wirth, B.; Herz, M.; Wetter, A.; Moskau, S.; Hahnen, E.; Rudnik-Schöneborn, S.; Wienker, T.; Zerres, K. Quantitative analysis of survival motor neuron copies: Identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype-phenotype correlation, and implications for genetic counseling. Am. J. Hum. Genet. 1999, 64, 1340–1356. [Google Scholar] [CrossRef]
- Aragon-Gawinska, K.; Mouraux, C.; Dangouloff, T.; Servais, L. Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review. Genes 2023, 14, 1377. [Google Scholar] [CrossRef] [PubMed]
- Proud, C.M.; Mercuri, E.; Finkel, R.S.; Kirschner, J.; De Vivo, D.C.; Muntoni, F.; Saito, K.; Tizzano, E.F.; Desguerre, I.; Quijano-Roy, S.; et al. Combination disease-modifying treatment in spinal muscular atrophy: A proposed classification. Ann. Clin. Transl. Neurol. 2023, 10, 2155–2160. [Google Scholar] [CrossRef]
- Ribero, V.A.; Daigl, M.; Martí, Y.; Gorni, K.; Evans, R.; Scott, D.A.; Mahajan, A.; Abrams, K.R.; Hawkins, N. How does risdiplam compare with other treatments for Types 1–3 spinal muscular atrophy: A systematic literature review and indirect treatment comparison. J. Comp. Eff. Res. 2022, 11, 347–370. [Google Scholar] [CrossRef]
- Erdos, J.; Wild, C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: A systematic review of real-world study data. Eur. J. Paediatr. Neurol. 2022, 39, 1–10. [Google Scholar] [CrossRef]
- Jiang, T.; Youn, B.; Paradis, A.D.; Beckerman, R.; Barnieh, L.; Johnson, N.B. A Critical Appraisal of Matching-Adjusted Indirect Comparisons in Spinal Muscular Atrophy. Adv. Ther. 2023, 40, 2985–3005. [Google Scholar] [CrossRef]
- Oechsel, K.F.; Cartwright, M.S. Combination therapy with onasemnogene and risdiplam in spinal muscular atrophy type 1. Muscle Nerve 2021, 64, 487–490. [Google Scholar] [CrossRef]
- Lee, B.H.; Collins, E.; Lewis, L.; Guntrum, D.; Eichinger, K.; Voter, K.; Abdel-Hamid, H.Z.; Ciafaloni, E. Combination therapy with nusinersen and AVXS-101 in SMA type 1. Neurology 2019, 93, 640–641. [Google Scholar] [CrossRef]
- Harada, Y.; Rao, V.K.; Arya, K.; Kuntz, N.L.; DiDonato, C.J.; Napchan-Pomerantz, G.; Agarwal, A.; Stefans, V.; Katsuno, M.; Veerapandiyan, A. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve 2020, 62, 550–554. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. Risdiplam in Patients Previously Treated with Other Therapies for Spinal Muscular Atrophy: An Interim Analysis from the JEWELFISH Study. Neurol. Ther. 2023, 12, 543–557. [Google Scholar]
- Parsons, J.; Kuntz, N.; Brandsema, J.; Proud, C.; Finkel, R.; Swoboda, K.; Masson, R.; Foster, R.; Liu, Y.; Makepeace, C.; et al. P210 Interim results from the RESPOND study evaluating nusinersen in children with spinal muscular atrophy previously treated with onasemnogene abeparvovec. Neuromuscul. Disord. 2023, 33, S87. [Google Scholar] [CrossRef]
| Infants Screened (N = 22, Number of States Responding) | Positive Screens (N = 27) | False-Positive Screens (N = 25) | False-Negative Screens (N = 25) | |
|---|---|---|---|---|
| Sample Total * | 2,536,709 | 228 | 393 | 0 |
| State Median (Range) | 78,022 (2000–363,131) | 7 (0–30) | 0 (0–364) | 0 (0–0) |
| # of States/# Responding (%) | ||
|---|---|---|
| Testing Methodology | ||
| Quantitative real-time PCR | 23/24 (96%) | |
| Perform SMN2 copy number testing | 12/28 (43%) | |
| Perform confirmatory testing of positive result | 4/28 (14%) | |
| Positive NBS Screen Tracking/Follow-Up | ||
| Communicate directly to both the primary care and SMA care provider | 20/27 (74%) | |
| Track short-term course (referral to treatment center and confirmatory testing results) | 24/27 (89%) | |
| Track treatment choice | 21/25 (84%) | |
| Track longer term outcomes/longitudinal data collection | 5/25 (20%) | |
| Participation in a NBS registry | 26/26 (100%) | |
| Total Respondents | N = 41 | |
|---|---|---|
| Practice setting | ||
| Tertiary care center—academic | 37 (90%) | |
| Mostly pediatric | 37 (90%) | |
| Provide care for newborns with neuromuscular disease | 40 (98%) | |
| Has pediatric hospital admitting privileges | 41 (100%) | |
| Participated in pediatric SMA clinical trials | 19 (46%) | |
| Training background | ||
| Child Neurology | 33 (81%) | |
| Adult Neurology | 5 (12%) | |
| Neuromuscular/EMG | 31 (76%) | |
| Infant SMA therapeutic experience | ||
| Nusinersen | 40 (98%) | |
| Onasemnogene abeparvovec | 39 (95%) | |
| Risdiplam | 34 (83%) | |
| None | 1 (2%) | |
| Time from referral to evaluation | ||
| <72 h | 28 (68%) | |
| Within one week | 10 (24%) | |
| No referrals received | 3 (7%) | |
| Average infant age at treatment | ||
| <1 week | 0 (0%) | |
| 1–2 weeks | 6 (14%) | |
| 2–3 weeks | 16 (39%) | |
| 3–4 weeks | 11 (27%) | |
| >5 weeks | 5 (12%) | |
| None treated | 3 (7%) | |
| Preferred first-line treatment | ||
| Onasemnogene abeparvovec | 33 (81%) | |
| No preference | 5 (12%) | |
| Combination onasemnogene abeparvovec and risdiplam | 2 (5%) | |
| Risdiplam | 1 (2%) | |
| Nusinersen | 0 (0%) | |
| Most time-consuming step in initiating treatment | ||
| Insurance approval | 34 (83%) | |
| Genetic/laboratory testing | 5 (12%) | |
| Time to referral | 1 (2%) | |
| Time from insurance approval to treatment | 1 (2%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidman, C.M.; Crockett, C.D.; Wedge, E.; Tabatabai, G.; Goedeker, N. Newborn Screening for Spinal Muscular Atrophy: Variations in Practice and Early Management of Infants with Spinal Muscular Atrophy in the United States. Int. J. Neonatal Screen. 2024, 10, 58. https://doi.org/10.3390/ijns10030058
Zaidman CM, Crockett CD, Wedge E, Tabatabai G, Goedeker N. Newborn Screening for Spinal Muscular Atrophy: Variations in Practice and Early Management of Infants with Spinal Muscular Atrophy in the United States. International Journal of Neonatal Screening. 2024; 10(3):58. https://doi.org/10.3390/ijns10030058
Chicago/Turabian StyleZaidman, Craig M., Cameron D. Crockett, Ethan Wedge, Grace Tabatabai, and Natalie Goedeker. 2024. "Newborn Screening for Spinal Muscular Atrophy: Variations in Practice and Early Management of Infants with Spinal Muscular Atrophy in the United States" International Journal of Neonatal Screening 10, no. 3: 58. https://doi.org/10.3390/ijns10030058
APA StyleZaidman, C. M., Crockett, C. D., Wedge, E., Tabatabai, G., & Goedeker, N. (2024). Newborn Screening for Spinal Muscular Atrophy: Variations in Practice and Early Management of Infants with Spinal Muscular Atrophy in the United States. International Journal of Neonatal Screening, 10(3), 58. https://doi.org/10.3390/ijns10030058






