Results of the Hungarian Newborn Screening Pilot Program for Spinal Muscular Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Initiation and Time Frame of the Pilot Program
2.2. Participants of the Program and Sample Collection
2.3. DNA Isolation and Real-Time PCR Assay
2.4. The Diagnostic Genetic Test
2.5. Ethics Approval
2.6. Data Analysis
3. Results
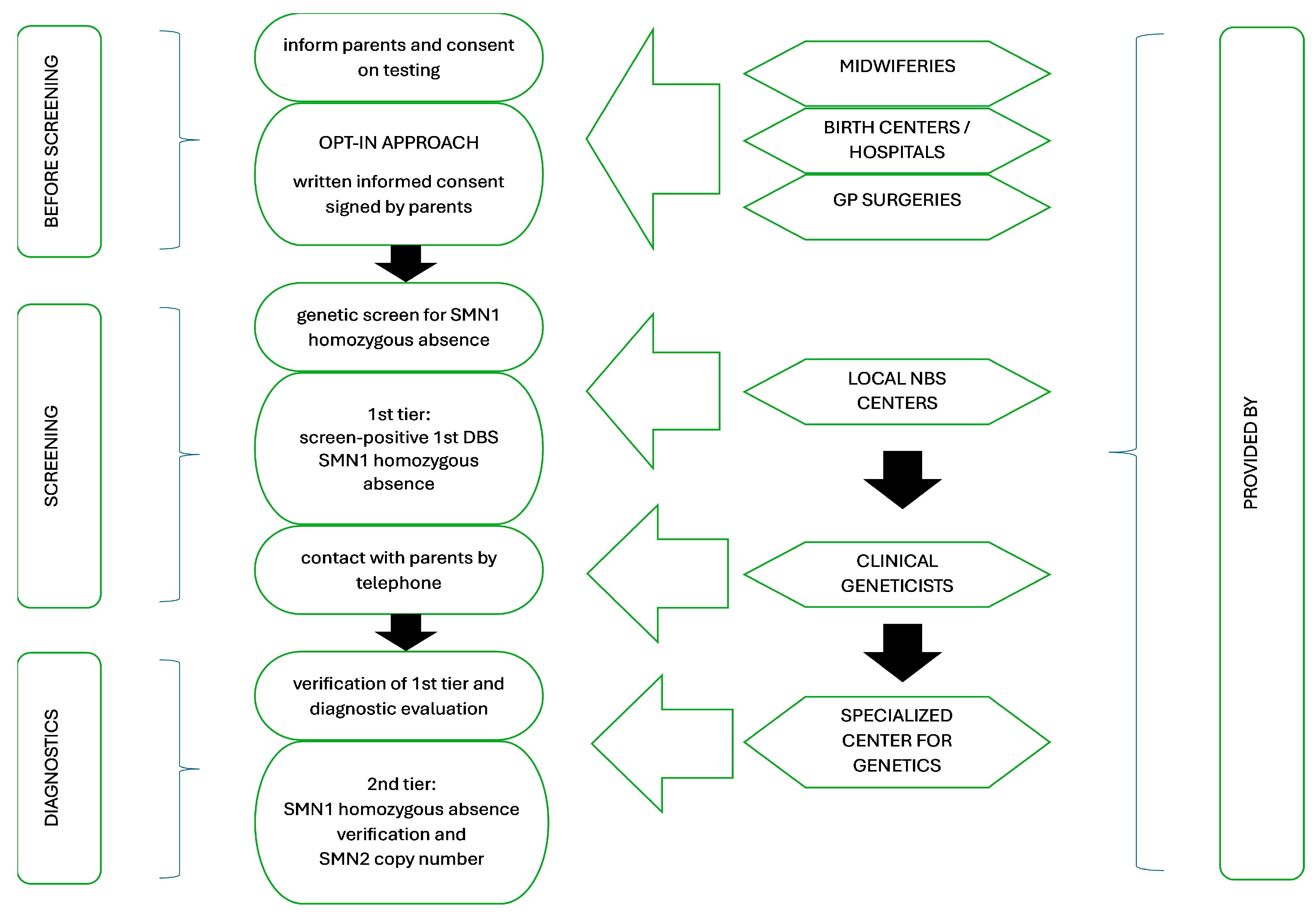
3.1. Screening Characteristics: Pilot Study Design
3.2. Screening Outline
3.3. Screening Timeline
3.4. Screening Performance
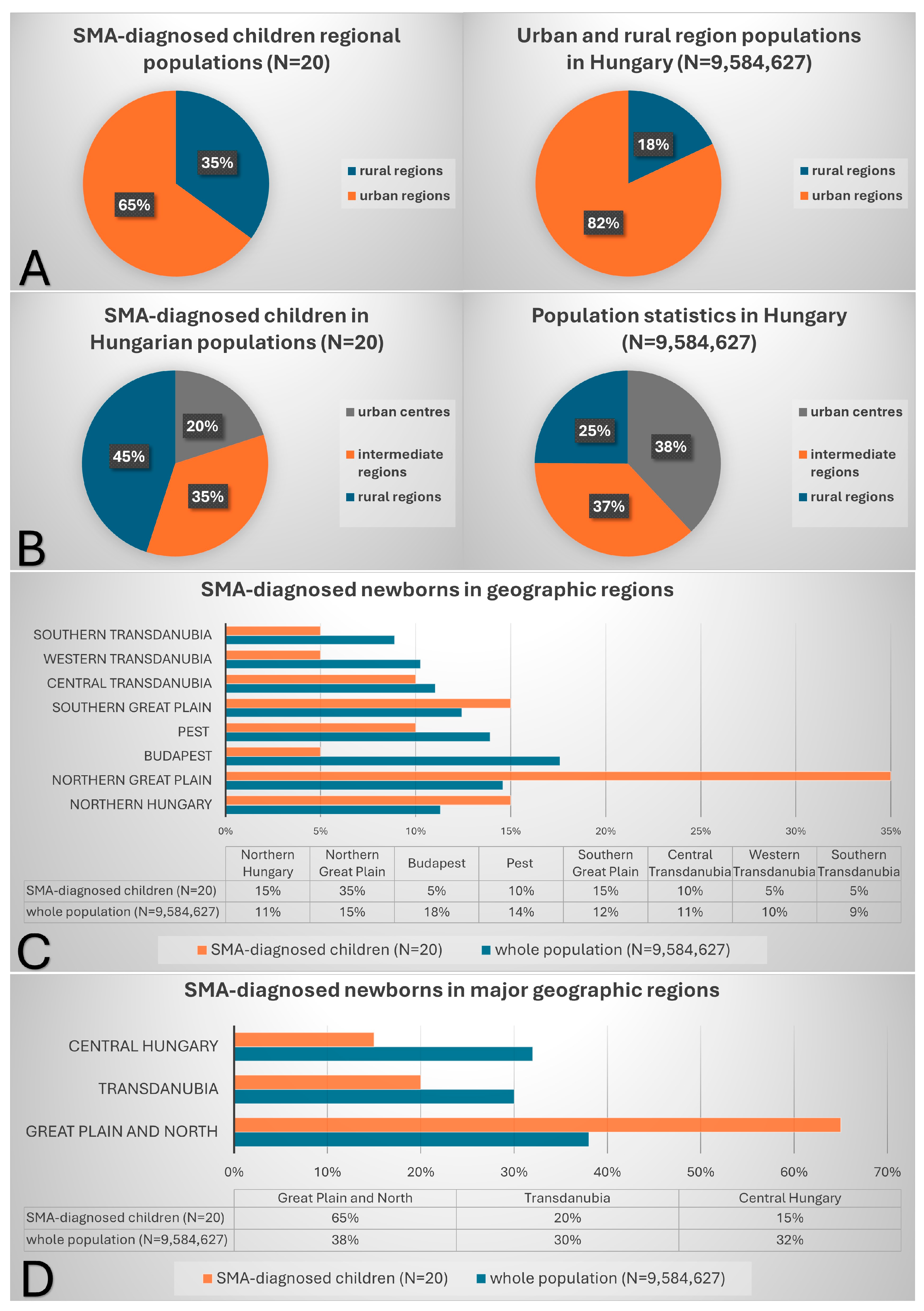
3.5. Distribution of Identified Patients Across Hungary
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koppaka, R.; Centers for Disease Control and Prevention (CDC). Ten Great Public Health Achievements—United States, 2001–2010. MMWR Morb. Mortal Wkly. Rep. 2011, 60, 619–623. [Google Scholar]
- Cipriano, L.E.; Rupar, C.A.; Zaric, G.S. The Cost-Effectiveness of Expanding Newborn Screening for up to 21 Inherited Metabolic Disorders Using Tandem Mass Spectrometry: Results from a Decision-Analytic Model. Value Health 2007, 10, 83–97. [Google Scholar] [CrossRef]
- Grosse, S. Showing Value in Newborn Screening: Challenges in Quantifying the Effectiveness and Cost-Effectiveness of Early Detection of Phenylketonuria and Cystic Fibrosis. Healthcare 2015, 3, 1133–1157. [Google Scholar] [CrossRef] [PubMed]
- Brzustowicz, L.M.; Lehner, T.; Castilla, L.H.; Penchaszadeh, G.K.; Wilhelmsen, K.C.; Daniels, R.; Davies, K.E.; Leppert, M.; Ziter, F.; Wood, D.; et al. Genetic Mapping of Chronic Childhood-Onset Spinal Muscular Atrophy to Chromosome 5q1 1.2–13.3. Nature 1990, 344, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B. An Update of the Mutation Spectrum of the Survival Motor Neuron Gene (SMN1) in Autosomal Recessive Spinal Muscular Atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Aragon-Gawinska, K.; Mouraux, C.; Dangouloff, T.; Servais, L. Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review. Genes 2023, 14, 1377. [Google Scholar] [CrossRef]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M.; et al. Correlation between SMA Type and SMN2 Copy Number Revisited: An Analysis of 625 Unrelated Spanish Patients and a Compilation of 2834 Reported Cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef]
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal Muscular Atrophy. Nat. Rev. Dis. Primers 2022, 8, 52. [Google Scholar] [CrossRef]
- Moultrie, F.; Chiverton, L.; Hatami, I.; Lilien, C.; Servais, L. Pushing the Boundaries: Future Directions in the Management of Spinal Muscular Atrophy. Trends Mol. Med. 2025, 31, 307–318. [Google Scholar] [CrossRef]
- Servais, L. Gene Therapy for Spinal Muscular Atrophy: Timing Is Key. Lancet Reg. Heal. Eur. 2024, 47, 101112. [Google Scholar] [CrossRef]
- Singh, S.; Ojodu, J.; Kemper, A.R.; Lam, W.K.K.; Grosse, S.D. Implementation of Newborn Screening for Conditions in the United States First Recommended during 2010–2018. Int. J. Neonatal Screen. 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.; Nalbant, G.; Sutton, A.; Harnan, S.; Thokala, P.; Chilcott, J.; McNeill, A.; Bessey, A. Systematic Review of Newborn Screening Programmes for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Gergely, A.; Jakus, R.; Fogarasi, A.; Grosz, Z.; Molnár, M.J.; Andor, I.; Schulcz, O.; Goschler, Á.; Medveczky, E.; et al. Efficacy of Nusinersen in Type 1, 2 and 3 Spinal Muscular Atrophy: Real World Data from Hungarian Patients. Eur. J. Paediatr. Neurol. 2020, 27, 37–42. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.-H.; Beckers, P.; Dideberg, V.; Di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N.; et al. Three Years Pilot of Spinal Muscular Atrophy Newborn Screening Turned into Official Program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef]
- Gailite, L.; Sterna, O.; Konika, M.; Isakovs, A.; Isakova, J.; Micule, I.; Setlere, S.; Diriks, M.; Auzenbaha, M. New-Born Screening for Spinal Muscular Atrophy: Results of a Latvian Pilot Study. Int. J. Neonatal Screen. 2022, 8, 15. [Google Scholar] [CrossRef]
- Šimić, D.; Šarić, A.; Škaričić, A.; Lehman, I.; Bunoza, B.; Rako, I.; Fumić, K. One-Year Pilot Study Results of Newborn Screening for Spinal Muscular Atrophy in the Republic of Croatia. Int. J. Neonatal Screen. 2024, 10, 50. [Google Scholar] [CrossRef]
- Belter, L.; Taylor, J.L.; Jorgensen, E.; Glascock, J.; Whitmire, S.M.; Tingey, J.J.; Schroth, M. Newborn Screening and Birth Prevalence for Spinal Muscular Atrophy in the US. JAMA Pediatr. 2024, 178, 946–949. [Google Scholar] [CrossRef]
- IJzebrink, A.; Van Dijk, T.; Franková, V.; Loeber, G.; Kožich, V.; Henneman, L.; Jansen, M. Informing Parents about Newborn Screening: A European Comparison Study. Int. J. Neonatal Screen. 2021, 7, 13. [Google Scholar] [CrossRef]
- Mikos, B.; Molnár, M.J.; Szatmári, I.; Monostori, P.; Bereczki, C.; Szabó, A.J.; Szabó, L.; Csősz, K.; Muzsik, B.; Velkey, G.J. Results of neonatal screening for spinal muscular atrophy in Hungary in 2023. Orv. Hetil. 2024, 165, 1122–1129. [Google Scholar] [CrossRef]
- Eurostat/Regions and Cities Illustrated (RCI). Available online: https://ec.europa.eu/eurostat/cache/RCI/#?vis=urbanrural.urb_typology&lang=en (accessed on 15 January 2025).
- Hungarian Central Statistical Office: Number of Livebirth and Population of Settlements by Legal Status. Available online: https://www.ksh.hu (accessed on 15 January 2025).
- Oskoui, M.; Dangouloff, T.; Servais, L. Universal Newborn Screening for Spinal Muscular Atrophy. JAMA Pediatr. 2024, 178, 520–521. [Google Scholar] [CrossRef]
- Bzdok, J.; Czibere, L.; Burggraf, S.; Pauly, N.; Maier, E.M.; Röschinger, W.; Becker, M.; Durner, J. A Modular Genetic Approach to Newborn Screening from Spinal Muscular Atrophy to Sickle Cell Disease—Results from Six Years of Genetic Newborn Screening. Genes 2024, 15, 1467. [Google Scholar] [CrossRef] [PubMed]
- Tãtaru, E.-A.; Ouillade, M.-C.; Chan, C.-H.; Pearce, D.A. Incorporating a New Disease in the Newborn Screening Programs in Europe: The Spinal Muscular Atrophy Case Study. Rare Dis. Orphan Drugs J. 2024, 3, 26. [Google Scholar] [CrossRef]
- Dangouloff, T.; Thokala, P.; Stevenson, M.D.; Deconinck, N.; D’Amico, A.; Daron, A.; Delstanche, S.; Servais, L.; Hiligsmann, M. Cost-Effectiveness of Spinal Muscular Atrophy Newborn Screening Based on Real-World Data in Belgium. Neuromuscul. Disord. 2024, 34, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Velikanova, R.; Van Der Schans, S.; Bischof, M.; Van Olden, R.W.; Postma, M.; Boersma, C. Cost-Effectiveness of Newborn Screening for Spinal Muscular Atrophy in The Netherlands. Value Health 2022, 25, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Cornel, M.C.; Van Der Meij, K.R.M.; Van El, C.G.; Rigter, T.; Henneman, L. Genetic Screening—Emerging Issues. Genes 2024, 15, 581. [Google Scholar] [CrossRef]
- Lee, F.K.; Greene, C.; Mercer, K.; Taylor, J.; Yazdanpanah, G.; Vogt, R.; Lee, R.; Cuthbert, C.; Cordovado, S. CDC’s Laboratory Activities to Support Newborn Screening for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2024, 10, 51. [Google Scholar] [CrossRef]
- Boyarchuk, O.; Yarema, N.; Kravets, V.; Shulhai, O.; Shymanska, I.; Chornomydz, I.; Hariyan, T.; Volianska, L.; Kinash, M.; Makukh, H. Newborn Screening for Severe Combined Immunodeficiency: The Results of the First Pilot TREC and KREC Study in Ukraine with Involving of 10,350 Neonates. Front. Immunol. 2022, 13, 999664. [Google Scholar] [CrossRef]
- Furnier, S.M.; Durkin, M.S.; Baker, M.W. Translating Molecular Technologies into Routine Newborn Screening Practice. Int. J. Neonatal Screen. 2020, 6, 80. [Google Scholar] [CrossRef]
- Tesorero, R.; Janda, J.; Hörster, F.; Feyh, P.; Mütze, U.; Hauke, J.; Schwarz, K.; Kunz, J.B.; Hoffmann, G.F.; Okun, J.G. A High-Throughput Newborn Screening Approach for SCID, SMA, and SCD Combining Multiplex qPCR and Tandem Mass Spectrometry. PLoS ONE 2023, 18, e0283024. [Google Scholar] [CrossRef]
- Taylor, J.L.; Lee, F.K.; Yazdanpanah, G.K.; Staropoli, J.F.; Liu, M.; Carulli, J.P.; Sun, C.; Dobrowolski, S.F.; Hannon, W.H.; Vogt, R.F. Newborn Blood Spot Screening Test Using Multiplexed Real-Time PCR to Simultaneously Screen for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. Clin. Chem. 2015, 61, 412–419. [Google Scholar] [CrossRef]
- Bækvad-Hansen, M.; Adamsen, D.; Bybjerg-Grauholm, J.; Hougaard, D.M. Implementation of SCID Screening in Denmark. Int. J. Neonatal Screen. 2021, 7, 54. [Google Scholar] [CrossRef]
- Gutierrez-Mateo, C.; Timonen, A.; Vaahtera, K.; Jaakkola, M.; Hougaard, D.M.; Bybjerg-Grauholm, J.; Baekvad-Hansen, M.; Adamsen, D.; Filippov, G.; Dallaire, S.; et al. Development of a Multiplex Real-Time PCR Assay for the Newborn Screening of SCID, SMA, and XLA. Int. J. Neonatal Screen. 2019, 5, 39. [Google Scholar] [CrossRef]
| SMA Pilot Study from November 2022 to December 2024 | ||||||
|---|---|---|---|---|---|---|
| Number of all neonates tested for SMA in | Number of all neonates tested in all NBS programs in | Number of SMA-diagnosed neonates in the program | ||||
| Budapest | Szeged | Budapest | Szeged | Budapest | Szeged | |
| 84,670 | 71,315 | 96,456 | 83,389 | 6 | 13 | |
| summary | 155,985 (87% of all NBS) | 179,845 | 19 + 1 | |||
| ID# | DBS Sampling (Age in Days) | DBS Received by NBS Centre (Age in Days) | First-Tier Result (Age in Days) | First-Tier Result Repeated (Age in Days) | Parents Contacted (Referral to Specialist) (Age in Days) | First Visit (Specialist Review) (Age in Days) | Second-Tier Result (Diagnostic Result) (Age in Days) | SMN2 Copy Numbers | Notes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 6 | 7 | 7 | 8 | 11 | 3 | |
| 2 | 3 | 7 | 7 | 8 | 8 | 9 | 12 | 3 | |
| 3 | 3 | 8 | 8 | 9 | 9 | 10 | 15 | 2 | |
| 4 | 3 | 6 | 6 | 7 | 7 | 8 | 12 | 3 | |
| 5 | 3 | 6 | 14 | 15 | 15 | 18 | 22 | 4 | shortage of PCR reagents 1 |
| 6 | 3 | 8 | 21 | 22 | 22 | 23 | 27 | 3 | shortage of PCR reagents 1 |
| 7 | 2 | 4 | 4 | 4 | 4 | 7 | 9 | 2 | |
| 8 | 4 | 5 | 5 | 5 | 8 | 9 | 11 | 2 | |
| 9 | 2 | 10 | 10 | 11 | 11 | 12 | 16 | 3 | |
| 10 | 2 | 6 | 61 | 61 | - | - | 50 | 2 | no NBS for SMA available 2 |
| 11 | 2 | 7 | 111 | 111 | 111 | 112 | 118 | 3 | no NBS for SMA available 3 |
| 12 | 3 | 6 | 109 | 109 | 109 | 110 | 113 | 4 | no NBS for SMA available 3 |
| 13 | 3 | 8 | 64 | 65 | 65 | 68 | 70 | 4 | no NBS for SMA available 3 |
| 14 | 3 | 5 | 6 | 9 | 9 | 10 | 18 | 3 | |
| 15 | 3 | 6 | 11 | 12 | 12 | 13 | 16 | 3 | |
| 16 | 2 | 8 | 9 | 10 | 10 | 11 | 12 | 3 | |
| 17 | 2 | 6 | 8 | 8 | 8 | 9 | 12 | 3 | |
| 18 | 3 | 7 | 11 | 12 | 12 | 13 | 14 | 2 | |
| 19 | 3 | 10 | 11 | 12 | 12 | 15 | 16 | 3 | |
| 20 | 2 | 11 | 12 | 13 | 13 | 14 | 15 | 2 | |
| median | 3.0 | 6.5 | 8.5 | 9.5 | 9.5 | 10.5 | 14.5 | ||
| range | 2–4 | 4–11 | 4–21 | 4–22 | 4–22 | 7–23 | 9–27 |
| Statistical Characterization of the Hungarian SMA Pilot Program from November 2022 to December 2024 | |
|---|---|
| Number of infants screened for SMA | 155,985 |
| Number of infants with positive tier 1 test among infants screened (% of infants screened) | 19 (0.0122%) |
| Number of infants with a confirmed SMA diagnosis among infants screened | 19 |
| Number of infants with a confirmed SMA diagnosis among infants not screened | 1 |
| Incidence of newly diagnosed SMA in the whole newborn population (screened and unscreened) | 1:7799 |
| Sensitivity | 100% |
| Specificity | 100% |
| Positive predictive value | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegedűs, K.; Lénárt, I.; Xue, A.; Monostori, P.B.; Baráth, Á.; Mikos, B.; Udvari, S.; Géresi, A.; Szabó, A.J.; Bereczki, C.; et al. Results of the Hungarian Newborn Screening Pilot Program for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2025, 11, 29. https://doi.org/10.3390/ijns11020029
Hegedűs K, Lénárt I, Xue A, Monostori PB, Baráth Á, Mikos B, Udvari S, Géresi A, Szabó AJ, Bereczki C, et al. Results of the Hungarian Newborn Screening Pilot Program for Spinal Muscular Atrophy. International Journal of Neonatal Screening. 2025; 11(2):29. https://doi.org/10.3390/ijns11020029
Chicago/Turabian StyleHegedűs, Krisztina, István Lénárt, Andrea Xue, Péter Béla Monostori, Ákos Baráth, Borbála Mikos, Szabolcs Udvari, Adrienn Géresi, Attila József Szabó, Csaba Bereczki, and et al. 2025. "Results of the Hungarian Newborn Screening Pilot Program for Spinal Muscular Atrophy" International Journal of Neonatal Screening 11, no. 2: 29. https://doi.org/10.3390/ijns11020029
APA StyleHegedűs, K., Lénárt, I., Xue, A., Monostori, P. B., Baráth, Á., Mikos, B., Udvari, S., Géresi, A., Szabó, A. J., Bereczki, C., Molnár, M. J., & Szatmári, I. (2025). Results of the Hungarian Newborn Screening Pilot Program for Spinal Muscular Atrophy. International Journal of Neonatal Screening, 11(2), 29. https://doi.org/10.3390/ijns11020029






