Pulse Oximetry Screening in Germany—Historical Aspects and Future Perspectives
Abstract
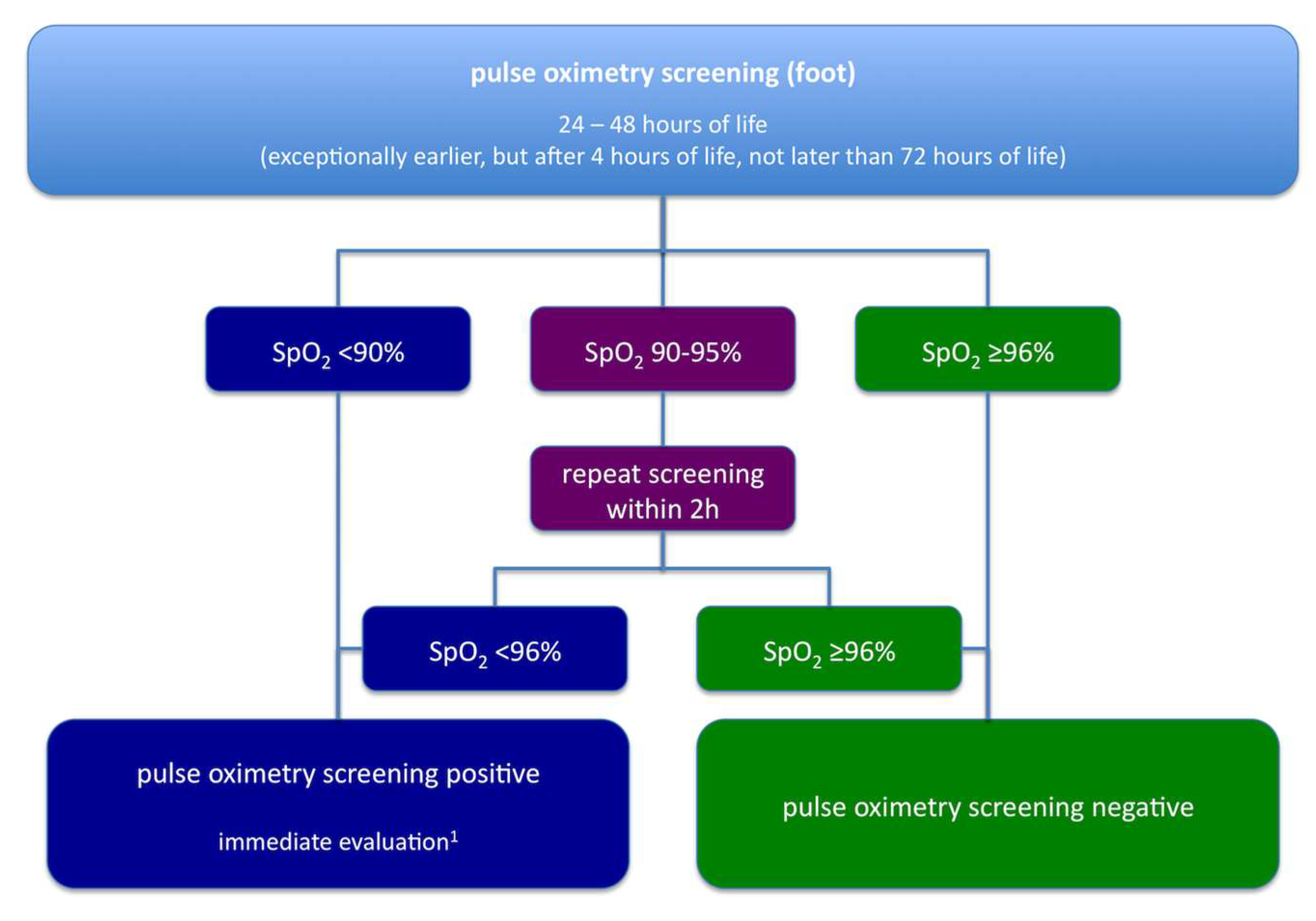
:1. Historical Aspects: From Clinical Data
2. To Legal Regulation
3. Future Perspectives
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schneider, P.; Kostelka, M.; Kändler, L.; Möckel, A.; Riede, F.T.; Dähnert, I. Die diagnostische Lücke bei neonatalen Herzerkrankungen—Herausforderung für Neonatologie und Kinderkardiologie. Kinder und Jugendmed. 2004, 4, 188–193. [Google Scholar] [CrossRef]
- Bakr, A.F.; Habib, H.S. Combining Pulse Oximetry and Clinical Examination in Screening for Congenital Heart Disease. Pediatr. Cardiol. 2005, 26, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Hoke, T.R.; Donohue, P.K.; Bawa, P.K.; Mitchell, R.D.; Pathak, A.; Rowe, P.C.; Byrne, B.J. Oxygen Saturation as a Screening Test for Critical Congenital Heart Disease: A Preliminary Study. Pediatr. Cardiol. 2002, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Koppel, R.I.; Druschel, C.M.; Carter, T.; Goldberg, B.E.; Mehta, P.N.; Talwar, R.; Bierman, F.Z. Effectiveness of Pulse Oximetry Screening for Congenital Heart Disease in Asymptomatic Newborns. Pediatrics 2003, 111, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Riede, F.T.; Wörner, C.; Dähnert, I.; Möckel, A.; Kostelka, M.; Schneider, P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—Results from a prospective multicenter study. Eur J. Pediatr. 2010, 169, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Meberg, A.; Brügmann-Pieper, S.; Due, R., Jr.; Eskedal, L.; Fagerli, I.; Farstad, T.; Frøisland, D.H.; Sannes, C.H.; Johansen, O.J.; Keljalic, J.; et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J. Pediatr. 2008, 152, 761–765. [Google Scholar] [CrossRef] [PubMed]
- De-Wahl Granelli, A.; Wennergren, M.; Sandberg, K.; Mellander, M.; Bejlum, C.; Inganäs, L.; Eriksson, M.; Segerdahl, N.; Agren, A.; Ekman-Joelsson, B.M.; et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns. BMJ 2009, 338, a3037. [Google Scholar] [CrossRef] [PubMed]
- Tautz, J.; Merkel, C.; Loersch, F.; Egen, O.; Hägele, F.; Thon, H.M.; Schaible, T. Implication of pulse oxymetry screening for detection of congenital heart defects. Klin. Padiatr. 2010, 222, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Bekanntmachung eines Beschlusses des Gemeinsamen Bundesausschusses nach §91 Abs. 7 des Fünften Buches Sozialgesetzbuch (SGB V) zur Vereinbarung über Maßnahmen zur Qualitätssicherung der Versorgung von Früh- und Neugeborenen nach §137 Abs. 1 Satz 3 Nr. 2 SGB V. Gemeinsamer Bundesausschuss. Available online: https://www.g-ba.de/downloads/39-261-229/2005-09-20-Vereinbarung-Frueh_Neu.pdf (accessed on 31 March 2018).
- Richtlinie des Gemeinsamen Bundesausschusses über Maßnahmen zur Qualitätssicherung der Versorgung von Früh- und Reifgeborenen. Gemeinsamer Bundesausschuss. Available online: https://www.g-ba.de/downloads/62-492-1487/QFR-RL_2017-10-19_iK-2018-01-01.pdf (accessed on 20 March 2018).
- Ewer, A.K.; Middleton, L.J.; Furmston, A.T.; Bhoyar, A.; Daniels, J.P.; Thangaratinam, S.; Deeks, J.J.; Khan, K.S. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): A test accuracy study. Lancet 2011, 378, 785–794. [Google Scholar] [CrossRef]
- Turska Kmieć, A.; Borszewska Kornacka, M.K.; Błaż, W.; Kawalec, W.; Zuk, M. Early screening for critical congenital heart defects in asymptomatic newborns in Mazovia province: Experience of the POLKARD pulse oximetry programme 2006–2008 in Poland. Kardiol. Pol. 2012, 70, 370–376. [Google Scholar] [PubMed]
- Abdul-Khaliq, H.; Berger, F. Die Diagnose wird häufig zu spät gestellt. Dtsch. Arztebl. 2011, 108, A1684. [Google Scholar]
- Lindinger, A.; Dähnert, I.; Riede, F.T. Stellungnahme zum Pulsoximetrie-Screening zur Erfassung von Kritischen Angeborenen Herzfehlern im Neugeborenenalter. Available online: http://www.kinderkardiologie.org/fileadmin/user_upload/Stellungnahmen/POS%20Stellungsnahme%20DGPK2%2011%2013%20final.pdf (accessed on 20 March 2018).
- Herting, E.; Vetter, K.; Gonser, M.; Bassler, D.; Hentschel, R.; Groneck, P. Betreuung von Gesunden Reifen Neugeborenen in der Geburtsklinik. Available online: http://www.awmf.org/uploads/tx_szleitlinien/024-005l_S2k_Betreuung_von_gesunden_reifen_Neugeborenen_2012-10-abgelaufen.pdf (accessed on 20 March 2018).
- Bärnighausen, T.; Sauerborn, R. One hundred and eighteen years of the German health insurance system: Are there any lessons for middle- and low-income countries? Soc. Sci. Med. 2002, 54, 1559–1587. [Google Scholar] [CrossRef]
- Screening auf Kritische Angeborene Herzfehler Mittels Pulsoxymetrie bei Neugeborenen. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Available online: https://www.iqwig.de/download/S13-01_Abschlussbericht_Pulsoxymetrie.pdf (accessed on 20 March 2018).
- Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Richtlinie über die Früherkennung von Krankheiten bei Kindern bis zur Vollendung des 6. Lebensjahres (Kinder-Richtlinie): Screening auf Kritische Angeborene Herzfehler Mittels Pulsoxymetrie bei Neugeborenen. Available online: https://www.g-ba.de/downloads/39-261-2762/2016-11-24_Kinder-RL_Pulsoxymetrie-Screening-Neugeborene_BAnz.pdf (accessed on 20 March 2018).
- Screening auf Kritische Angeborene Herzfehler Mittels Pulsoxymetrie bei Neugeborenen–Zusammenfassende Dokumentation. Available online: https://www.g-ba.de/downloads/40-268-4066/2016-11-24_Kinder-RL_Pulsoxymetrie-Screening-Neugeborene_ZD.pdf (accessed on 20 March 2018).
- Kemper, A.R.; Mahle, W.T.; Martin, G.R.; Cooley, W.C.; Kumar, P.; Morrow, W.R.; Kelm, K.; Pearson, G.D.; Glidewell, J.; Grosse, S.D.; et al. Strategies for Implementing Screening for Critical Congenital Heart Disease. Pediatrics 2011, 128, e1259–e1267. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Brown, K.; Zamora, J.; Khan, K.S.; Ewer, A.K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. Lancet 2012, 379, 2459–2464. [Google Scholar] [CrossRef]
- Manzoni, P.; Martin, G.R.; Luna, M.S.; Mestrovic, J.; Simeoni, U.; Zimmermann, L.; Ewer, A.K.; Manzoni, P.; Martin, G.R.; Granelli, A.D.W.; et al. Pulse oximetry screening for critical congenital heart defects: A European consensus statement. Lancet Child. Adolesc. Health 2017, 1, 88–90. [Google Scholar] [CrossRef]
- Narayen, I.C.; Blom, N.A.; Ewer, A.K.; Vento, M.; Manzoni, P.; te Pas, A.B. Aspects of pulse oximetry screening for critical congenital heart defects: When, how and why? Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F162–F167. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.C.; Lieberman, E.; O’Leary, E.; Geggel, R.L. Prenatal and newborn screening for critical congenital heart disease: Findings from a nursery. Pediatrics 2014, 134, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Quartermain, M.D.; Pasquali, S.K.; Hill, K.D.; Goldberg, D.J.; Huhta, J.C.; Jacobs, J.P.; Jacobs, M.L.; Kim, S.; Ungerleider, R.M. Variation in Prenatal Diagnosis of Congenital Heart Disease in Infants. Pediatrics 2015, 136, e378–e385. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, A.; Schwedler, G.; Hense, H.W. Prevalence of Congenital Heart Defects in Newborns in Germany: Results of the First Registration Year of the PAN Study (July 2006 to June 2007). Klin. Padiatr. 2010, 222, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Abouk, R.; Grosse, S.D.; Ailes, E.C.; Oster, M.E. Association of US State Implementation of Newborn Screening Policies for Critical Congenital Heart Disease With Early Infant Cardiac Deaths. JAMA 2017, 318, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Wren, C.; Reinhardt, Z.; Khawaja, K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F33–F35. [Google Scholar] [CrossRef] [PubMed]


| Level of Care | Admission Criteria |
|---|---|
| Perinatal centre level 1 | Expected prematurity with a birth weight of <1250 g or a gestational age of <29 weeks triplet pregnancy and gestational age <33 weeks; multiple pregnancy Prenatal diagnosis of any fetal or maternal condition necessitating immediate postnatal intensive care (critical congenital heart disease, diaphragmatic hernia, myelomeningocele, gastroschisis) |
| Perinatal centre level 2 | Expected prematurity with a birth weight of 1250–1499 g or a gestational age of ≥29 to <32 weeks HELLP syndrome Intrauterine growth restriction <3rd percentile Insulin-dependent gestational diabetes with elevated risk for the fetus/newborn |
| Perinatal clinic | Expected prematurity with a birth weight of ≥1500 g or a gestational age of ≥32 to <36 weeks Intrauterine growth restriction between third and tenth percentile Insulin-dependent gestational diabetes without elevated risk for the fetus/newborn |
| Obstetrical clinic | Gestational age ≥36 weeks, uncomplicated delivery expected |
| Number and Percentage of Newborns |
|
| False positive results |
| Number of newborns with CCHD detected by pulse oximetry screening |
| Timing of diagnostic and therapeutic procedures in newborns with CCHD |
| Number and Percentage of Newborns |
|
| False negative results |
| Detection of neonatal diseases in newborns with false positive screening results (with respect to CCHD) |
| Reasons for not performing pulse oximetry screening in eligible newborns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riede, F.-T.; Paech, C.; Orlikowsky, T. Pulse Oximetry Screening in Germany—Historical Aspects and Future Perspectives. Int. J. Neonatal Screen. 2018, 4, 15. https://doi.org/10.3390/ijns4020015
Riede F-T, Paech C, Orlikowsky T. Pulse Oximetry Screening in Germany—Historical Aspects and Future Perspectives. International Journal of Neonatal Screening. 2018; 4(2):15. https://doi.org/10.3390/ijns4020015
Chicago/Turabian StyleRiede, Frank-Thomas, Christian Paech, and Thorsten Orlikowsky. 2018. "Pulse Oximetry Screening in Germany—Historical Aspects and Future Perspectives" International Journal of Neonatal Screening 4, no. 2: 15. https://doi.org/10.3390/ijns4020015




