Using a Model of Germ-Free Animals to Study the Impact of Gut Microbiome in Research: A Step by Step Sterility Setting and Management
Abstract
:1. Introduction
2. Experimental Design
Materials and Equipment
- A devoted room and technical team are basic requirements. GF mice can be housed in sterile semirigid isolators (SRI) or in flexible isolators (Park Bioservices, LLC, Groveland, MA, USA) (Figure 1C). We chose to work with SRI. Each isolator, depending on its size, can hold up to 12 cages, each housing four mice.
- C57 BL/6 GF mice, as well as their control C57BL/6 littermates, can be purchased from several authorized vendors, as well as other germ-free strains such as BALB/c, Swiss Webster, or strains bred in-house. However, the latter poses a challenge due to the poor reproductive performance of the mice, attributable to restricted abdominal space from an enlarged cecum. In our studies, mice were purchased from Taconic (Taconic Bioscience, Rensselaer, NY, USA).
- All animal procedures and Animal Study Proposals (ASP) were reviewed and approved by an Institutional Animal Care and Use Committee (FDA White Oak IACUC for our studies).
3. Procedure
3.1. Setting up a Sterile Environment: (Figure 1)
3.1.1. Set up and Preparation
3.1.2. Experimental Design
3.2. Working in the Sterile Environment (Figure 2)
3.2.1. Procedure: Introduction of the Mice
3.2.2. Identification of the Mice
3.2.3. Animals Observation
3.2.4. Fecal Sample Collection
3.2.5. Mice Injections
Biologic or Small Molecule Therapeutics
Mice Can be Fecal Transplanted (Fecal Matter Transplant, FMT) Using a Similar Protocol
3.2.6. Mice Bleeding, Tissue Collection
3.2.7. End of the Study
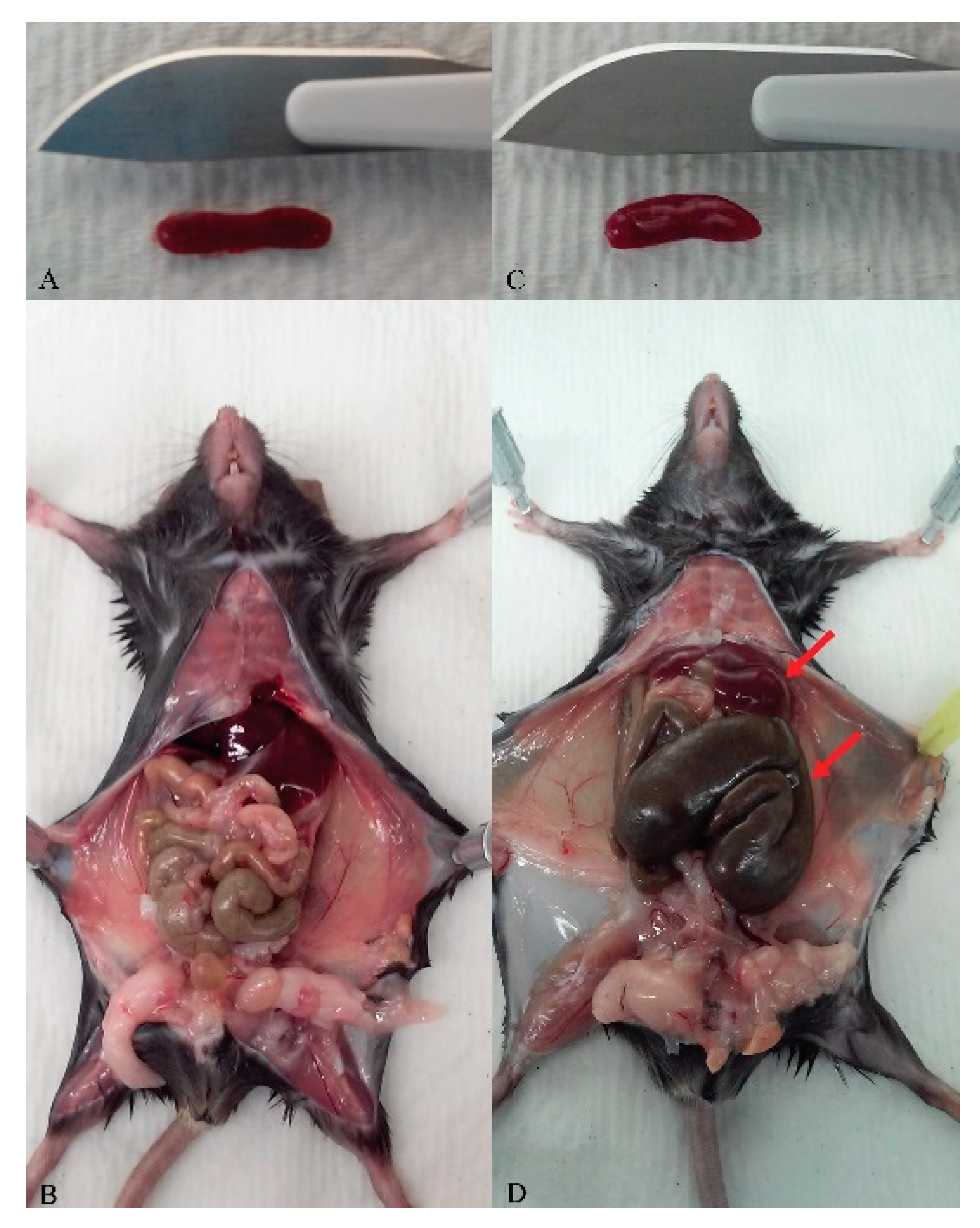
4. Expected Results and Discussion
Characterization of phenotype (Figure 3)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Xiao, L.; Feng, Q.; Liang, S.; Sonne, S.B.; Xia, Z.; Qiu, X.; Li, X.; Long, H.; Zhang, J.; Zhang, D.; et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015, 33, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Duerkop, B.A.; Hooper, L.V. Resident viruses and their interactions with the immune system. Nat. Immunol. 2013, 14, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Scher, J.U.; Littman, D.R.; Abramson, S.B. Microbiome in Inflammatory Arthritis and Human Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi-Roodsaz, S.; Abramson, S.B.; Scher, J.U. The metabolic role of the gut microbiota in health and rheumatic disease: Mechanisms and interventions. Nat. Rev. Rheumatol. 2016, 12, 446–455. [Google Scholar] [CrossRef]
- Wu, H.J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016, 19, 8. [Google Scholar] [CrossRef]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, G.R.; Trexler, P.C. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut 1971, 12, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrand, F.; Nguyen, T.L.; Brinkman, B.; Yunta, R.G.; Cauwe, B.; Vandenabeele, P.; Liston, A.; Raes, J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013, 14, R4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zaheer, M.; Bian, F.; Quach, D.; Swennes, A.G.; Britton, R.A.; Pflugfelder, S.C.; de Paiva, C.S. Sjogren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice. Int. J. Mol. Sci. 2018, 19, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Han, W.; Zhan, G.; Li, S.; Jiang, X.; Wang, L.; Xiang, S.; Zhu, B.; Yang, L.; Luo, A.; et al. Abnormal gut microbiota composition contributes to the development of type 2 diabetes mellitus in db/db mice. Aging 2019, 11, 10454–10467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Rao, X.; Wang, H.; Zeng, B.; Yu, Y.; Chen, J.; Zhong, J.; Qi, X.; Zeng, L.; Zheng, P.; et al. Hippocampus-specific regulation of long non-coding RNA and mRNA expression in germ-free mice. Funct. Integr. Genomics 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.M.; Roy, S.; Tomcho, J.C.; Schreckenberger, Z.J.; Chakraborty, S.; Bearss, N.R.; Saha, P.; McCarthy, C.G.; Vijay-Kumar, M.; Joe, B.; et al. Microbiota are critical for vascular physiology: Germ-free status weakens contractility and induces sex-specific vascular remodeling in mice. Vascul. Pharmacol. 2019, 125–126, 106633. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferrau, F.; Libra, M. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabay, O.; Vicenty, J.; Zack-Taylor, A.; Tiffany, L.; Wunderlin, G.; Smith, D.; Reyes-Munoz, L.; Edwards, V.; Wu, W.W.; Phue, J.N.; et al. Exposure to TNF antagonist therapies induces variations of the gut microbiota in an in vivo model using healthy mice. Jt. Bone Spine 2019. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabay, O.; Vicenty, J.; Smith, D.; Tiffany, L.; Ascher, J.; Curry, T.; Dennis, J.; Clouse, K.A. Using a Model of Germ-Free Animals to Study the Impact of Gut Microbiome in Research: A Step by Step Sterility Setting and Management. Methods Protoc. 2020, 3, 18. https://doi.org/10.3390/mps3010018
Gabay O, Vicenty J, Smith D, Tiffany L, Ascher J, Curry T, Dennis J, Clouse KA. Using a Model of Germ-Free Animals to Study the Impact of Gut Microbiome in Research: A Step by Step Sterility Setting and Management. Methods and Protocols. 2020; 3(1):18. https://doi.org/10.3390/mps3010018
Chicago/Turabian StyleGabay, Odile, Jonathan Vicenty, Dylan Smith, Linda Tiffany, Jill Ascher, Tina Curry, John Dennis, and Kathleen A. Clouse. 2020. "Using a Model of Germ-Free Animals to Study the Impact of Gut Microbiome in Research: A Step by Step Sterility Setting and Management" Methods and Protocols 3, no. 1: 18. https://doi.org/10.3390/mps3010018




