Development of In Vitro Corneal Models: Opportunity for Pharmacological Testing
Abstract
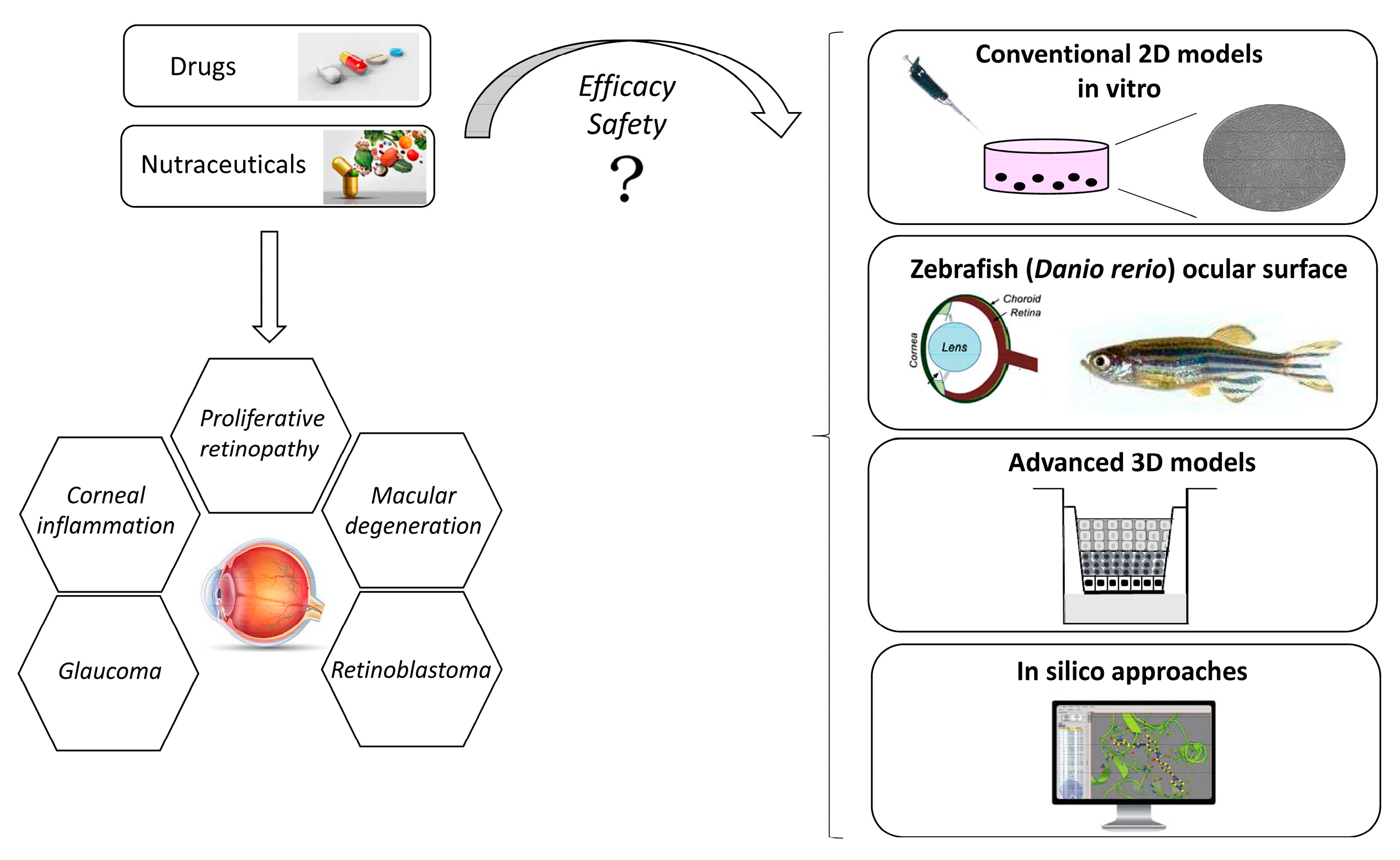
1. Introduction
2. In Vitro Ocular Models
2.1. Opportunity and Application
2.2. Conventional 2D Models
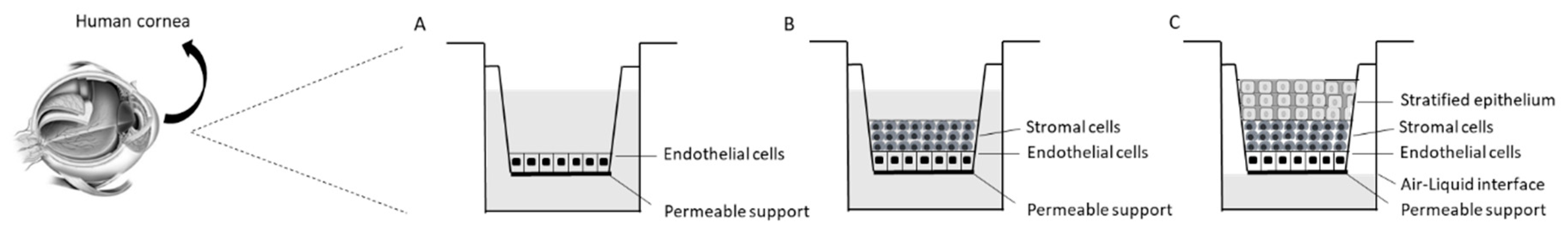
2.3. Advanced Corneal 3D Models
2.3.1. Application of Human Cornea-Like Epithelium: Irritation Test Following OECD TG 492
2.3.2. Zebrafish Ocular Surface: A Model for Human Corneal Diseases?
2.4. Pharmacological Application of 3D Reconstructed Human Corneal Tissues: The Dry Eye Model
3. Computational Aspects for the Ocular Pharmacology and Toxicology
4. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barar, J.; Asadi, M.; Mortazavi-Tabatabaei, S.A.; Omidi, Y. Ocular Drug Delivery; Impact of in vitro Cell Culture Models. J. Ophthalmic Vis. Res. 2009, 4, 238–252. [Google Scholar] [PubMed]
- Kasthurirangan, S.; Markwell, E.L.; Atchison, D.A.; Pope, J.M. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Estlack, Z.; Bennet, D.; Reid, T.; Kim, J. Microengineered biomimetic ocular models for ophthalmological drug development. Lab Chip 2017, 17, 1539–1551. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Trans. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- Dartt, D.A.; Willcox, M.D. Complexity of the tear film: Importance in homeostasis and dysfunction during disease. Exp. Eye Res. 2013, 117, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; Li, W. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, T.; Mehra, D.; Le, P.H. Histology, Eye. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544343/ (accessed on 3 July 2020).
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef]
- Correia Barao, R.; Pinto Ferreira, N.; Abegao Pinto, L. A Camera without a Diaphragm. Ophthalmol. Glaucoma 2020, 3, 138. [Google Scholar] [CrossRef]
- Kels, B.D.; Grzybowski, A.; Grant-Kels, J.M. Human ocular anatomy. Clin. Dermatol. 2015, 33, 140–146. [Google Scholar] [CrossRef]
- Haderspeck, J.C.; Chuchuy, J.; Kustermann, S.; Liebau, S.; Loskill, P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert Opin. Drug Discov. 2019, 14, 47–57. [Google Scholar] [CrossRef]
- Chemi, G.; Brindisi, M.; Brogi, S.; Relitti, N.; Butini, S.; Gemma, S.; Campiani, G. A light in the dark: State of the art and perspectives in optogenetics and optopharmacology for restoring vision. Future Med. Chem. 2019, 11, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Shafaie, S.; Hutter, V.; Cook, M.T.; Brown, M.B.; Chau, D.Y. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores. Open Access 2016, 5, 94–108. [Google Scholar] [CrossRef]
- Vasconcelos, T.; da Silva, S.B.; Ferreira, D.; Pintado, M.; Marques, S. Cell-based in vitro models for ocular permeability studies. In Concepts and Models for Drug Permeability Studies; Woodhead Publishing: Cambridge, UK, 2016; pp. 129–154. [Google Scholar]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef]
- Swaminathan, S.; Kumar, V.; Kaul, R. Need for alternatives to animals in experimentation: An Indian perspective. Indian J. Med. Res. 2019, 149, 584–592. [Google Scholar]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef]
- Burden, N.; Benstead, R.; Benyon, K.; Clook, M.; Green, C.; Handley, J.; Harper, N.; Maynard, S.K.; Mead, C.; Pearson, A.; et al. Key Opportunities to Replace, Reduce, and Refine Regulatory Fish Acute Toxicity Tests. Environ. Toxicol. Chem. 2020, 39, 2076–2089. [Google Scholar] [CrossRef]
- Piccinno, M.S.; Petrachi, T.; Resca, E.; Strusi, V.; Bergamini, V.; Mulas, G.A.; Mari, G.; Dominici, M.; Veronesi, E. Label-free toxicology screening of primary human mesenchymal cells and iPS-derived neurons. PLoS ONE 2018, 13, e0201671. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Crespo-Moral, M.; Garcia-Posadas, L.; Lopez-Garcia, A.; Diebold, Y. Histological and immunohistochemical characterization of the porcine ocular surface. PLoS ONE 2020, 15, e0227732. [Google Scholar] [CrossRef]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Diebold, Y.; Calonge, M.; Gao, J.; Stern, M.E.; Beuerman, R.W. Comparison of gene expression profiles of conjunctival cell lines with primary cultured conjunctival epithelial cells and human conjunctival tissue. Gene Exp. 2009, 14, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Blazejewska, E.A.; Schlotzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009, 27, 642–652. [Google Scholar] [CrossRef]
- Kolle, S.N.; Van Cott, A.; van Ravenzwaay, B.; Landsiedel, R. Lacking applicability of in vitro eye irritation methods to identify seriously eye irritating agrochemical formulations: Results of bovine cornea opacity and permeability assay, isolated chicken eye test and the EpiOcular ET-50 method to classify according to UN GHS. Regul. Toxicol. Pharmacol. 2017, 85, 33–47. [Google Scholar] [PubMed]
- Kaluzhny, Y.; Kandarova, H.; d’Argembeau-Thornton, L.; Kearney, P.; Klausner, M. Eye Irritation Test (EIT) for Hazard Identification of Eye Irritating Chemicals using Reconstructed Human Cornea-like Epithelial (RhCE) Tissue Model. J. Vis. Exp. 2015, 23, e52979. [Google Scholar] [CrossRef]
- Ronkko, S.; Vellonen, K.S.; Jarvinen, K.; Toropainen, E.; Urtti, A. Human corneal cell culture models for drug toxicity studies. Drug Deliv. Trans. Res. 2016, 6, 660–675. [Google Scholar] [CrossRef]
- Wilson, S.L.; Ahearne, M.; Hopkinson, A. An overview of current techniques for ocular toxicity testing. Toxicology 2015, 327, 32–46. [Google Scholar] [CrossRef]
- Kolle, S.N.; Sauer, U.G.; Moreno, M.C.; Teubner, W.; Wohlleben, W.; Landsiedel, R. Eye irritation testing of nanomaterials using the EpiOcular eye irritation test and the bovine corneal opacity and permeability assay. Part. Fibre Toxicol. 2016, 13, 18. [Google Scholar] [CrossRef]
- McNamee, P.; Hibatallah, J.; Costabel-Farkas, M.; Goebel, C.; Araki, D.; Dufour, E.; Hewitt, N.J.; Jones, P.; Kirst, A.; Le Varlet, B.; et al. A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: Eye irritation. Regul. Toxicol. Pharmacol. 2009, 54, 197–209. [Google Scholar] [CrossRef]
- Zorn-Kruppa, M.; Houdek, P.; Wladykowski, E.; Engelke, M.; Bartok, M.; Mewes, K.R.; Moll, I.; Brandner, J.M. Determining the Depth of Injury in Bioengineered Tissue Models of Cornea and Conjunctiva for the Prediction of All Three Ocular GHS Categories. PLoS ONE 2014, 9, e114181. [Google Scholar] [CrossRef] [PubMed]
- Rosdy, M.; Beuerman, R.; Nguyen, D.; DeWever, B. Three-Dimensional Construct of the Human Corneal Epithelium for In Vitro Toxicology. In Alternative Toxicological Methods; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease insights from the zebrafish, Danio rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar] [PubMed]
- Gestri, G.; Link, B.A.; Neuhauss, S.C. The visual system of zebrafish and its use to model human ocular diseases. Dev. Neurobiol. 2012, 72, 302–327. [Google Scholar] [CrossRef]
- Puzzolo, D.; Pisani, A.; Malta, C.; Santoro, G.; Meduri, A.; Abbate, F.; Montalbano, G.; Wylegala, E.; Rana, R.A.; Bucchieri, F.; et al. Structural, ultrastructural, and morphometric study of the zebrafish ocular surface: A model for human corneal diseases? Curr. Eye Res. 2018, 43, 175–185. [Google Scholar] [CrossRef]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef]
- Rolando, M.; Cantera, E.; Mencucci, R.; Rubino, P.; Aragona, P. The correct diagnosis and therapeutic management of tear dysfunction: Recommendations of the P.I.C.A.S.S.O. board. Int. Ophthalmol. 2018, 38, 875–895. [Google Scholar] [CrossRef]
- Ziaragkali, S.; Kotsalidou, A.; Trakos, N. Dry Eye Disease in Routine Rheumatology Practice. Mediterr. J. Rheumatol. 2018, 29, 127–139. [Google Scholar] [CrossRef]
- Uchino, M.; Schaumberg, D.A. Dry Eye Disease: Impact on Quality of Life and Vision. Curr. Ophthalmol. Rep. 2013, 1, 51–57. [Google Scholar] [CrossRef]
- Javadi, M.A.; Feizi, S. Dry eye syndrome. J. Ophthalmic Vis. Res. 2011, 6, 192–198. [Google Scholar]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Abusharha, A.A.; Pearce, E.I. The effect of low humidity on the human tear film. Cornea 2013, 32, 429–434. [Google Scholar] [CrossRef]
- Acera, A.; Vecino, E.; Duran, J.A. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8285–8291. [Google Scholar] [CrossRef]
- Raies, A.B.; Bajic, V.B. In silico toxicology: Computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2016, 6, 147–172. [Google Scholar] [CrossRef]
- Sirous, H.; Campiani, G.; Brogi, S.; Calderone, V.; Chemi, G. Computer-Driven Development of an in Silico Tool for Finding Selective Histone Deacetylase 1 Inhibitors. Molecules 2020, 25, 1952. [Google Scholar] [CrossRef]
- Brogi, S.; Papazafiri, P.; Roussis, V.; Tafi, A. 3D-QSAR using pharmacophore-based alignment and virtual screening for discovery of novel MCF-7 cell line inhibitors. Eur. J. Med. Chem. 2013, 67, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Corelli, F.; Di Marzo, V.; Ligresti, A.; Mugnaini, C.; Pasquini, S.; Tafi, A. Three-dimensional quantitative structure-selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2. Eur. J. Med. Chem. 2011, 46, 547–555. [Google Scholar] [CrossRef]
- Chemi, G.; Gemma, S.; Campiani, G.; Brogi, S.; Butini, S.; Brindisi, M. Computational Tool for Fast in silico Evaluation of hERG K(+) Channel Affinity. Front. Chem. 2017, 5, 7. [Google Scholar] [CrossRef]
- Zaccagnini, L.; Brogi, S.; Brindisi, M.; Gemma, S.; Chemi, G.; Legname, G.; Campiani, G.; Butini, S. Identification of novel fluorescent probes preventing PrP(Sc) replication in prion diseases. Eur. J. Med. Chem. 2017, 127, 859–873. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Halsey, L.G.; Bury, N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017, 220, 3007–3016. [Google Scholar] [CrossRef]
- Verheyen, G.R.; Braeken, E.; Van Deun, K.; Van Miert, S. Evaluation of existing (Q)SAR models for skin and eye irritation and corrosion to use for REACH registration. Toxicol. Lett. 2017, 265, 47–52. [Google Scholar] [CrossRef]
- Abraham, M.H.; Kumarsingh, R.; Cometto-Muniz, J.E.; Cain, W.S. A Quantitative Structure–Activity Relationship (QSAR) for a Draize Eye Irritation Database. Toxicol. In Vitro 1998, 12, 201–207. [Google Scholar] [CrossRef]
- Abraham, M.H.; Kumarsingh, R.; Cometto-Muniz, J.E.; Cain, W.S. Draize eye scores and eye irritation thresholds in man can be combined into one QSAR. Ann. N. Y. Acad. Sci. 1998, 855, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Pan, D.; Hopfinger, A.J. A study of the relationship between cornea permeability and eye irritation using membrane-interaction QSAR analysis. Toxicol. Sci. 2005, 88, 434–446. [Google Scholar] [CrossRef][Green Version]
- Zhu, H.; Tropsha, A.; Fourches, D.; Varnek, A.; Papa, E.; Gramatica, P.; Oberg, T.; Dao, P.; Cherkasov, A.; Tetko, I.V. Combinatorial QSAR modeling of chemical toxicants tested against Tetrahymena pyriformis. J. Chem. Inf. Model. 2008, 48, 766–784. [Google Scholar] [CrossRef]
- Solimeo, R.; Zhang, J.; Kim, M.; Sedykh, A.; Zhu, H. Predicting chemical ocular toxicity using a combinatorial QSAR approach. Chem. Res. Toxicol. 2012, 25, 2763–2769. [Google Scholar] [CrossRef]
- Baskin, I.I. Machine Learning Methods in Computational Toxicology. In Computational Toxicology. Methods in Molecular Biology; Nicolotti, O., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1800, pp. 119–139. [Google Scholar]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Sadeghipour, A.; Gerendas, B.S.; Waldstein, S.M.; Bogunovic, H. Artificial intelligence in retina. Prog. Retin. Eye Res. 2018, 67, 1–29. [Google Scholar] [CrossRef]
- Luechtefeld, T. Analysis of Draize eye irritation testing and its prediction by mining publicly available 2008-2014 REACH data. Altex 2016, 33, 123. [Google Scholar] [CrossRef]
- Esposito, L.; Clemente, C.; Bonora, N.; Rossi, T. Modelling human eye under blast loading. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 107–115. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citi, V.; Piragine, E.; Brogi, S.; Ottino, S.; Calderone, V. Development of In Vitro Corneal Models: Opportunity for Pharmacological Testing. Methods Protoc. 2020, 3, 74. https://doi.org/10.3390/mps3040074
Citi V, Piragine E, Brogi S, Ottino S, Calderone V. Development of In Vitro Corneal Models: Opportunity for Pharmacological Testing. Methods and Protocols. 2020; 3(4):74. https://doi.org/10.3390/mps3040074
Chicago/Turabian StyleCiti, Valentina, Eugenia Piragine, Simone Brogi, Sara Ottino, and Vincenzo Calderone. 2020. "Development of In Vitro Corneal Models: Opportunity for Pharmacological Testing" Methods and Protocols 3, no. 4: 74. https://doi.org/10.3390/mps3040074
APA StyleCiti, V., Piragine, E., Brogi, S., Ottino, S., & Calderone, V. (2020). Development of In Vitro Corneal Models: Opportunity for Pharmacological Testing. Methods and Protocols, 3(4), 74. https://doi.org/10.3390/mps3040074






