A Simple and Effective Bioassay Method Suitable to Comparative In Vitro Study of Tomato Salt Tolerance at Early Development Stages
Abstract
:1. Introduction
2. Materials and Methods
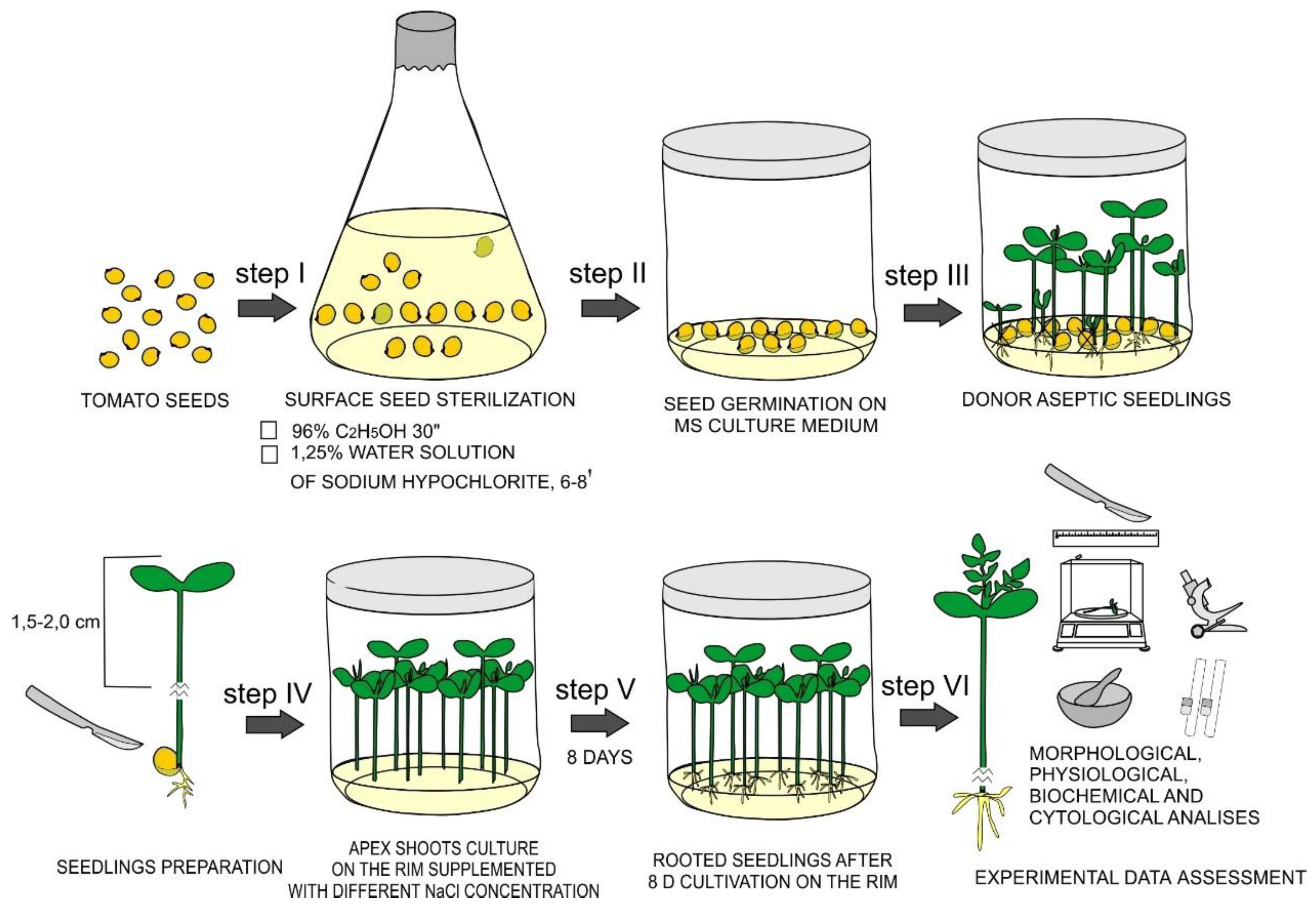
2.1. Plant Material and Obtaining of Aseptic Donor Seedlings
2.2. Effects of NaCl Treatments on De Novo Root Formation in In Vitro Tomato Seedlings and Its Morphological Characteristics
2.3. Measurements of CO2 Gas Exchange
2.4. Proline and Photosynthetic Pigment Contents
2.5. Preparation of Cotyledon Samples for Light and Transmission Electron Microscopy (TEM)
2.6. Statistical Treatments of Experimental Data
3. Results
3.1. Influence of Different NaCl Concentrations on the Number of Regenerated Roots and Their Morphological Characteristics
3.2. Influence of Different NaCl Concentrations on the Shoot Fresh Weight (SFW) and Shoot Dry Weight (SDW)
3.3. Influence of NaCl Salinity on the DRR and TPR
3.4. Influence of NaCl Salinity on the Proline and Photosynthetic Pigment Contents
3.5. The Morphological Response of Epidermal and Mesophylls Cotyledonary Tomato Cells to NaCl Salinity In Vitro
3.6. Ultrastructure of Cotyledonary SM Cells from Control and Salt-Treated (150 mM NaCl) Tomato Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Massantini, R.; Radicetti, E.; Frangipane, M.T.; Campiglia, E. Quality of Tomato (Solanum lycopersicum L.) changes under different cover crops, soil tillage and nitrogen fertilization management. Agriculture 2021, 11, 106. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Tomato Production in Russian Federation. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 10 December 2021).
- Abbasi, S.; Sadeghi, A.; Omidvari, M.; Tahan, V. The stimulators and responsive genes to induce systemic resistance against pathogens: An exclusive focus on tomato as a model plant. Biocatal. Agric. Biotechnol. 2021, 33, 101993. [Google Scholar] [CrossRef]
- Anwar, R.; Fatima, T.; Mattoo, A. Tomatoes: A Model Crop of Solanaceous Plants; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Salim, M.M.R.; Rashid, M.H.; Hossain, M.M.; Zakaria, M. Morphological characterization of tomato (Solanum lycopersicum L.) genotypes. J. Saudi Soc. Agric. Sci. 2020, 19, 233–240. [Google Scholar] [CrossRef]
- Athinodorou, F.; Foukas, P.; Tsaniklidis, G.; Kotsiras, A.; Chrysargyris, A.; Delis, C.; Kyratzis, A.C.; Tzortzakis, N.; Nikoloudakis, N. morphological diversity, genetic characterization, and phytochemical assessment of the cypriot tomato germplasm. Plants 2021, 10, 1698. [Google Scholar] [CrossRef]
- Foolad, M.R. Genome mapping and molecular breeding of tomato. Int. J. Plant Genom. 2007, 2007, 064358. [Google Scholar] [CrossRef]
- Szczechura, W.; Staniaszek, M.; Habdas, H. Tomato Molecular Markers. J. Fruit Ornam. Plant Res. 2011, 74, 5–23. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tissue Organ Cult. 2015, 120, 881–902. [Google Scholar] [CrossRef]
- Bhatia, P.; Ashwath, N.; Senaratna, T.; Midmore, D. Tissue culture studies of tomato (Lycopersicon esculentum). Plant Cell Tissue Organ Cult. 2004, 78, 1–21. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Shirokova, Y.; Forkutsa, I.; Sharafutdinova, N. Use of electrical conductivity instead of soluble salts for soil salinity monitoring in central asia. Irrig. Drain. Syst. 2000, 14, 199–206. [Google Scholar] [CrossRef]
- Singh, M.; Nara, U.; Kumar, A.; Choudhary, A.; Singh, H.; Thapa, S. Salinity tolerance mechanisms and their breeding implications. J. Gen. Eng. Biotechnol. 2021, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Bai, Y.; Zhang, P.; Finkers, R.; Du, Y.; Visser, R.G.F.; van Heusden, A.W. Seedling salt tolerance in tomato. Euphytica 2011, 178, 403–414. [Google Scholar] [CrossRef]
- Kaveh, H.; Nemati, H.; Farsi, M.; Jartoodeh, S.V. How salinity affect germination and emergence of tomato lines. J. Biol. Environ. Sci. 2011, 5, 159–163. [Google Scholar]
- Cuartero, J.; Bolarin, M.C.; Asins, M.J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef]
- Alfocea, F.P.; Estan, M.T.; Caro, M.; Bolarín, M.C. Response of tomato cultivars to salinity. Plant Soil 1993, 150, 203–211. [Google Scholar] [CrossRef]
- Cano, E.A.; Pérez-Alfocea, F.; Moreno, V.; Caro, M.; Bolarín, M.C. Evaluation of salt tolerance in cultivated and wild tomato species through in vitro shoot apex culture. Plant cell, tissue and organ culture. Plant Cell Tissue Organ Cult. 1998, 53, 19–26. [Google Scholar] [CrossRef]
- Rofekuggaman, M.; Kubra, K.; Mahmood, S. Effect of different salt concentrations (NaCl) on seed germination and seedling growth of tomato cv. BINA Tomato-10. Asian Plant Res. J. 2020, 5, 38–44. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, K.; Kumar, M.; Shamim, M.; Kumar, J.; Kumar, A. Assessment of NaCl-induced stress in tomato (Lycopersicon esculentum L.). Eur. J. Med. Plants 2021, 32, 41–47. [Google Scholar] [CrossRef]
- Chakma, P.; Hossain, M.M.; Rabbani, M.G. Effects of salinity stress on seed germination and seedling growth of tomato. J. Bangladesh Agric. Univ. 2019, 17, 490–499. [Google Scholar] [CrossRef]
- Singh, J.; Sastry, E.D.; Singh, V. Effect of salinity on tomato (Lycopersicon esculentum Mill.) during seed germination stage. Physiol. Mol. Biol. Plants 2012, 18, 45–50. [Google Scholar] [CrossRef]
- Sané, A.K.; Diallo, B.; Kane, A.; Sagna, M.; Sané, D.; Sy, M.O. In vitro germination and early vegetative growth of five tomato (Solanum lycopersicum L.) varieties under salt stress conditions. Am. J. Plant Sci. 2021, 12, 796–817. [Google Scholar] [CrossRef]
- Yokaş, İ.; Tuna, A.L.; Bürün, B.; Altunlu, H.; Altan, F.; Kaya, C. Responses of the tomato (Lycopersicon esculentum Mill.) plant to exposure to different salt forms and rates. Turk. J. Agricult. For. 2008, 32, 319–329. [Google Scholar]
- Seth, R.; Kendurkar, S. In vitro screening: An effective method for evaluation of commercial cultivars of tomato towards salinity stress. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 725–730. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Baranova, E.N.; Khaliluev, M.R.; Akanov, E.N.; Gulevich, A.A.; Khalilueva, N.M.; Kurenina, L.V. System for Control of Photosynthetic and Respiratory CO2-Gas Exchange of Plants, Isolated Organs and Tissues in Vitro. RU Patent 2572349, 10 January 2016. [Google Scholar]
- Baranova, Y.N.; Akanov, E.N.; Gulevich, A.A.; Kurenina, L.V.; Danilova, S.A.; Khaliluev, M.R. Dark respiration rate of transgenic tomato plants expressing FeSOD1 gene under chloride and sulfate salinity. Russ. Agric. Sci. 2014, 40, 14–17. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shlyk, A.A. Definition of a Chlorophyll and Carotenoids in Extracts of Green Leaves. Biochemical Methods in Physiology of Plants; Nauka: Moscow, Russia, 1971. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Alsadon, A.A.; Ibrahim, A.A.; Wahb-Allah, M.A.; Ali, A.A.M.; Sadder, M.T. Tomato under salinity stress: Correlation between growth and yield components and responsive genes. Acta Hortic. 2014, 1081, 111–119. [Google Scholar] [CrossRef]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Huang, C.; Peng, F.; You, Q.; Xue, X.; Wang, T.; Liao, J. Growth, yield and fruit quality of cherry tomato irrigated with saline water at different developmental stages. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 317–324. [Google Scholar] [CrossRef]
- Kahlaoui, B.; Hachicha, M.; Rejeb, S.; Rejeb, M.N.; Hanchi, B.; Misle, E. Effects of saline water on tomato under subsurface drip irrigation: Nutritional and foliar aspects. J. Soil Sci. Plant Nutr. 2011, 11, 69–86. [Google Scholar] [CrossRef]
- Rahil, M.; Hajjeh, H.; Qanadillo, A. Effect of saline water application through different irrigation intervals on tomato yield and soil properties. Open J. Soil Sci. 2013, 3, 143–147. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Martinez, V.; Cerda, A. Salt tolerance of tomato plants as affected by stage of plant development. Hortic. Sci. 2001, 36, 1260–1263. [Google Scholar] [CrossRef]
- Suarez, D.L.; Celis, N.; Ferreira, J.F.; Reynolds, T.; Sandhu, D. Linking genetic determinants with salinity tolerance and ion relationships in eggplant, tomato and pepper. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Ahmad, M.; Shoukat, A.; Anjum, M.; Kamran, M.; Saeed, M.Q.; Hussain, R.; Abbas, M.H. Assessment of tomato genotypes against salinity on the basis of morphological and physiological parameters in hydroponic conditions. Nat. Sci. 2019, 17, 56–70. [Google Scholar]
- Pailles, Y.; Awlia, M.; Julkowska, M.; Passone, L.; Zemmouri, K.; Negrão, S.; Schmöckel, S.M.; Tester, M. Diverse traits contribute to salinity tolerance of wild tomato seedlings from the Galapagos Islands. Plant Physiol. 2020, 182, 534–546. [Google Scholar] [CrossRef]
- Ali, A.A.M.; Romdhane, W.B.; Tarroum, M.; Al-Dakhil, M.; Al-Doss, A.; Alsadon, A.A.; Hassairi, A. Analysis of salinity tolerance in tomato introgression lines based on morpho-physiological and molecular traits. Plants 2021, 10, 2594. [Google Scholar] [CrossRef]
- Loudari, A.; Benadis, C.; Naciri, R.; Soulaimani, A.; Zeroual, Y.; Gharous, M.E.; Kalaji, H.M.; Oukarroum, A. Salt stress affects mineral nutrition in shoots and roots and chlorophyll a fluorescence of tomato plants grown in hydroponic culture. J. Plant Int. 2020, 15, 398–405. [Google Scholar] [CrossRef]
- Zaki, H.E.; Yokoi, S. A comparative in vitro study of salt tolerance in cultivated tomato and related wild species. Plant Biotech. 2016, 33, 361–372. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, M.; Hossain, K.F.B.; Sikder, M.T.; Shammi, M.; Rasheduzzaman, M.; Hossain, M.A.; Alam, M.; Uddin, M.K. Effects of NaCl-salinity on tomato (Lycopersicon esculentum Mill.) plants in a pot experiment. Open Agric. 2018, 3, 578–585. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H. Ultrastructural changes in shoot apical meristem of canola (Brassica napus cv. Symbol) treated with sodium chloride. Pak. J. Biol. Sci. 2008, 11, 1161–1164. [Google Scholar] [CrossRef]
- de Jonge, J. Shoot Apical Meristem Arrest in Brassica and Tomato. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 16 December 2013. [Google Scholar]
- El-Meleigy, E.A.; Gabr, M.F.; Mohamed, F.H.; Ismail, M.A. Responses to NaCl salinity of tomato cultivated and breeding lines differing in salt tolerance in callus cultures. Int. J. Agric. Biol. 2004, 6, 19–26. [Google Scholar]
- Mahdi, E.F.M.; Idris, T.I.M. The effects of NaCl pre-treatment on salt tolerance of tomato (Lycopersicon esculentum Mill.) callus grown under elevated saline conditions. Int. Res. J. Biotech. 2013, 4, 61–67. [Google Scholar]
- Nahar, L.L.; Nasar, M.Z.; Al Nayem, C.; Ashrafuzzaman, M. In Vitro growth media effect for regeneration of tomato (Lycopersicon esculentum) and evaluation of the salt tolerance activity of callus. J. Agric. Sustain. 2013, 3, 132–143. [Google Scholar]
- Cano, E.A.; Pérez-Alfocea, F.; Moreno, V.; Bolarin, M.C. Responses to NaCl stress of cultivated and wild tomato species and their hybrids in callus cultures. Plant Cell Rep. 1996, 15, 791–794. [Google Scholar] [CrossRef]
- Salina, Y.B.; Tyutyuma, N.V.; Tyutyuma, A.V. Salinization as a critical factor of land fertility in the Astrakhan region. Dostizheniya Nauk. Tekh. APK 2018, 32, 5–8. (In Russian) [Google Scholar]
- Bogoutdinova, L.R.; Baranova, E.N.; Baranova, G.B.; Kononenko, N.V.; Lazareva, E.M.; Smirnova, E.A.; Khaliluev, M.R. Morpho-biological and cytological characterization of tomato roots (Solanum lycopersicum L., cv. Rekordsmen) regenerated under NaCl salinity in vitro. Cell Tissue Biol. 2020, 14, 228–242. [Google Scholar] [CrossRef]
- Bogoutdinova, L.R.; Baranova, G.B.; Baranova, E.N.; Khaliluev, M.R. Comparative anatomical and morphological studies of the epidermal and cortical parenchyma hypocotyl cells of two tomato genotypes (Solanum lycopersicum L.) under sodium chloride stress in vitro. Sel’skokhozyaistvennaya Biol. 2016, 51, 318–326. [Google Scholar] [CrossRef]








| NaCl Concentrations, mM | Rhizogenesis Frequency, % | Time of the Beginning Root Formation, Days | ||
|---|---|---|---|---|
| Line YaLF | cv. Rekordsmen | Line YaLF | cv. Rekordsmen | |
| 0 | 100 | 100 | 4 | 4 |
| 25 | 100 | 100 | 4 | 4 |
| 50 | 100 | 100 | 4 | 4 |
| 75 | 100 | 100 | 4 | 4 |
| 100 | 100 | 100 | 4 | 4 |
| 150 | 100 | 100 | 5 | 5 |
| 200 | 16,7 | 100 | 7 | 6 |
| 250 | 0 | 93,3 | – | 7 |
| 300 | nd | 0 | nd | – |
| NaCl Concentrations, mM | RFW, mg | RDW, mg | ||
|---|---|---|---|---|
| Line YaLF | cv. Rekordsmen | Line YaLF | cv. Rekordsmen | |
| 0 | 17.68 l | 15.97 kl | 1.19 l | 0.95 k |
| 25 | 14.08 jk | 11.61 ij | 0.78 ghijk | 0.78 hijk |
| 50 | 7.44 fgh | 8.37 gh | 0.67 fghi | 0.85 ijk |
| 75 | 6.54 efgh | 8.37 h | 0.57 fg | 0.91 jk |
| 100 | 4.78 cdef | 5.39 defg | 0.47 cdef | 0.55 def |
| 150 | 1.77 ab | 3.35 bcd | 0.24 b | 0.55 ef |
| 200 | 0.07 a | 0.91 ab | 0.02a | 0.14 ab |
| 250 | – | 0.70 ab | – | 0.08 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliluev, M.R.; Bogoutdinova, L.R.; Raldugina, G.N.; Baranova, E.N. A Simple and Effective Bioassay Method Suitable to Comparative In Vitro Study of Tomato Salt Tolerance at Early Development Stages. Methods Protoc. 2022, 5, 11. https://doi.org/10.3390/mps5010011
Khaliluev MR, Bogoutdinova LR, Raldugina GN, Baranova EN. A Simple and Effective Bioassay Method Suitable to Comparative In Vitro Study of Tomato Salt Tolerance at Early Development Stages. Methods and Protocols. 2022; 5(1):11. https://doi.org/10.3390/mps5010011
Chicago/Turabian StyleKhaliluev, Marat R., Liliya R. Bogoutdinova, Galina N. Raldugina, and Ekaterina N. Baranova. 2022. "A Simple and Effective Bioassay Method Suitable to Comparative In Vitro Study of Tomato Salt Tolerance at Early Development Stages" Methods and Protocols 5, no. 1: 11. https://doi.org/10.3390/mps5010011
APA StyleKhaliluev, M. R., Bogoutdinova, L. R., Raldugina, G. N., & Baranova, E. N. (2022). A Simple and Effective Bioassay Method Suitable to Comparative In Vitro Study of Tomato Salt Tolerance at Early Development Stages. Methods and Protocols, 5(1), 11. https://doi.org/10.3390/mps5010011








