Optimized Protocol for Isolation and Culture of Murine Neonatal Primary Lung Fibroblasts
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Sterile surgical scissors and forceps;
- 70% Ethanol;
- Hank’s Balanced Salt Solution (HBSS) 10X (Gibco, 14175095);
- Sterile HBSS 1X diluted with MiliQ water and with pH adjusted to 7.2;
- Sterile phosphate-buffered saline (PBS) 1X;
- MiliQ water;
- Collagenase/Dispase® (Roche, 10269638001);
- 0.25% Trypsin-EDTA (Gibco, 25200-056);
- Sterile Red Blood Cell lysis (RBC) buffer (0.155 M NH4Cl, 10 mM KHCO3, 10 mM EDTA, pH 7.4);
- DMEM (Gibco, 41966-029);
- Foetal Bovine serum (FBS) (Gibco, 10270-106);
- Penicillin/Streptomycin (P/S) (Gibco, 15140-122);
- Amphotericin B (Gibco, 15290-026);
- 6-well cell culture plates;
- 100 mm cell culture Petri plates;
- 1.5 mL Eppendorf tubes;
- Dimethyl sulfoxide (DMSO);
- Sterile glass coverslips;
- Fibronectin solution for coating (Sigma, F0556);
- Bovine serum albumin (BSA) (Sigma, 9048-46-8);
- Goat serum (Sigma, G9023);
- Triton X-100 (Sigma, X100);
- Alexa Fluor™ 488 phalloidin (Invitrogen, A12379);
- Alpha-smooth muscle actin (GeneTex, GTX100034);
- Cy3-AffiniPure Goat Anti-Rabbit IgG (H + L) (Jackson ImmunoResearch, 111-165-003);
- ProLong Diamond antifade reagent (Life Technologies, P36970);
- NZYol (NZYtech, MB18501);
- Qiagen RNeasy Mini Kit (Qiagen, 74104);
- Luna Universal One-Step RT-qPCR kit (New England Biolabs, E3005);
- 96 or 384-well plate suitable for Real-time PCR System.
2.2. Equipment
- Thermo-shaker for Eppendorf tubes;
- Ultracentrifuge for Eppendorf tubes;
- Biological culture hood;
- Cell culture incubator;
- Confocal microscope;
- Nanodrop;
- Real-Time PCR System.
2.3. Freshly Prepared Solutions (to Be Made Fresh Immediately before the Experiment)
- Collagenase/Dispase® 1 mg/mL diluted in sterile HBSS 1X. Keep in ice until use.
- Freezing media: 10% DMSO, 20% FBS in DMEM;
- Blocking solution for immunofluorescence staining (5% BSA, 2% goat serum, 1% Triton X-100 in PBS);
- Antibody solution for immunofluorescence staining (2% BSA, 2% goat serum, 1% Triton X-100 in PBS).
3. Procedure
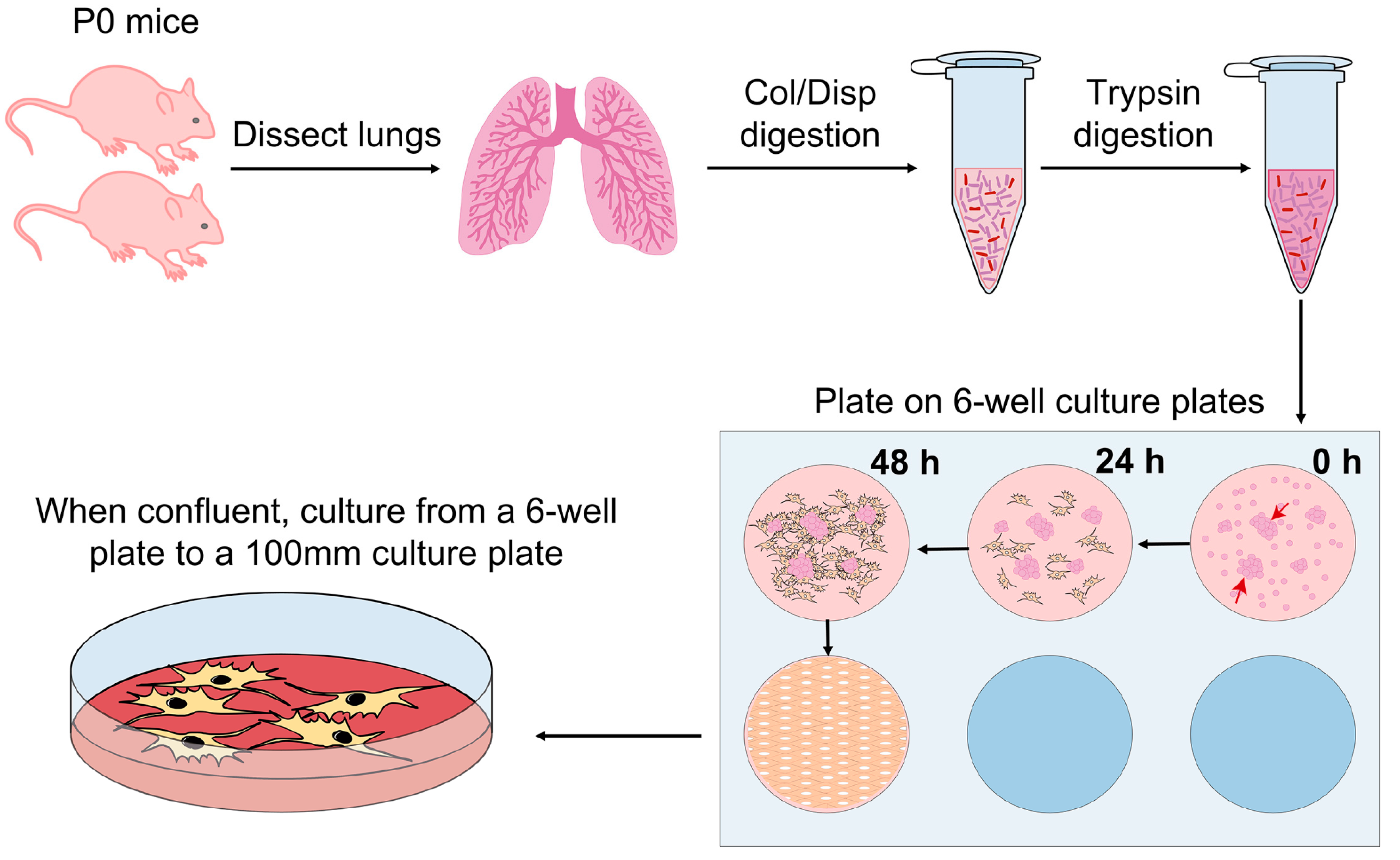
3.1. Tissue Collection and Dissociation
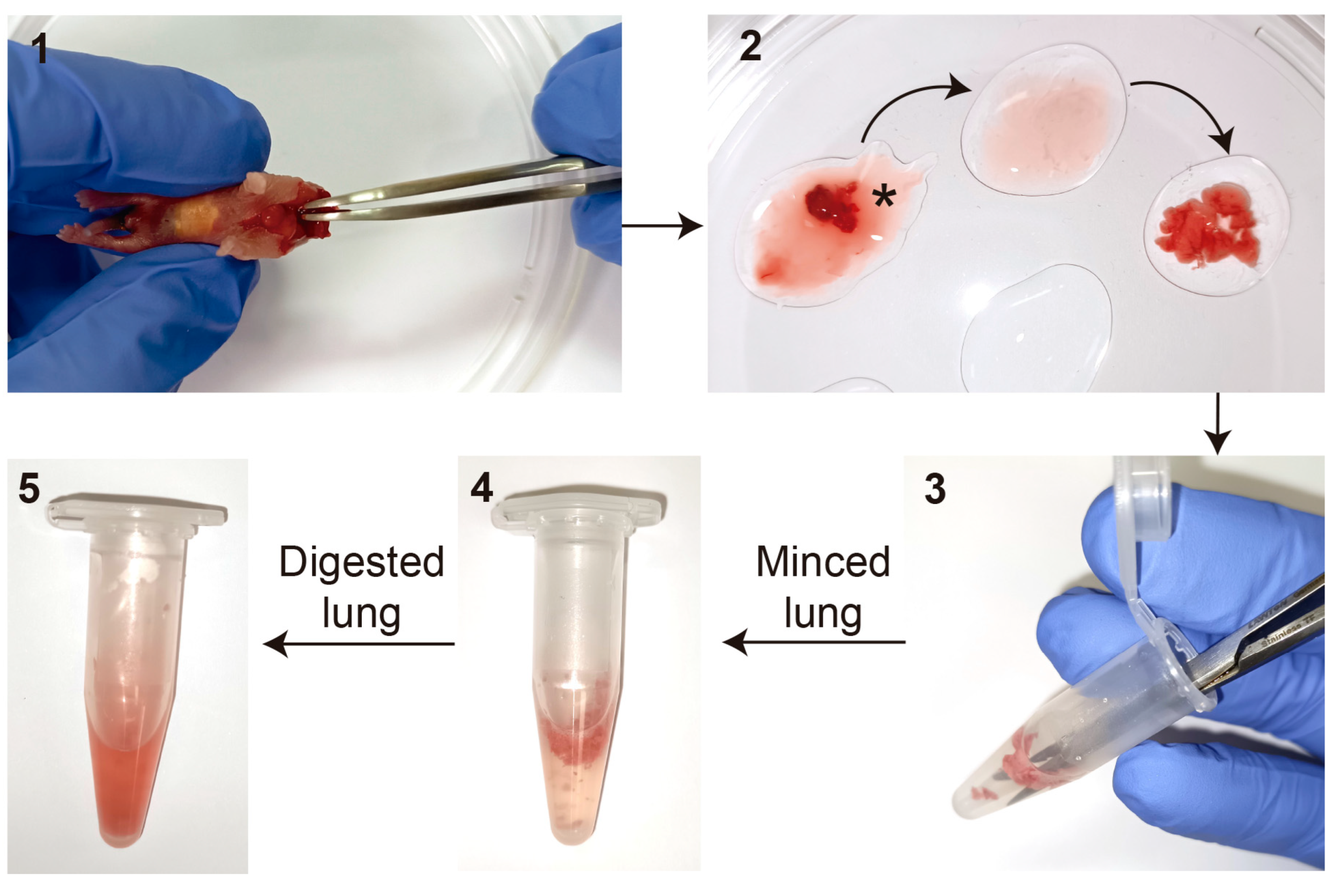
- Briefly wash newborn mice skin with 70% Ethanol. Euthanize newborn pups by decapitation. Make a small incision with the scissors from the neck to the chest without reaching the peritoneal cavity, and use forceps to remove the lungs attached to the heart;
CRITICAL STEP For multiple pups, euthanize one after another. Do not reach the peritoneal cavity to avoid contaminations;
- Place the lung lobes attached to the heart into a drop of HBSS in a sterile 100 mm culture plate containing multiple drops of HBSS. Remove the heart and other non-lung tissue, including the trachea and bronchi, and collect the 5 lung lobes individually one by one. Wash out the blood as much as possible by passing the tissue from drop to drop of HBSS at least 3 times (Figure 2).
3.2. Enzymatic Digestion
- For the first enzymatic digestion, place the lung lobes into a 1.5 mL Eppendorf tube containing 0.5 mL of 1 mg/mL Collagenase/Dispase® and mince thoroughly with small surgical scissors. Incubate at 37 °C for 30 min (min) with gentle agitation;
- After the incubation, vortex the Eppendorf briefly and centrifuge for 5 min at 1000 × g at room temperature (RT). Carefully decant and discard the supernatant;
CRITICAL STEP The digested lung is viscous and does not remain strongly attached at the bottom of the tube. Thus, to avoid losing material, special care is needed when decanting the supernatant;
- Wash the pellet by adding 1 mL of HBSS and centrifuge for 5 min at 1000 × g. Carefully decant and discard the supernatant;
- For the second enzymatic digestion, add 0.5 mL of 0.25% Trypsin-EDTA to each Eppendorf and mix thoroughly by pipetting up and down several times. Incubate at 37 °C for 20 min with gentle agitation;
CRITICAL STEP Do not exceed 20 min digestion time with trypsin, otherwise, the final cell recovery will be extremely low;
- Centrifuge for 5 min at 1000 × g at RT. Carefully decant and discard the supernatant.
3.3. Red Blood Cell (RBC) Removal
- Resuspend the pellet in 0.1 mL of RBC at RT and incubate for 1 min. Quickly, neutralize by adding 1.2 mL of 1X PBS;
CRITICAL STEP If the erythrocytes are not eliminated, the efficiency will be very low. Do not exceed the lysis time, otherwise, the viability of the fibroblasts will be affected;
- Centrifuge for 5 min at 1000 × g at RT. Decant and discard supernatant;
3.4. Platting and Freezing
- Resuspend the pellet in 0.5 mL of fibroblast culture media (DMEM supplemented with 10% of FBS, 1% P/S, 0.2% Amphotericin B). Gently pipette up and down to break big aggregates and plate the suspension into a 6-well tissue culture plate;
CRITICAL STEP Do not discard tissue pieces as lung fibroblasts will crawl out from those pieces, see Figure 3;
- Add 2 mL of fibroblast culture media so the final volume in each well will be about 2.5 mL;
- Culture cells at 37 °C and 5% CO2;
- After 24 h, change the media. If erythrocytes are still present in the well, carefully wash with 2 mL of 1X PBS, aspirate PBS and add 2.5 mL of fresh fibroblasts media. After the first change of media, change it every other day. Split to a 100 mm culture plate when the cells are confluent;
- Freeze 1 vial of cells from 1 confluent 100 mm culture plate (containing about 5 million cells). To detach cells, remove media, wash with sterile PBS to remove FBS residues, aspirate PBS and add 1 mL of 0.25% Trypsin-EDTA. Incubate for 2 min at 37 °C, then, add at least 2 mL of media to the 100 mm culture plate to neutralize the effect of the trypsin;
- Place the 3 mL of cells into a 15 mL tube and centrifuge for 5 min at 1200 rpm. Remove the supernatant and resuspend the cell pellet in lung fibroblasts freezing media (10% DMSO, 20% FBS in DMEM). Place cryovial swiftly into ice and freeze up to −80 °C using a cell freezing container. After 24 h, place cryovials into liquid nitrogen.
3.5. Immunofluorescence (IF)
- Place sterile glass coverslips on a 24-well culture plate and coat them with 40 µL of 0.2 µg/mL fibronectin diluted in sterile 1X PBS overnight at 37 °C;
- Plate 1.5 × 104 cells (cell density must be determined experimentally) on each fibronectin-coated coverslip. Final volume in each well should be approximately 0.5 mL. Incubate 12–24 h in a cell incubator;
- Aspirate media and wash each well with 1X PBS. Aspirate PBS and fix the cells with 0.5 mL of 4% paraformaldehyde (PFA) for 15 min at 37 °C. Eliminate PFA and wash 2 times with 1X PBS;
- Permeabilize the cells by washing them for 10 min with 0.1% Triton X-100 in PBS (PBS-Triton);
- Incubate cells with blocking solution (5% Bovine serum albumin (BSA), 2% goat serum, 1% Triton X-100 in PBS) for at least 30 min at RT;
- Incubate with the actin primary antibody (1:100) diluted in antibody solution (2% BSA, 2% goat serum, 1% Triton X-100 in PBS) overnight at 4 °C;
- Wash 3 × 5 min with 1X PBS and permeabilize with PBS-Triton for 10 min. Then, incubate the secondary antibody (Cy3 Goat anti-rabbit, 1:500) together with phalloidin (1:1000) and DAPI diluted in antibody solution for 1 h at RT;
- Wash 3 × 5 min with 1X PBS, mount coverslips with ProLong Diamond antifading reagent and examine them using a confocal microscope.
3.6. qPCR Assays
- Grow the cells in a 100 mm culture plate up to 70–80% confluency;
- Remove the media and wash 2x with 1X PBS. Completely aspirate the PBS and add 0.5 mL of NZYol. Detach and lysate the cells using a cell scraper and collect the sample into a 1.5 mL tube;
- (Note: at this point, samples can be stored at −80 °C prior to RNA isolation);
- Isolate RNA with NZYol following the manufacture’s indications, and further purify it using the Qiagen RNeasy Mini Kit columns;
- RNA concentration and quality can be assessed by RNA capillary electrophoresis columns, or using a Nanodrop;
- RT-qPCR assays to detect expression levels of genes of interest (Table 1) can be assessed using the Luna Universal One-Step RT-qPCR kit following the manufacture’s protocol. β2-microglobulin is used as a housekeeping gene to have an endogenous control to normalize results.
4. Expected Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, W.V.; Kotton, D.N. Specification and Patterning of the Respiratory System. StemBook 2008. [Google Scholar] [CrossRef] [PubMed]
- Herriges, M.; Morrisey, E.E. Lung Development: Orchestrating the Generation and Regeneration of a Complex Organ. Development 2014, 141, 502–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrisey, E.E.; Hogan, B.L.M. Preparing for the First Breath: Genetic and Cellular Mechanisms in Lung Development. Dev. Cell 2010, 18, 8–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlfeld, S.K.; Conway, S.J. Aberrant Signaling Pathways of the Lung Mesenchyme and Their Contributions to the Pathogenesis of Bronchopulmonary Dysplasia. Birth Defects Res. Part A-Clin. Mol. Teratol. 2012, 94, 3–15. [Google Scholar]
- Chao, C.M.; El Agha, E.; Tiozzo, C.; Minoo, P.; Bellusci, S. A Breath of Fresh Air on the Mesenchyme: Impact of Impaired Mesenchymal Development on the Pathogenesis of Bronchopulmonary Dysplasia. Front. Med. 2015, 2, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Volckaert, T.; Chanda, D.; Thannickal, V.J.; De Langhe, S.P. Fgf10 Signaling in Lung Development, Homeostasis, Disease, and Repair After Injury. Front. Genet. 2018, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biasin, V.; Crnkovic, S.; Sahu-Osen, A.; Birnhuber, A.; El Agha, E.; Sinn, K.; Klepetko, W.; Olschewski, A.; Bellusci, S.; Marsh, L.M.; et al. PDGFRα and ASMA Mark Two Distinct Mesenchymal Cell Populations Involved in Parenchymal and Vascular Remodeling in Pulmonary Fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L684–L697. [Google Scholar] [CrossRef] [PubMed]
- Melo-Narváez, M.C.; Stegmayr, J.; Wagner, D.E.; Lehmann, M. Lung Regeneration: Implications of the Diseased Niche and Ageing. Eur. Respir. Rev. 2020, 29, 157. [Google Scholar] [CrossRef]
- Nasri, A.; Foisset, F.; Ahmed, E.; Lahmar, Z.; Vachier, I.; Jorgensen, C.; Assou, S.; Bourdin, A.; De Vos, J. Roles of Mesenchymal Cells in the Lung: From Lung Development to Chronic Obstructive Pulmonary Disease. Cells 2021, 10, 3467. [Google Scholar] [CrossRef] [PubMed]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170, 1134–1148.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samarelli, A.V.; Tonelli, R.; Heijink, I.; Martin Medina, A.; Marchioni, A.; Bruzzi, G.; Castaniere, I.; Andrisani, D.; Gozzi, F.; Manicardi, L.; et al. Dissecting the Role of Mesenchymal Stem Cells in Idiopathic Pulmonary Fibrosis: Cause or Solution. Front. Pharmacol. 2021, 12, 1656. [Google Scholar] [CrossRef]
- Kruk, D.M.L.W.; Heijink, I.H.; Slebos, D.J.; Timens, W.; Ten Hacken, N.H. Mesenchymal Stromal Cells to Regenerate Emphysema: On the Horizon? Respiration 2018, 96, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.A.; Lapa e Silva, J.R.; Rocco, P.R.M. Mesenchymal Stromal Cell Therapy in COPD: From Bench to Bedside. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3017–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.J.; Segal, K.; Casaburi, R.; Hayes, J.; Tashkin, D. Effect of Mesenchymal Stromal Cell Infusions on Lung Function in COPD Patients with High CRP Levels. Respir. Res. 2021, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Mateos, R.; Jimeno, D.; Gómez, C.; Calzada, N.; Fernández-Medarde, A.; Santos, E. Concomitant Deletion of HRAS and NRAS Leads to Pulmonary Immaturity, Respiratory Failure and Neonatal Death in Mice. Cell Death Dis. 2019, 10, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelman, B.L.; Redente, E.F. Isolation and Characterization of Mouse Fibroblasts. In Lung Innate Immunity and Inflammation; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Seluanov, A.; Vaidya, A.; Gorbunova, V. Establishing Primary Adult Fibroblast Cultures from Rodents. J. Vis. Exp. 2010, 44, e2033. [Google Scholar] [CrossRef] [Green Version]


| Gene | Accession Number | Sequence |
|---|---|---|
| Hoxa5 | P09021 | F- CAGGGTCTGGTAGCGAGTGT R- CTCAGCCCCAGATCTACCC |
| Hoxb5 | P09079 | F- CTGGTAGCGAGTATAGGCGG R- AGGGGCAGACTCCACAGATA |
| Hoxc5 | P32043 | F- TTCTCGAGTTCCAGGGTCTG R- ATTTACCCGTGGATGACCAA |
| Wnt2 | P21552 | F- TCTTGAAACAAGAATGCAAGTGTCA R- GAGATAGTCGCCTGTTTTCCTGAA |
| Wnt2b | O70283 | F- CTGCTGCTGCTACTCCTGACT R- GGGGATGTTGTCACAGATCA |
| FGF7 | Q544I6 | F- CTGCTCCACGCTAACTTCCA R- GAGTTTACGCACCAGCACAC |
| FGF9 | P54130 | F- TTCATGCGGTGGGTTCTTATT R- TCCTCATCCAAGCTTCCATCA |
| FGF10 | O35565 | F- GTCAGCGGGACCAAGAATGA R- GTCGTTGTTAAACTCTTTTGAGCC |
| Lef1 | P27782 | F- AAATGGGTCCCTTTCTCCAC R- CTCGTCGCTGTAGGTGATGA |
| Axin2 | O88566 | F- CAGTGAGCTGGTTGTCACCT R- TCCTCAAAAACTGCTCCGCA |
| Nkd1 | Q99MH6 | F- TAGACCTGGCGGGGATAGAG R- GTCAAGGAGGTGGAAGGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Mateos, R.; Santos, E.; Fernández-Medarde, A. Optimized Protocol for Isolation and Culture of Murine Neonatal Primary Lung Fibroblasts. Methods Protoc. 2023, 6, 14. https://doi.org/10.3390/mps6010014
Fuentes-Mateos R, Santos E, Fernández-Medarde A. Optimized Protocol for Isolation and Culture of Murine Neonatal Primary Lung Fibroblasts. Methods and Protocols. 2023; 6(1):14. https://doi.org/10.3390/mps6010014
Chicago/Turabian StyleFuentes-Mateos, Rocío, Eugenio Santos, and Alberto Fernández-Medarde. 2023. "Optimized Protocol for Isolation and Culture of Murine Neonatal Primary Lung Fibroblasts" Methods and Protocols 6, no. 1: 14. https://doi.org/10.3390/mps6010014








