Magnetic Resonance Imaging as a Tool for Monitoring Intratibial Growth of Experimental Prostate Cancer Metastases in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture
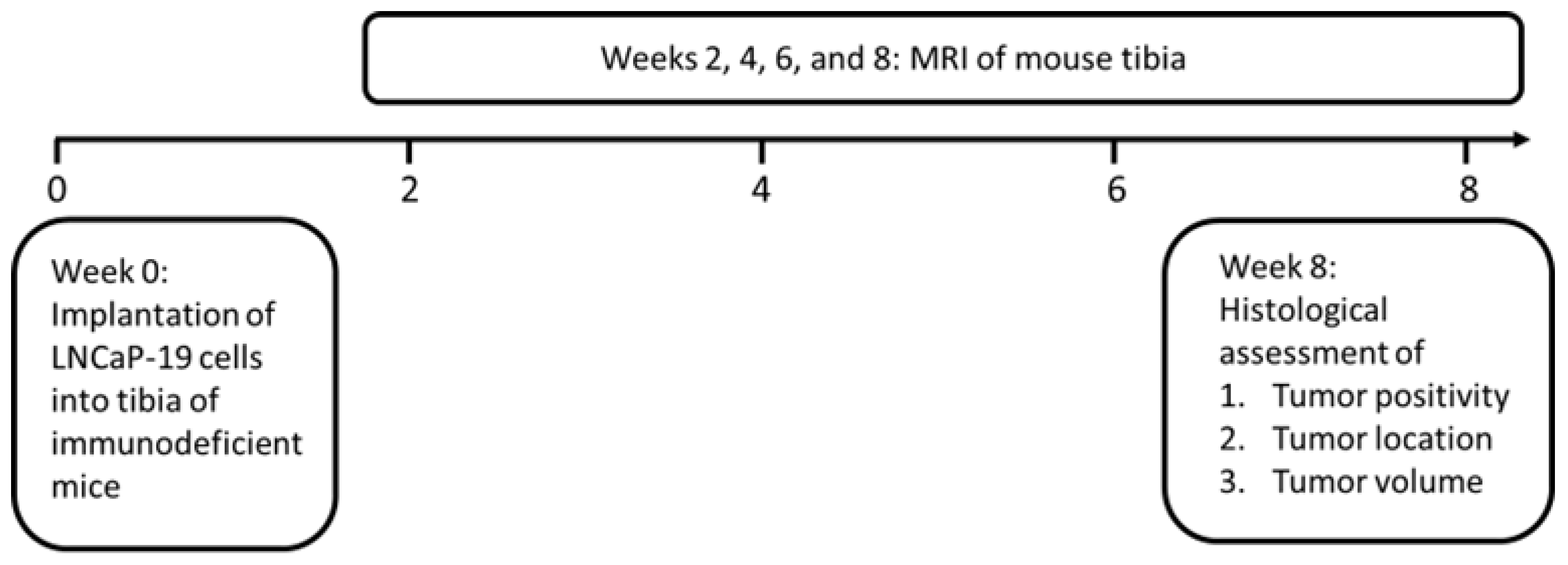
2.2. In Vivo Intratibial Tumors
2.3. Magnetic Resonance Imaging
2.4. Histological Evaluation
2.5. Statistics
3. Results
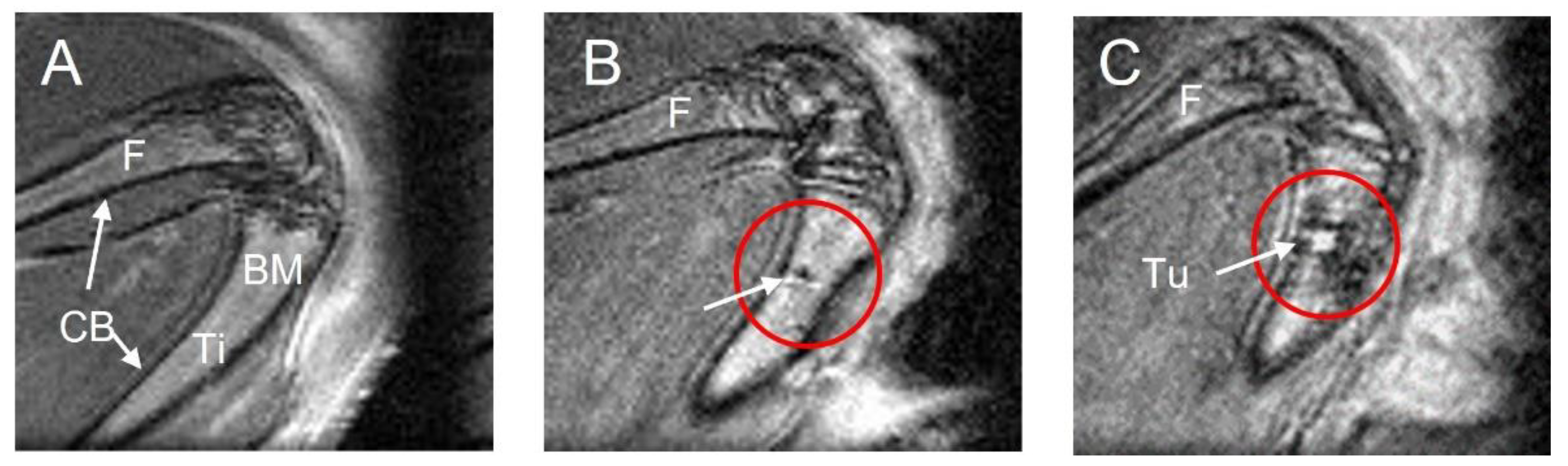
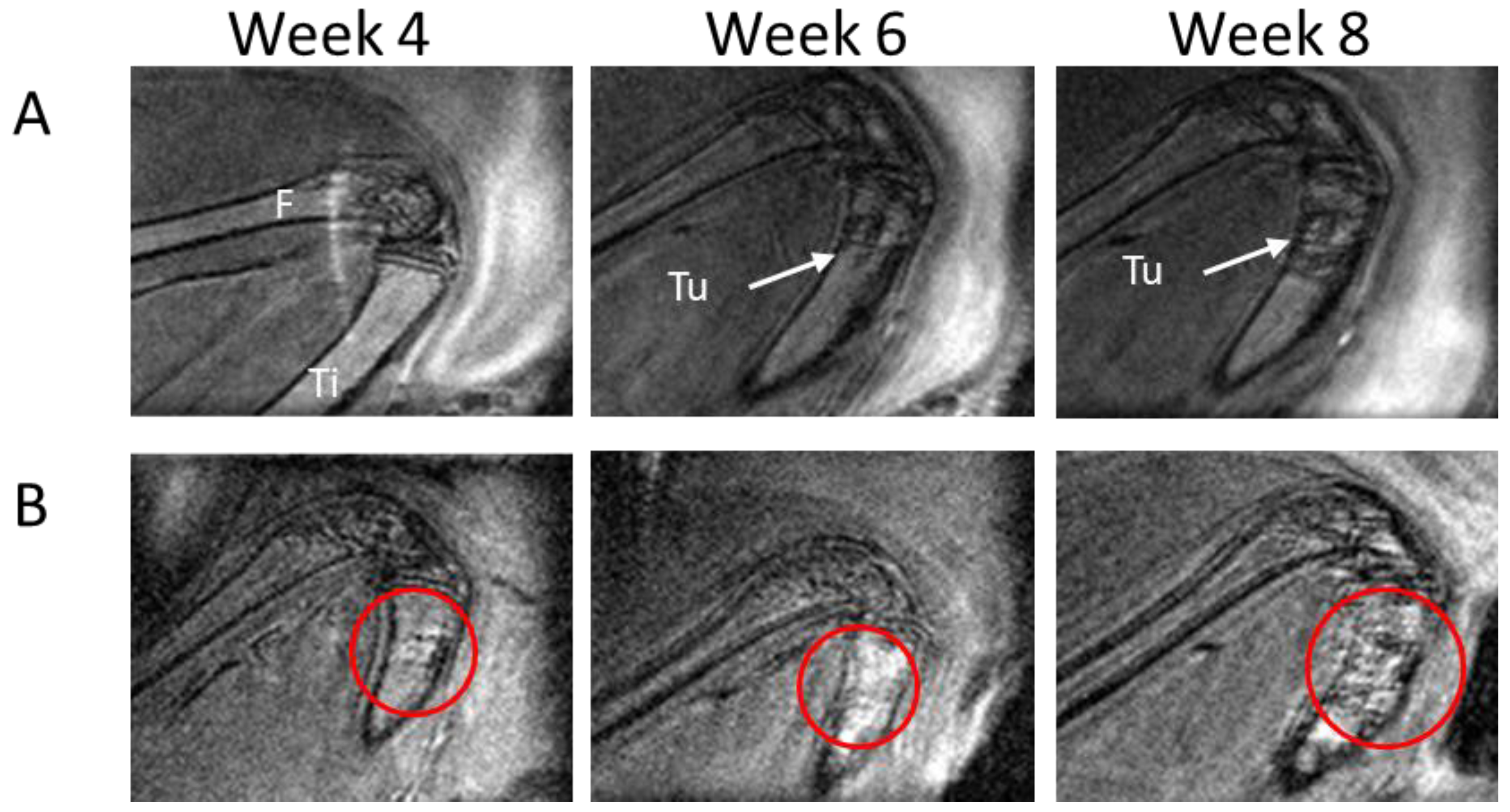
3.1. MRI Performance for Tumor Location and Phenotype Definition
3.2. MRI Performance in Evaluating Tumor Positivity
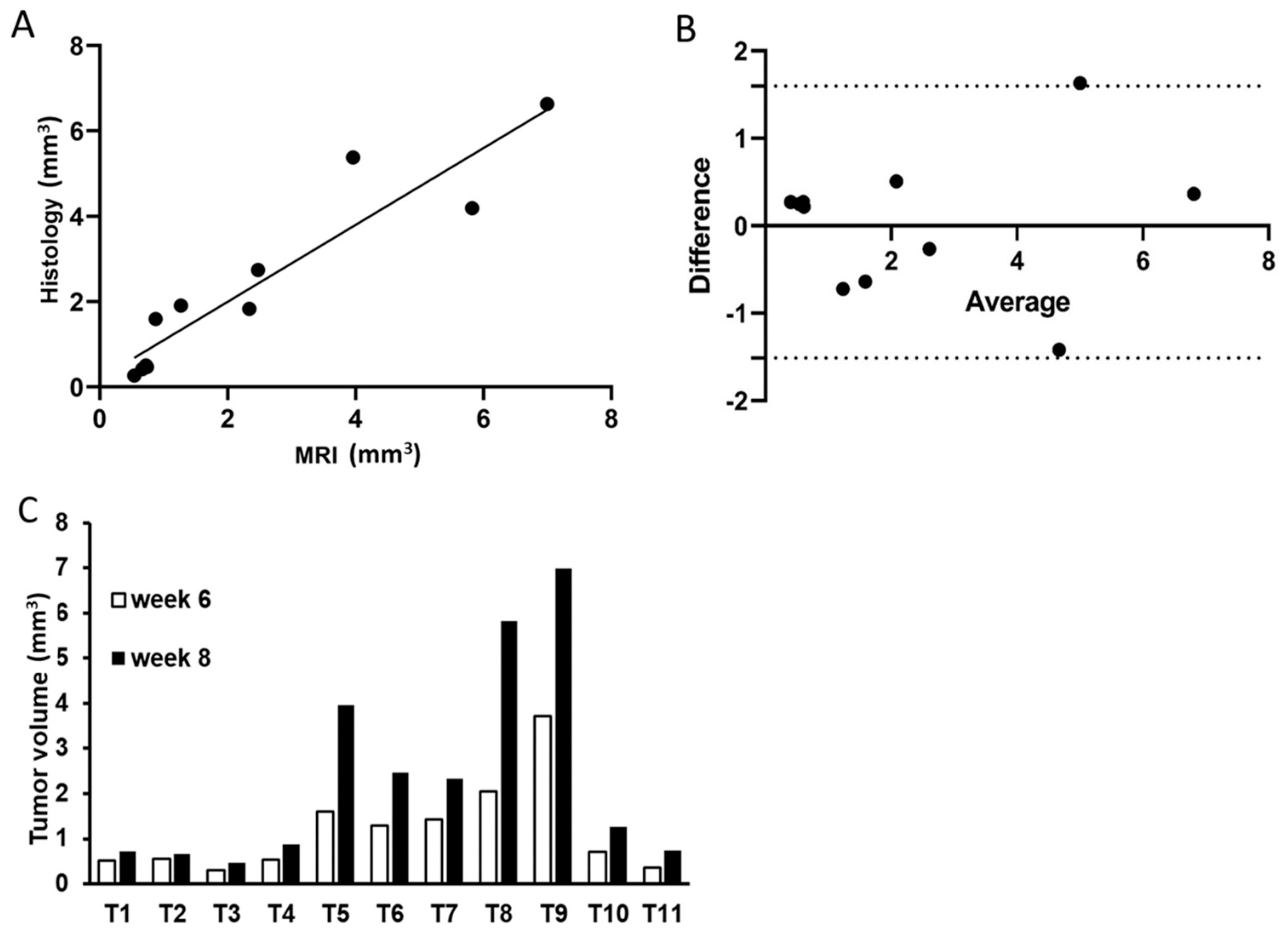
3.3. MRI Performance in Assessing Tumor Growth
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, T.; Shin, T. Current magnetic resonance imaging-based diagnostic strategies for prostate cancer. Int. J. Urol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.M.; Blackledge, M.D.; Tunariu, N.; Koh, D.M.; Messiou, C. Whole-body MRI: A practical guide for imaging patients with malignant bone disease. Clin. Radiol. 2021, 76, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Tae, J.H.; Chang, I.H. Animal models of bone metastatic prostate cancer. Investig. Clin. Urol. 2023, 64, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, M.F.; Cavaliere, C.; Auletta, L.; Basso, L.; Salvatore, M. Magnetic Resonance Imaging for Translational Research in Oncology. J. Clin. Med. 2019, 8, 1883. [Google Scholar] [CrossRef] [PubMed]
- Montelius, M.; Ljungberg, M.; Horn, M.; Forssell-Aronsson, E. Tumour size measurement in a mouse model using high resolution MRI. BMC Med. Imaging 2012, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Blattmann, C.; Thiemann, M.; Stenzinger, A.; Roth, E.K.; Dittmar, A.; Witt, H.; Lehner, B.; Renker, E.; Jugold, M.; Eichwald, V.; et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J. Transl. Med. 2015, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, D. Ultrasound imaging-guided intracardiac injection to develop a mouse model of breast cancer brain metastases followed by longitudinal MRI. J. Vis. Exp. 2014, 85, 51146. [Google Scholar] [CrossRef]
- Graham, T.J.; Box, G.; Tunariu, N.; Crespo, M.; Spinks, T.J.; Miranda, S.; Attard, G.; de Bono, J.; Eccles, S.A.; Davies, F.E.; et al. Preclinical evaluation of imaging biomarkers for prostate cancer bone metastasis and response to cabozantinib. J. Natl. Cancer Inst. 2014, 106, dju033. [Google Scholar] [CrossRef] [PubMed]
- Hoff, B.A.; Chughtai, K.; Jeon, Y.H.; Kozloff, K.; Galban, S.; Rehemtulla, A.; Ross, B.D.; Galban, C.J. Multimodality imaging of tumor and bone response in a mouse model of bony metastasis. Transl. Oncol. 2012, 5, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, H.; Welen, K.; Damber, J.E. Transition of an androgen-dependent human prostate cancer cell line into an androgen-independent subline is associated with increased angiogenesis. Prostate 2005, 62, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Hagberg Thulin, M.; Jennbacken, K.; Damber, J.E.; Welen, K. Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies. Clin. Exp. Metastasis 2014, 31, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Back, T.A.; Jennbacken, K.; Hagberg Thulin, M.; Lindegren, S.; Jensen, H.; Olafsen, T.; Yazaki, P.J.; Palm, S.; Albertsson, P.; Damber, J.E.; et al. Targeted alpha therapy with astatine-211-labeled anti-PSCA A11 minibody shows antitumor efficacy in prostate cancer xenografts and bone microtumors. EJNMMI Res. 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Jennbacken, K.; Gustavsson, H.; Tesan, T.; Horn, M.; Vallbo, C.; Welen, K.; Damber, J.E. The prostatic environment suppresses growth of androgen-independent prostate cancer xenografts: An effect influenced by testosterone. Prostate 2009, 69, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Shiirevnyamba, A.; Takahashi, T.; Shan, H.; Ogawa, H.; Yano, S.; Kanayama, H.; Izumi, K.; Uehara, H. Enhancement of osteoclastogenic activity in osteolytic prostate cancer cells by physical contact with osteoblasts. Br. J. Cancer 2011, 104, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.R.; West, J.L.; Badea, C.T. In vivo small animal micro-CT using nanoparticle contrast agents. Front. Pharmacol. 2015, 6, 256. [Google Scholar] [CrossRef] [PubMed]

| Imaging Parameters | Transversal | Sagittal |
|---|---|---|
| Repetition time (ms) | 2500 | 2700 |
| Effective echo time (ms) | 28 | 18.5 |
| Turbo factor | 6 | 4 |
| Number of averages | 4 | 20 |
| FOV (read × phase) (mm) | 19 × 13 | 16.2 × 13.5 |
| Matrix size (read × phase) | 120 × 80 | 134 × 160 |
| Pixel size (read × phase) (mm) | 0.16 × 0.16 | 0.12 × 0.09 |
| Number of slices | 16 | 9 |
| Slice thickness (mm) | 0.5 | 0.23 |
| Slice gap | 0.5 | 0.1 |
| Fat suppression | yes | yes |
| Respiratory triggering | no | no |
| Scan time | 2 min 10 s | 36 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Montelius, M.; Damber, J.-E.; Welén, K. Magnetic Resonance Imaging as a Tool for Monitoring Intratibial Growth of Experimental Prostate Cancer Metastases in Mice. Methods Protoc. 2023, 6, 118. https://doi.org/10.3390/mps6060118
Huang J, Montelius M, Damber J-E, Welén K. Magnetic Resonance Imaging as a Tool for Monitoring Intratibial Growth of Experimental Prostate Cancer Metastases in Mice. Methods and Protocols. 2023; 6(6):118. https://doi.org/10.3390/mps6060118
Chicago/Turabian StyleHuang, Junchi, Mikael Montelius, Jan-Erik Damber, and Karin Welén. 2023. "Magnetic Resonance Imaging as a Tool for Monitoring Intratibial Growth of Experimental Prostate Cancer Metastases in Mice" Methods and Protocols 6, no. 6: 118. https://doi.org/10.3390/mps6060118
APA StyleHuang, J., Montelius, M., Damber, J.-E., & Welén, K. (2023). Magnetic Resonance Imaging as a Tool for Monitoring Intratibial Growth of Experimental Prostate Cancer Metastases in Mice. Methods and Protocols, 6(6), 118. https://doi.org/10.3390/mps6060118






