Nanoparticle Lysis of Cryptosporidium Oocysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Protozoa
2.3. NP Exposures
2.4. Oocyst Detection
3. Results
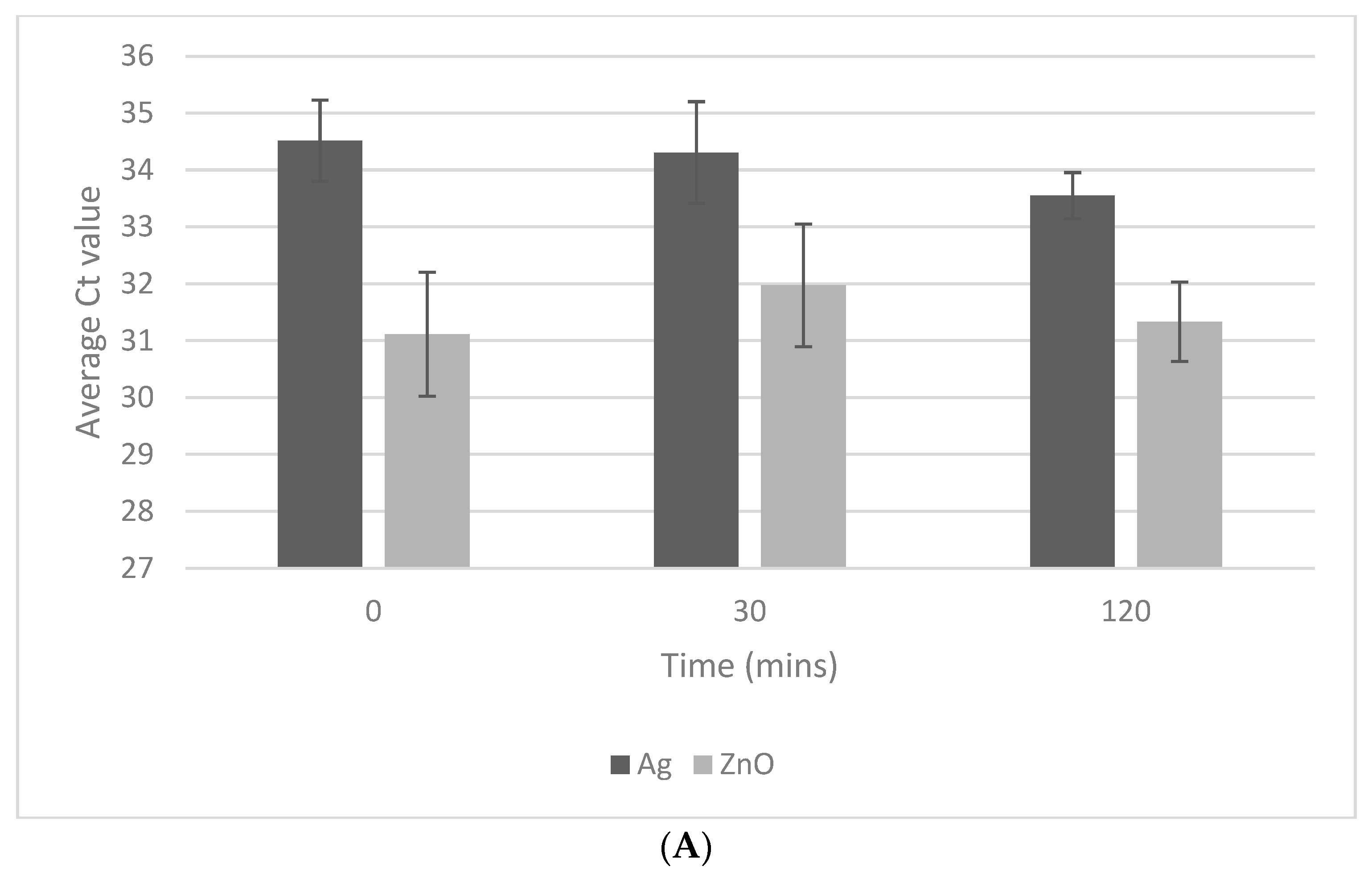
3.1. Exposure Time
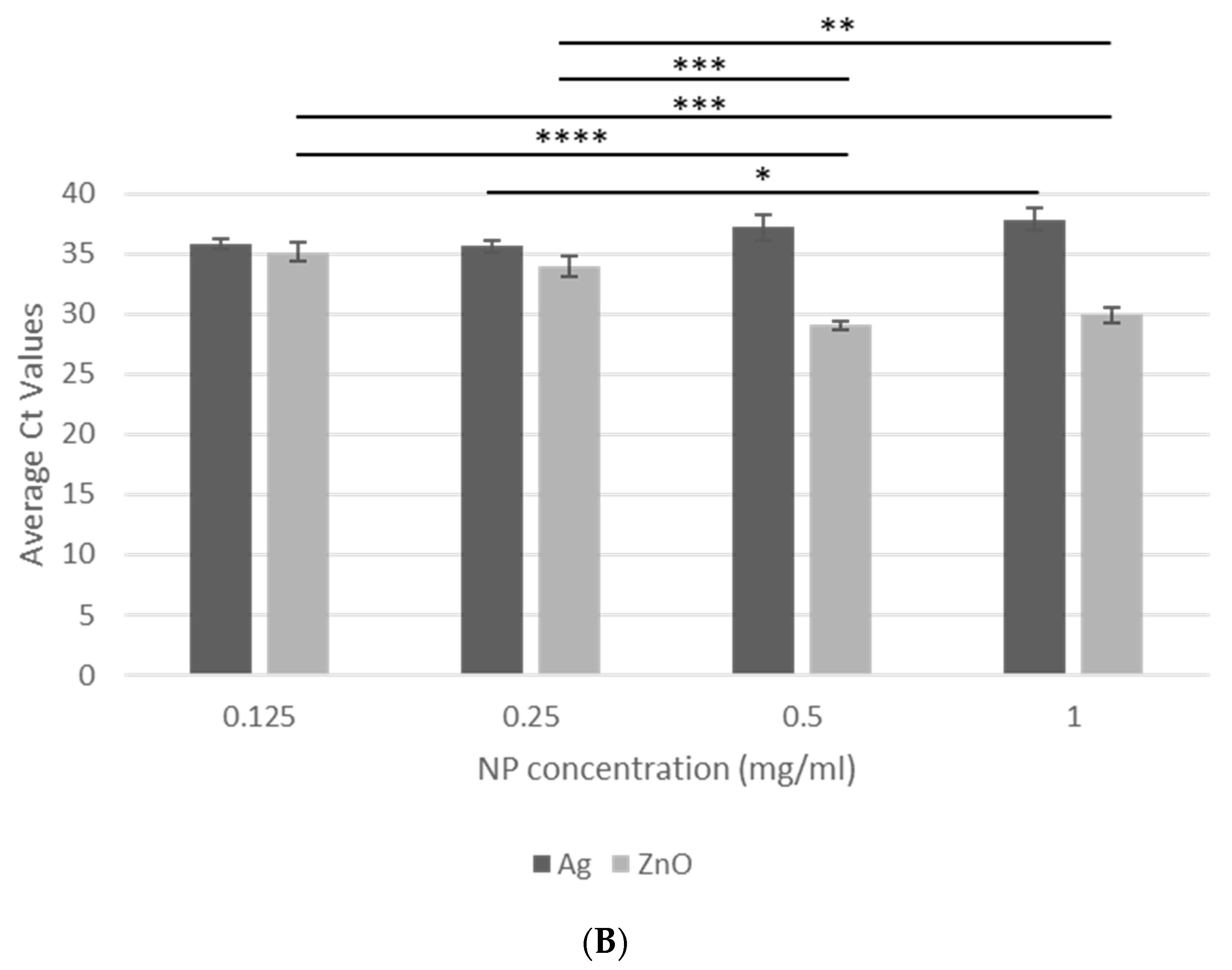
3.2. NP Concentration
3.3. Oocyst Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, B.L.; Chalmers, R.M.; Davies, A.P. Health sequelae of human cryptosporidiosis in industrialised countries: A systematic review. Parasites Vectors 2020, 13, 443. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yang, Y.; Wang, Y.; Yang, D.; Yang, Y.; Shi, Y.; Li, C.; Li, L.; Chen, Y.; Jiang, Q.; et al. Prevalence of Cryptosporidium Infection in the Global Population: A Systematic Review and Meta-analysis. Acta Parasitol. 2020, 65, 882–889. [Google Scholar] [CrossRef]
- Corso, P.S.; Kramer, M.H.; Blair, K.A.; Addiss, D.G.; Davis, J.P.; Haddix, A.C. Cost of illness in the 1993 waterborne Cryptosporidium outbreak, Milwaukee, Wisconsin. Emerg. Infect. Dis. 2003, 9, 426–431. [Google Scholar] [CrossRef]
- Widerström, M.; Schönning, C.; Lilja, M.; Lebbad, M.; Ljung, T.; Allestam, G.; Ferm, M.; Björkholm, B.; Hansen, A.; Hiltula, J.; et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg. Infect. Dis. 2014, 20, 581–589. [Google Scholar] [CrossRef]
- Widmer, G.; Carmenax, D.; Kváč, M.; Chalmers, R.M.; Kissinger, J.C.; Xiao, L.; Sateriale, A.; Striepen, B.; Laurent, F.; Lacroix-Lamande, S.; et al. Update on Cryptosporidium spp.: Highlights from the Seventh International Giardia and Cryptosporidium Conference. Parasite 2020, 27, 14. [Google Scholar] [CrossRef] [PubMed]
- Innes, E.A.; Chalmers, R.M.; Wells, B.; Pawlowic, M.C. A One Health Approach to Tackle Cryptosporidiosis. Trends Parasitol. 2020, 36, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.J.; Innes, E.A.; Morrison, L.J.; Katzer, F.; Wells, B. Long-term production effects of clinical cryptosporidiosis in neonatal calves. Int. J. Parasitol. 2020, 50, 371–376. [Google Scholar] [CrossRef]
- Destura, R.V.; Cena, R.B.; Galarion, M.J.H.; Pangilinan, M.; Arevlao, G.M.; Alba, R.O.C.; Petronio, J.A.G.; Salem, G.M.; Schwem, B.; Sevilleja, J.E.A.D. Advancing Cryptosporidium Diagnostics from Bench to Bedside. Curr. Trop. Med. Rep. 2015, 2, 150–160. [Google Scholar] [CrossRef]
- Valeix, N.; Costa, D.; Basmaciyan, L.; Valot, S.; Vincent, A.; Razakandrainibe, R.; Rovert-Gangneux, F.; Nourrisson, C.; Pereira, B.; Frealle, E.; et al. Multicenter Comparative Study of Six Cryptosporidium parvum DNA Extraction Protocols Including Mechanical Pretreatment from Stool Samples. Microorganisms 2020, 8, 1450. [Google Scholar] [CrossRef]
- Sekikawa, T.; Toshiki, K. A new method for efficient detection of Cryptosporidium RNA by real-time reverse transcription-PCR with surfactants. Water Supply 2015, 15, 1061–1068. [Google Scholar] [CrossRef]
- Hawash, Y. DNA extraction from protozoan oocysts/cysts in feces for diagnostic PCR. Korean J. Parasitol. 2014, 52, 263–271. [Google Scholar] [CrossRef]
- Wells, B.; Shaw, H.; Hotchkiss, E.; Gilray, J.; Ayton, R.; Green, J.; Katzer, F.; Wells, A.; Innes, E. Prevalence, species identification and genotyping Cryptosporidium from livestock and deer in a catchment in the Cairngorms with a history of a contaminated public water supply. Parasites Vectors 2015, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Koken, E.; Darnault, C.J.G.; Jacobson, A.R.; Powelson, D.; Hendrickson, W. Quantification of Cryptosporidium parvum in natural soil matrices and soil solutions using qPCR. J. Microbiol. Methods 2013, 92, 135–144. [Google Scholar] [CrossRef]
- Najdrowski, M.; Joachim, A.; Daugschies, A. An improved in vitro infection model for viability testing of Cryptosporidium parvum oocysts. Vet. Parasitol. 2007, 150, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Sanchez, G.; Wilderer, P.A.; Munch, J.C.; Horn, H.; Lebuhn, M. Evaluation of two methods for quantification of hsp70 mRNA from the waterborne pathogen Cryptosporidium parvum by reverse transcription real-time PCR in environmental samples. Water Res. 2009, 43, 2669–2678. [Google Scholar] [CrossRef]
- Halstead, F.D.; Lee, A.V.; Couto-Parada, X.; Polley, S.D.; Ling, C.; Jenkins, C.; Chalmers, R.M.; Elwin, K.; Gray, J.J.; Iturriza-Gómara, M.; et al. Universal extraction method for gastrointestinal pathogens. J. Med. Microbiol. 2013, 62, 1535–1539. [Google Scholar] [CrossRef]
- Anceno, A.J.; Katayama, H.; Houpt, E.R.; Chavalitshewinkoon-Petmitr, P.; Chuluun, B.; Shipin, O.V. IMS-free DNA extraction for the PCR-based quantification of Cryptosporidium parvum and Giardia lamblia in surface and waste water. Int. J. Environ. Health Res. 2007, 17, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lindergard, G.; Nydam, D.V.; Wade, S.E.; Schaaf, S.L.; Mohammed, H.O. The Sensitivity of PCR Detection of Cryptosporidium Oocysts in Fecal Samples Using Two DNA Extraction Methods. Mol. Diagn. 2003, 7, 147–153. [Google Scholar] [CrossRef]
- Elwin, K.; Robinson, G.; Hadfield, S.J.; Fairclough, H.V.; Iturriza-Gomara, M.; Chalmers, R.M. A comparison of two approaches to extracting Cryptosporidium DNA from human stools as measured by a real-time PCR assay. J. Microbiol. Methods 2012, 89, 38–40. [Google Scholar] [CrossRef]
- Altammar, K. A review on nanoparticles: Characteristics, synthesis, applications and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Plachá, D.; Jampílek, J. Impact of Nanoparticles on Protozoa. In Nanotechnology in Medicine; Rai, M., Patel, M., Patel, R., Eds.; Springer Nature: London, UK, 2021. [Google Scholar] [CrossRef]
- Cameron, P.; Gaiser, B.K.; Bhandar, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-H.; Tsegaye, M.; Varhue, W.; Liao, K.-T.; Abebe, L.S.; Smith, J.A.; Guerrant, R.L.; Swami, N.S. Quantitative dielectrophoretic tracking for characterization and separation of persistent subpopulations of Cryptosporidium parvum. Analyst 2014, 139, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Haasan, D.; Farghali, M.; Eldeek, H.; Gaber, M.; Elossily, N.; Ismail, T. Antiprotozoal activity of silver nanoparticles against Cryptosporidium parvum oocysts: New insights on their feasibility as a water disinfectant. J. Microbiol. Methods 2019, 165, 105698. [Google Scholar] [CrossRef]
- Abou Elez, R.M.M.; Attia, A.S.A.; Tolba, H.M.N.; Anter, R.G.A.; Elsohaby, I. Molecular identification and antiprotozoal activity of silver nanoparticles on viability of Cryptosporidium parvum isolated from pigeons, pigeon fanciers and water. Sci. Rep. 2023, 13, 3109. [Google Scholar] [CrossRef]
- Abebe, L.S.; Su, Y.-H.; Guerrant, R.L.; Swami, N.S.; Smith, J.A. Point-of-Use Removal of Cryptosporidium parvum from Water: Independent Effects of Disinfection by Silver Nanoparticles and Silver Ions and by Physical Filtration in Ceramic Porous Media. Environ. Sci. Technol. 2015, 49, 12958–12967. [Google Scholar] [CrossRef]
- Ahmed, S.A.; El-Mahallawy, H.S.; Karanis, P. Inhibitory activity of chitosan nanoparticles against Cryptosporidium parvum oocysts. Parasitol. Res. 2019, 118, 2053–2063. [Google Scholar] [CrossRef]
- Hamdy, D.A.; Ismail, M.A.M.; El-Askary, H.M.; Abdel-Tawab, H.; Ahmed, M.M.; Fouad, F.M.; Mohamed, F. Newly fabricated zinc oxide nanoparticles loaded materials for therapeutic nano delivery in experimental cryptosporidiosis. Sci. Rep. 2023, 13, 19650. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalai, M.; Zarrintan, M.H.; Adibka, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Gaiser, B.; Hutchison, G.R.; Stone, V. An in vitro liver model—Assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part. Fibre Toxicol. 2012, 9, 28. [Google Scholar] [CrossRef]
- Donnellan, S.; Tran, L.; Johnston, H.; McLuckie, J.; Stevenson, K.; Stone, V. A rapid screening assay for identifying mycobacteria targeted nanoparticle antibiotics. Nanotoxicology 2016, 10, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbu, G.D.; Baskar, R.; Anusuya, T.; Seshan, C.A.; Chelliah, R. Toxicity mechanism of titanium dioxide and zinc oxide nanoparticles against food pathogens. Colloids Surf. B Biointerfaces 2016, 148, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.-B.; Li, Z.-X.; Guo, Z.-Y. Silicon inhibition effects on the polymerase chain reaction: A real-time detection approach. J. Biomed. Mater. Res. Part A 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Wan, W.; Yeow, J.T.W.; Dyke, M.I.V. Effect of silver and titanium dioxide nanoparticles on PCR efficiency. In Proceedings of the 2009 9th IEEE Conference on Nanotechnology (IEEE-NANO), Genoa, Italy, 26–30 July 2009. [Google Scholar]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Naqvi, S.; Samim, M.; Abdin, M.Z.; Ahmed, F.J.; Maitra, A.N.; Prashant, C.K.; Dinda, A.K. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int. J. Nanomed. 2010, 5, 983–989. [Google Scholar] [CrossRef]
- Kalbassi, M.R.; Johari, S.A.; Soltani, M.; Yu, I. Particle Size and Agglomeration Affect the Toxicity levels of Silver Nanoparticle types in Aquatic Environment. Ecopersia 2013, 1, 247–264. [Google Scholar]
- Nair, S.; Sasidharan, A.; Rani, V.V.D.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2008, 20, 235. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Silva, T.; Pokhrel, L.R.; Dubey, B.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: Comparison between general linear model-predicted and observed toxicity. Sci. Total Environ. 2014, 468–469, 968–976. [Google Scholar] [CrossRef]
- Mallevre, F.; Fernandes, T.F.; Aspray, T.J. Silver, zinc oxide and titanium dioxide nanoparticle ecotoxicity to bioluminescent Pseudomonas putida in laboratory medium and artificial wastewater. Environ. Pollut. 2014, 195, 218–225. [Google Scholar] [CrossRef]
- Wu, J.; Kodzius, R.; Cao, W.; Wen, W. Extraction, amplification and detection of DNA in microfluidic chip-based assays. Microchim. Acta 2014, 181, 1611–1631. [Google Scholar] [CrossRef]

| NP Concentration (µg/mL) | Ag NPs | ZnO NPs | ||||
|---|---|---|---|---|---|---|
| Z-Ave (nm) | Pdl | Zeta Potential (mV) | Z-Ave (nm) | Pdl | Zeta Potential (mV) | |
| 1 | 44.1 | 0.39 | −14.1 | 305.5 | 0.37 | −4.7 |
| 5 | 47.06 | 0.35 | −6.3 | 369.9 | 0.42 | −12.6 |
| 10 | 79.29 | 0.28 | −7.5 | 396.1 | 0.35 | −11.5 |
| 20 | 94.31 | 0.42 | −15.2 | 747.1 | 0.38 | −5.8 |
| 50 | 130.27 | 0.25 | −11.3 | 887.1 | 0.49 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaidya, A.; Bankier, C.; Johnston, H.; Bridle, H. Nanoparticle Lysis of Cryptosporidium Oocysts. Methods Protoc. 2024, 7, 66. https://doi.org/10.3390/mps7050066
Vaidya A, Bankier C, Johnston H, Bridle H. Nanoparticle Lysis of Cryptosporidium Oocysts. Methods and Protocols. 2024; 7(5):66. https://doi.org/10.3390/mps7050066
Chicago/Turabian StyleVaidya, Ameya, Claire Bankier, Helinor Johnston, and Helen Bridle. 2024. "Nanoparticle Lysis of Cryptosporidium Oocysts" Methods and Protocols 7, no. 5: 66. https://doi.org/10.3390/mps7050066
APA StyleVaidya, A., Bankier, C., Johnston, H., & Bridle, H. (2024). Nanoparticle Lysis of Cryptosporidium Oocysts. Methods and Protocols, 7(5), 66. https://doi.org/10.3390/mps7050066








