Evaluation of Protocols for DNA Extraction from Individual Culex pipiens to Assess Pyrethroid Resistance Using Genotyping Real-Time Polymerase Chain Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Collection and Sorting
2.2. DNA Extraction Methods
2.2.1. QIAsymphony Sample Preparation Protocol
2.2.2. DNAzol Direct Reagent
2.2.3. Chelex® 100
2.2.4. PrepMan® Ultra Sample Preparation Reagent
2.3. Method Comparison
2.4. Real-Time PCR
3. Results
3.1. Entomological Analysis
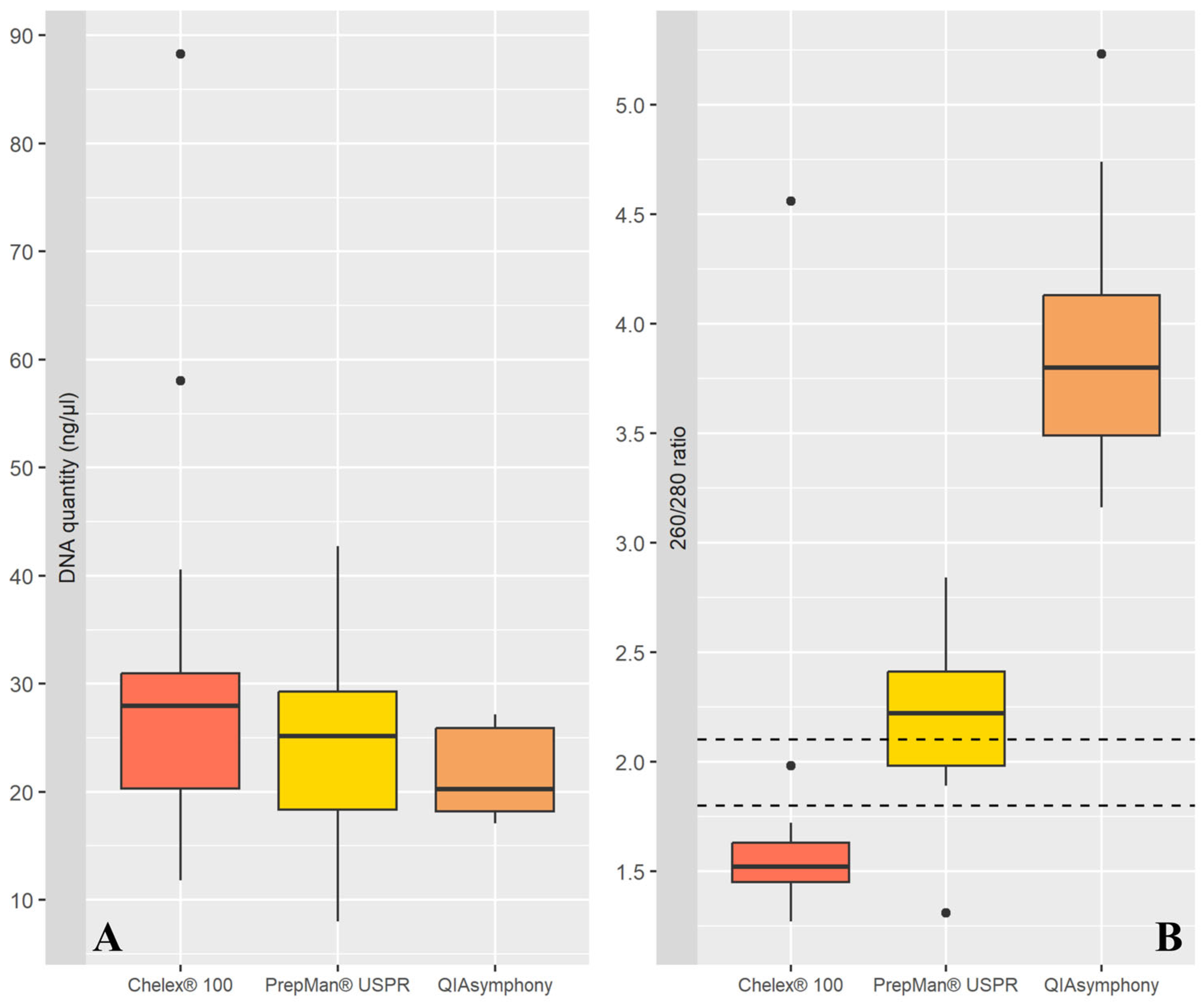
3.2. Comparison of Extraction Methods
3.3. Statistical Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinogradova, E.B. Culex pipiens pipiens mosquitoes: Taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Pensoft Ser. Parasitol. 2000, 2, 250. [Google Scholar]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Brugman, V.A.; Hernández-Triana, L.M.; Medlock, J.M.; Fooks, A.R.; Carpenter, S.; Johnson, N. The role of Culex pipiens L. (Diptera: Culicidae) in virus transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Autorino, G.L.; Battisti, A.; Deubel, V.; Ferrari, G.; Forletta, R.; Giovannini, A.; Lelli, R.; Murri, S.; Scicluna, M.T. West Nile virus epidemic in horses, Tuscany region, Italy. Emerg. Infect. Dis. 2002, 8, 1372–1378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef]
- Italian Ministry of Health. National Surveillance and Response Integrated Plan for West Nile and Usutu Viruses—2018; Italian Ministry of Health: Rome, Italy, 2018. (In Italian)
- Scaramozzino, P.; Carvelli, A.; Bruni, G.; Cappiello, G.; Censi, F.; Magliano, A.; Manna, G.; Ricci, I.; Rombolà, P.; Romiti, F.; et al. West Nile and Usutu viruses co-circulation in central Italy: Outcomes of the 2018 integrated surveillance. Parasites Vectors 2021, 14, 243. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. La Sorveglianza dei Casi Umani di Infezione da West Nile e Usutu Virus. 2024. Available online: https://www.epicentro.iss.it/westNile/bollettino (accessed on 2 September 2024).
- Bkhache, M.; Tmimi, F.-Z.; Charafeddine, O.; Faraj, C.; Failloux, A.-B.; Sarih, M. First report of L1014F-kdr mutation in Culex pipiens complex from Morocco. Parasites Vectors 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Piano Nazionale di Prevenzione, Sorveglianza e Risposta alle Arbovirosi (PNA) 2020–2025. 2019. Available online: https://www.salute.gov.it/portale/malattieInfettive/dettaglioPubblicazioniMalattieInfettive.jsp?lingua=italiano&id=2947 (accessed on 14 October 2021).
- Pichler, V.; Giammarioli, C.; Bellini, R.; Veronesi, R.; Arnoldi, D.; Rizzoli, A.; Lia, R.P.; Otranto, D.; Ballardini, M.; Cobre, P.; et al. First evidence of pyrethroid resistance in Italian populations of West Nile virus vector Culex pipiens. Med. Vet. Entomol. 2022, 36, 390–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chrustek, A.; Hołyńska-Iwan, I.; Dziembowska, I.; Bogusiewicz, J.; Wróblewski, M.; Cwynar, A.; Olszewska-Słonina, D. Current Research on the Safety of Pyrethroids Used as Insecticides. Medicina 2018, 54, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tmimi, F.Z.; Faraj, C.; Bkhace, M.; Mounaji, K.; Failloux, A.B.; Sarih, M. Insecticide resistance and target site mutations (G119S ace-1 and L1014F kdr) of Culex pipiens in Morocco. Parasite Vectors 2018, 22, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soderlund, D.; Knipple, D. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem. Mol. 2003, 33, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, R.; Dong, K. The biochemical and molecular genetic basis of resistance to pesticides in arthropods. In Global Pesticide Resistance in Arthropods; CAB International: Wallingford, UK, 2008; pp. 40–89. [Google Scholar]
- Cantu, C.; Bucheli, S.; Houston, R. Comparison of DNA extraction techniques for the recovery of bovine DNA from fly larvae crops. J. Forensic Sci. 2022, 67, 1651–1659. [Google Scholar] [CrossRef]
- Jangra, S.; Ghosh, A. Rapid and zero-cost DNA extraction from soft-bodied insects for routine PCR-based applications. PLoS ONE 2022, 17, e0271312. [Google Scholar] [CrossRef]
- Firmansyah, N.E.; Thongseesuksai, T.; Boonmars, T.; Sungkar, S.; Laummaunwai, P. Comparison of 3 DNA extraction methods for extracting DNA from an adult Culex quinquefasciatus (Diptera: Culicidae). J. Insect Sci. 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Severini, F.; Toma, L.; Di Luca, M. Zanzare in Italia: Raccolta, Identificazione e Conservazione Delle Specie più Comuni; Istituto Superiore di Sanità: Roma, Italy, 2022; Rapporti ISTISAN 22/3; Available online: https://www.iss.it/-/rapporto_istisan_22_3 (accessed on 14 September 2024).
- Nieman, C.C.; Yamasaki, Y.; Collier, T.C.; Lee, Y. A DNA extraction protocol for improved DNA yield from individual mosquitoes. F1000Research 2015, 20, 1314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iurescia, M.; Romiti, F.; Cocumelli, C.; Diaconu, E.L.; Stravino, F.; Onorati, R.; Alba, P.; Friedrich, K.G.; Maggi, F.; Magliano, A.; et al. Plasmodium matutinum transmitted by Culex pipiens as a cause of avian malaria in captive african penguins (Spheniscus demersus) in Italy. Front. Veter-Sci. 2021, 8, 621974. [Google Scholar] [CrossRef]
- Cocumelli, C.; Iurescia, M.; Diaconu, E.L.; Galietta, V.; Raso, C.; Buccella, C.; Stravino, F.; Grande, F.; Fiorucci, L.; De Liberato, C.; et al. Plasmodium matutinum causing avian malaria in lovebirds (Agapornis roseicollis) hosted in an Italian zoo. Microorganisms 2021, 9, 1356. [Google Scholar] [CrossRef]
- Musapa, M.; Kumwenda, T.; Mkulama, M.; Chishimba, S.; Norris, D.E.; Thuma, P.E.; Mharakurwa, S. A Simple Chelex Protocol for DNA Extraction from Anopheles spp. J. Vis. Exp. 2013, 71, e3281. [Google Scholar] [CrossRef]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. JoVE J. 2010, 45, e2565. [Google Scholar] [CrossRef]
- Lucena-Aguilar, G.; Sánchez-López, A.M.; Barberán-Aceituno, C.; Carrillo-Ávila, J.A.; López-Guerrero, J.A.; Aguilar-Quesada, R. DNA Source Selection for Downstream Applications Based on DNA Quality Indicators Analysis. Biopreserv. Biobank. 2016, 14, 264–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 September 2024).
- Hardstone Yoshimizu, M.; Padgett, K.; Kramer, V. Surveillance of a kdr Resistance Mutation in Culex pipiens (Diptera: Culicidae) and Culex quinquefasciatus in California. J. Med. Entomol. 2020, 57, 645–648. [Google Scholar] [CrossRef]
- Asghar, U.; Malik, M.; Anwar, F.; Javed, A.; Raza, A. DNA Extraction from Insects by Using Different Techniques: A Review. Adv. Entomol. 2005, 3, 132–138. [Google Scholar] [CrossRef]
- A Rider, M.; Byrd, B.D.; Keating, J.; Wesson, D.M.; A Caillouet, K. PCR detection of malaria parasites in desiccated Anopheles mosquitoes is uninhibited by storage time and temperature. Malar. J. 2012, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Sidstedt, M.; Rådström, P.; Hedman, J. PCR inhibition in qPCR, dPCR and MPS-mechanisms and solutions. Anal. Bioanal. Chem. 2020, 412, 2009–2023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Primers | Sequence |
| Culex Fw | 5′-TTCGTTCCCACCTTTTCTTG-3′ |
| Culex Rw | 5′-TTCGTTCCCACCTTTTCTTG-3′ |
| Probes | Sequence |
| Culex WT | 5′-YY-CTCACGACTAAATTTC-MGB-3′ |
| Culex Res (L1014F) | 5′-FAM-CACGACGAAATTTC-MGB-3′ |
| Extraction Method | Median (ng/μL) | IQR (Interquartile Range) | Minimum (ng/μL) | Maximum (ng/μL) | High Quality Sample (%) | Amplification Success Rate (%) |
|---|---|---|---|---|---|---|
| Chelex® 100 | 27.18 | 9.84 | 11.8 | 58.05 | 0.00 | 50.00 |
| DNAzol® Direct reagent | 2.10 | 2.92 | 0.10 | 21.01 | 5.88 | 58.82 |
| PrepMan® USPR | 25.18 | 10.93 | 8.00 | 42.75 | 23.53 | 76.47 |
| QIAsymphony | 20.27 | 7.69 | 17.08 | 27.18 | 0.00 | 64.71 |
| Extraction Method | Time | Cost |
|---|---|---|
| Chelex® 100 | 90 min | 6 ×X |
| DNAzol® Direct reagent | 90 min | 1.6 × |
| PrepMan® USPR | 15 min | × |
| QIAsymphony | 105 min (+ON * incubation) | 12 × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Congiu, I.; Cugini, E.; Smedile, D.; Romiti, F.; Iurescia, M.; Donati, V.; De Liberato, C.; Battisti, A. Evaluation of Protocols for DNA Extraction from Individual Culex pipiens to Assess Pyrethroid Resistance Using Genotyping Real-Time Polymerase Chain Reaction. Methods Protoc. 2024, 7, 106. https://doi.org/10.3390/mps7060106
Congiu I, Cugini E, Smedile D, Romiti F, Iurescia M, Donati V, De Liberato C, Battisti A. Evaluation of Protocols for DNA Extraction from Individual Culex pipiens to Assess Pyrethroid Resistance Using Genotyping Real-Time Polymerase Chain Reaction. Methods and Protocols. 2024; 7(6):106. https://doi.org/10.3390/mps7060106
Chicago/Turabian StyleCongiu, Ilaria, Elisa Cugini, Daniele Smedile, Federico Romiti, Manuela Iurescia, Valentina Donati, Claudio De Liberato, and Antonio Battisti. 2024. "Evaluation of Protocols for DNA Extraction from Individual Culex pipiens to Assess Pyrethroid Resistance Using Genotyping Real-Time Polymerase Chain Reaction" Methods and Protocols 7, no. 6: 106. https://doi.org/10.3390/mps7060106
APA StyleCongiu, I., Cugini, E., Smedile, D., Romiti, F., Iurescia, M., Donati, V., De Liberato, C., & Battisti, A. (2024). Evaluation of Protocols for DNA Extraction from Individual Culex pipiens to Assess Pyrethroid Resistance Using Genotyping Real-Time Polymerase Chain Reaction. Methods and Protocols, 7(6), 106. https://doi.org/10.3390/mps7060106






