Optimized Methods to Quantify Tumor Treating Fields (TTFields)-Induced Permeabilization of Glioblastoma Cell Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
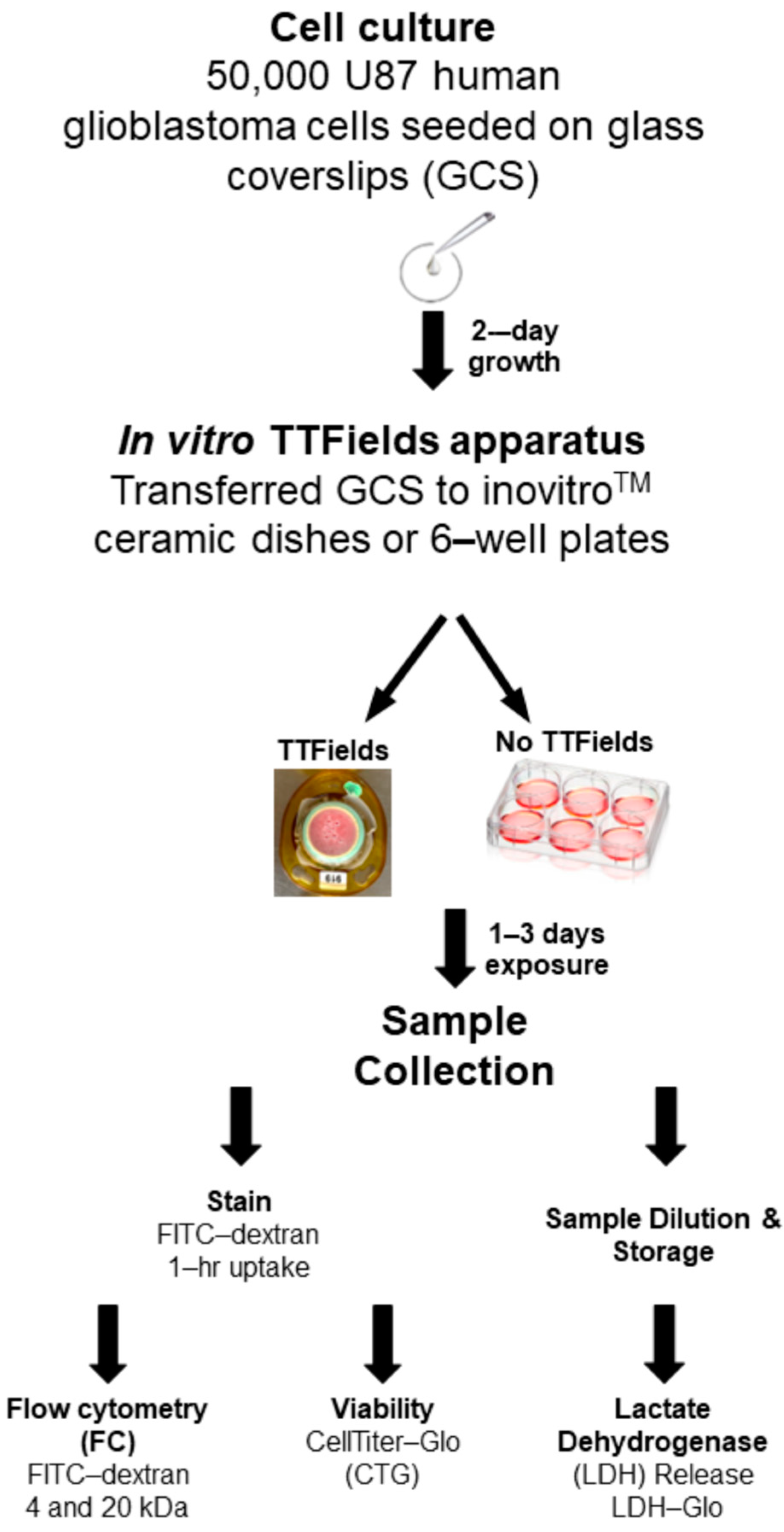
2.2. GCS Preparation for the TTFields Experiment
2.3. In Vitro TTFields Apparatus
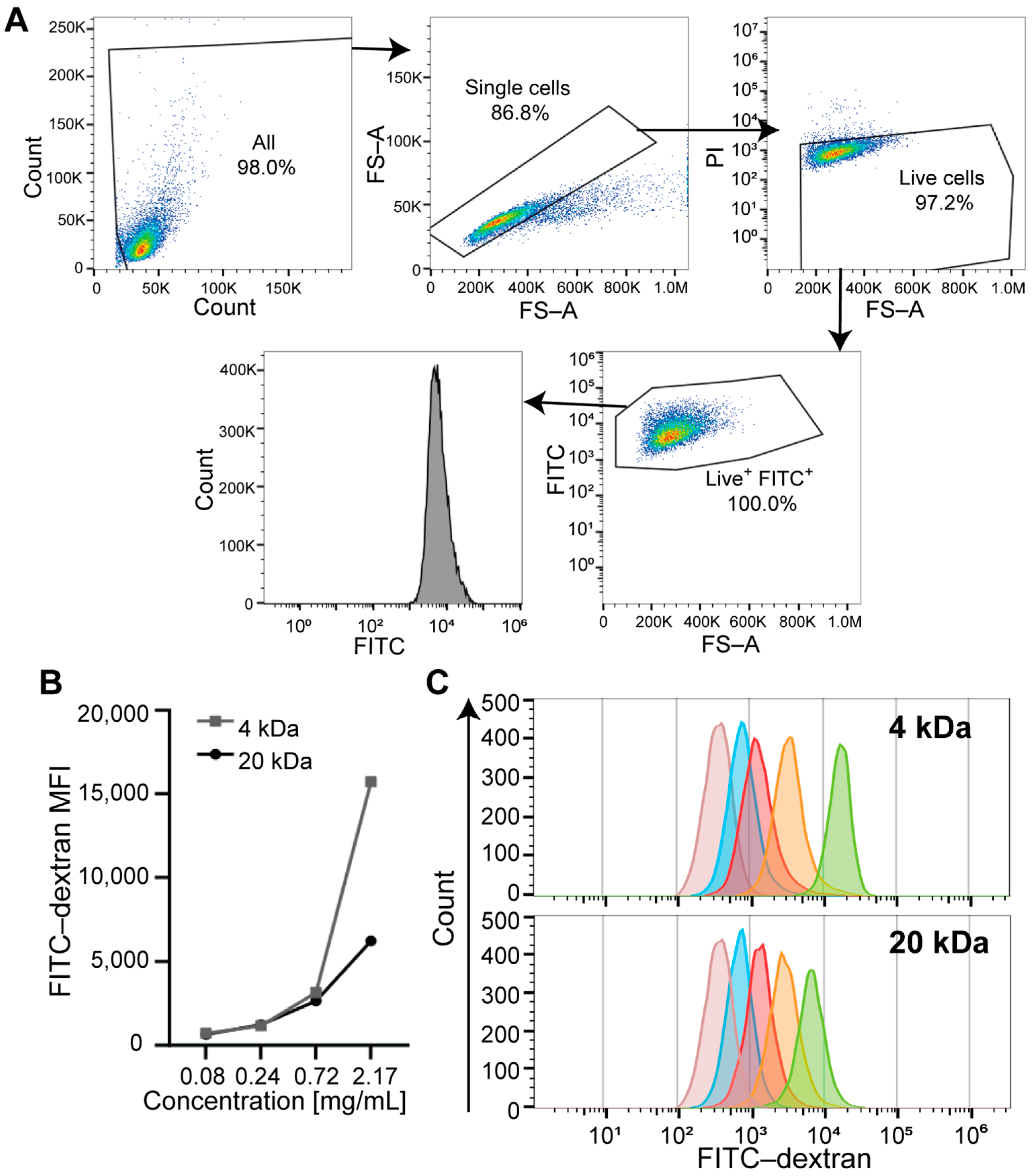
2.4. Flow Cytometry
2.5. Cell Viability Measurement by CellTiter-Glo® Assay
2.6. LDH Release Assay
2.7. Triton-X-100 Concentration Optimization by Light Microscopy
2.8. Data and Statistical Analyses
3. Results
3.1. Identifying the Optimal FITC-Dextran Concentration for the FC-Based Approach (Titration Range)
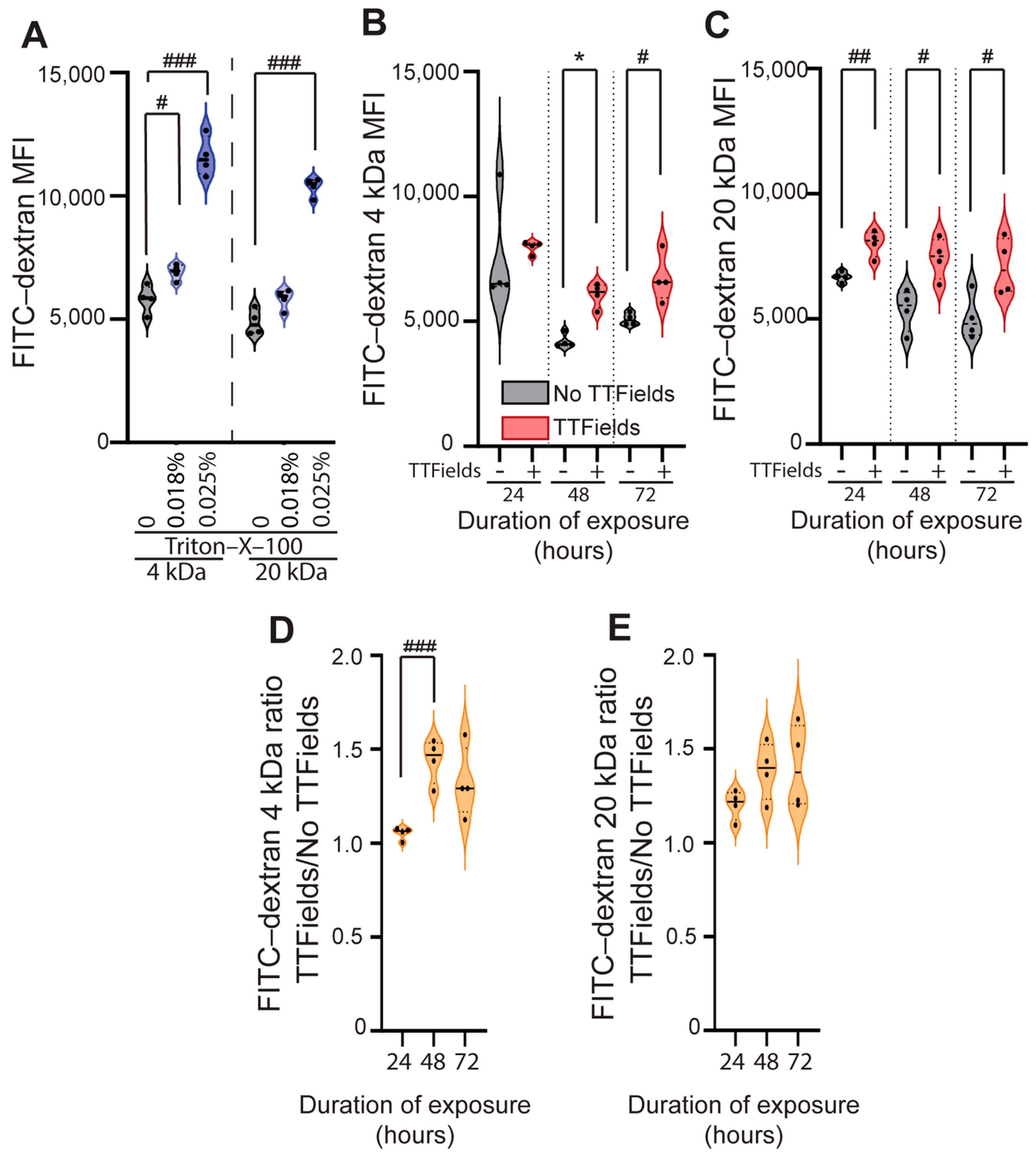
3.2. TTFields Exposure Significantly Increases the Uptake of Different-Sized FITC-Dextran Probes (4 and 20 kDa) in U87 Human GBM Cells
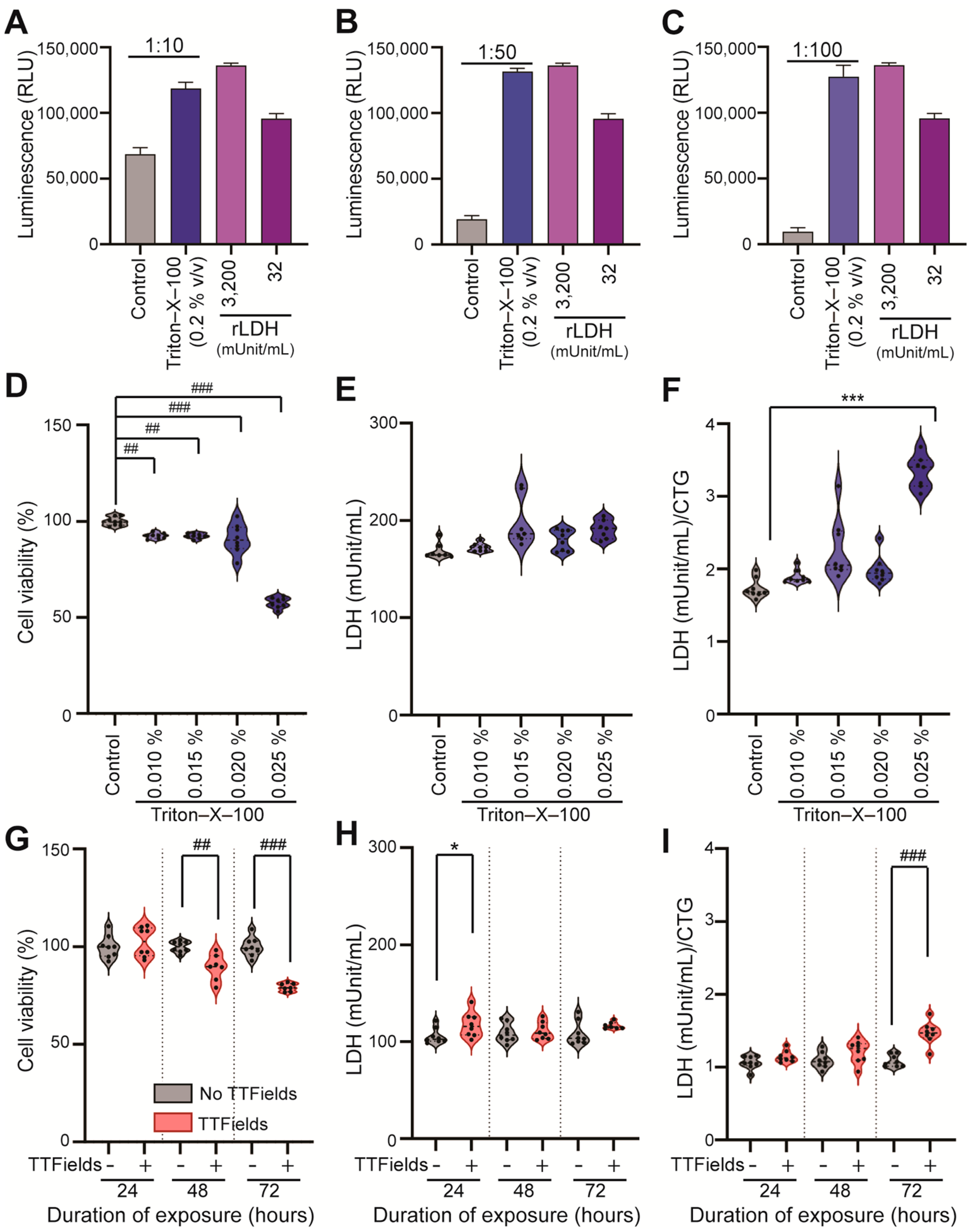
3.3. Optimized LDH Release Assay Detects TTFields-Induced Human GBM Cell Membrane Permeabilization in a Time-Dependent Manner, with High Sensitivity
3.4. Normalized LDH/CTG Ratio Detects GBM Cell Membrane Permeabilization Induced by the Combination of TTFields with Chemotherapy in Surviving Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTG | CellTiter-Glo® |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| FC | flow cytometry |
| FITC | fluorescein isothiocyanate |
| GBM | glioblastoma |
| GCS | glass coverslip |
| LDH | lactate dehydrogenase |
| MFI | mean fluorescence intensity |
| MGMT | O6-methylguanine DNA methyltransferase |
| TTFields | tumor treating fields |
References
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V. Novottf-100a Versus Physician’s Choice Chemotherapy in Recurrent Glioblastoma: A Randomised Phase Iii Trial of a Novel Treatment Modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide Vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide Vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Goldlust, S.A.; Kleinberg, L.R.; Honnorat, J.; Bush, N.A.O.; Ram, Z. Tumor Treating Fields (Ttfields) Therapy Vs Physicians’ Choice Standard-of-Care Treatment in Patients with Recurrent Glioblastoma: A Post-Approval Registry Study (Ef-19). Discov. Oncol. 2022, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Kazda, T.; Novak, T.; Slanina, P.; Sroubek, J.; Klener, J.; Hrbac, T.; Syrucek, M.; Rulseh, A.M. Eighteen Years’ Experience with Tumor Treating Fields in the Treatment of Newly Diagnosed Glioblastoma. Front. Oncol. 2023, 12, 1014455. [Google Scholar] [CrossRef]
- Ballo, M.T.; Conlon, P.; Lavy-Shahaf, G.; Kinzel, A.; Vymazal, J.; Rulseh, A.M. Association of Tumor Treating Fields (Ttfields) Therapy with Survival in Newly Diagnosed Glioblastoma: A Systematic Review and Meta-Analysis. J. Neurooncol. 2023, 164, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crino, L.; et al. Tumour Treating Fields in Combination with Pemetrexed and Cisplatin or Carboplatin as First-Line Treatment for Unresectable Malignant Pleural Mesothelioma (Stellar): A Multicentre, Single-Arm Phase 2 Trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.; Kotecha, R.; Ramlau, R.; Zhang, L.; Milanowski, J.; Cobo, M.; Roubec, J.; Petruzelka, L.; Havel, L.; Kalmadi, S.; et al. Tumor Treating Fields Therapy with Standard Systemic Therapy Versus Standard Systemic Therapy Alone in Metastatic Non-Small-Cell Lung Cancer Following Progression on or after Platinum-Based Therapy (Lunar): A Randomised, Open-Label, Pivotal Phase 3 Study. Lancet Oncol. 2023, 24, 1002–1017. [Google Scholar] [CrossRef]
- Mun, E.J.; Babiker, H.M.; Weinberg, U.; Kirson, E.D.; Von Hoff, D.D. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clin. Cancer Res. 2018, 24, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Anadkat, M.J.; Ballo, M.T.; Iwamoto, F.; Jeyapalan, S.A.; La Rocca, R.V.; Schwartz, M.; Serventi, J.N.; Glas, M. Prevention and Management of Dermatologic Adverse Events Associated with Tumor Treating Fields in Patients with Glioblastoma. Front. Oncol. 2020, 10, 1045. [Google Scholar] [CrossRef]
- Anadkat, M.J.; Lacouture, M.; Friedman, A.; Horne, Z.D.; Jung, J.; Kaffenberger, B.; Kalmadi, S.; Ovington, L.; Kotecha, R.; Abdullah, H.I.; et al. Expert Guidance on Prophylaxis and Treatment of Dermatologic Adverse Events with Tumor Treating Fields (Ttfields) Therapy in the Thoracic Region. Front. Oncol. 2022, 12, 975473. [Google Scholar] [CrossRef] [PubMed]
- Gatson, N.T.; Leese, E.; Ornelas, S.; Tadipatri, R.; Manikowski, J.; Patel, C.; Odia, Y. Innv-38. 3 Days-on-1 Day-Off Fractionated Use Protocol for Improved Compliance and Preventative Skin Safety for Use of Tumor Treating Fields: Practice Improvement Strategy in Neuro-Oncology. Neuro-Oncology 2024, 26 (Suppl. 8), viii177. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Davis, M.E.; Elzinga, G.; Butowski, N.; Tran, D.; Villano, J.L.; DiMeglio, L.; Davies, A.M.; Wong, E.T. Characterization and Management of Dermatologic Adverse Events with the Novottf-100a System, a Novel Anti-Mitotic Electric Field Device for the Treatment of Recurrent Glioblastoma. Semin. Oncol. 2014, 41 (Suppl. 4), S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.C.; Salvador, E.; Deniz, K.; Swanson, K.; Tuszynski, J.; Carlson, K.W.; Karanam, N.K.; Patel, C.B.; Story, M.; Lou, E. The Mechanisms of Action of Tumor Treating Fields. Cancer Res. 2022, 82, 3650–3658. [Google Scholar] [CrossRef]
- Shams, S.; Patel, C.B. Anti-Cancer Mechanisms of Action of Therapeutic Alternating Electric Fields (Tumor Treating Fields [Ttfields]). J. Mol. Cell Biol. 2022, 14, mjac047. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Kessler, A.F.; Domröse, D.; Hörmann, J.; Schaeffer, C.; Giniunaite, A.; Burek, M.; Tempel-Brami, C.; Voloshin, T.; Volodin, A. Tumor Treating Fields (Ttfields) Reversibly Permeabilize the Blood–Brain Barrier in Vitro and in Vivo. Biomolecules 2022, 12, 1348. [Google Scholar] [CrossRef]
- Iv, M.; Naya, L.; Sanan, S.; Van Buskirk, S.L.; Nagpal, S.; Thomas, R.P.; Recht, L.D.; Patel, C.B. Tumor Treating Fields Increases Blood-Brain Barrier Permeability and Relative Cerebral Blood Volume in Patients with Glioblastoma. Neuroradiol. J. 2024, 37, 107–118. [Google Scholar] [CrossRef]
- Chang, E.; Patel, C.B.; Pohling, C.; Young, C.; Song, J.; Flores, T.A.; Zeng, Y.; Joubert, L.M.; Arami, H.; Natarajan, A.; et al. Tumor Treating Fields Increases Membrane Permeability in Glioblastoma Cells. Cell Death Discov. 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.A.; Ho, M.C.; Chang, E.; Carlson, K.W.; Natarajan, A.; Marciano, T.; Bomzon, Z.; Patel, C.B. Permeabilizing Cell Membranes with Electric Fields. Cancers 2021, 13, 2283. [Google Scholar] [CrossRef]
- Robinson, J.P. Flow Cytometry: Past and Future. Biotechniques 2022, 72, 159–169. [Google Scholar] [CrossRef]
- Costigan, A.; Hollville, E.; Martin, S.J. Discriminating between Apoptosis, Necrosis, Necroptosis, and Ferroptosis by Microscopy and Flow Cytometry. Curr. Protoc. 2023, 3, e951. [Google Scholar] [CrossRef]
- Izumi, Y.; Izumi, M.; Benz, A.M.; Zorumski, C.F. Lactate Dehydrogenase Release Is Facilitated by Brief Sonication of Rat Hippocampal Slices and Isolated Retinas Following Acute Neuronal Damage. J. Neurosci. Methods 2001, 108, 49–55. [Google Scholar] [CrossRef]
- Kaja, S.; Payne, A.J.; Singh, T.; Ghuman, J.K.; Sieck, E.G.; Koulen, P. An Optimized Lactate Dehydrogenase Release Assay for Screening of Drug Candidates in Neuroscience. J. Pharmacol. Toxicol. Methods 2015, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Pohling, C.; Beygui, N.; Patel, C.B.; Rosenberg, J.; Ha, D.H.; Gambhir, S.S. Synergistic Inhibition of Glioma Cell Proliferation by Withaferin a and Tumor Treating Fields. J. Neurooncol. 2017, 134, n259–n268. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Koppl, T.; Hormann, J.; Schonharl, S.; Bugaeva, P.; Kessler, A.F.; Burek, M.; Ernestus, R.I.; Lohr, M.; Hagemann, C. Tumor Treating Fields (Ttfields) Induce Cell Junction Alterations in a Human 3d in Vitro Model of the Blood-Brain Barrier. Pharmaceutics 2023, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, O.E. The Fundamental Principles of Reproducibility. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2021, 379, 20200210. [Google Scholar] [CrossRef]
- Diaba-Nuhoho, P.; Amponsah-Offeh, M. Reproducibility and Research Integrity: The Role of Scientists and Institutions. BMC Res. Notes 2021, 14, 451. [Google Scholar] [CrossRef]
- Nitsch, A.; Haralambiev, L.; Einenkel, R.; Muzzio, D.O.; Zygmunt, M.T.; Ekkernkamp, A.; Burchardt, M.; Stope, M.B. Determination of in Vitro Membrane Permeability by Analysis of Intracellular and Extracellular Fluorescein Signals in Renal Cells. In Vivo 2019, 33, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Mendes, R.; Silva, F.; Mendes, T.F.; Zelaya-Lazo, A.; Halwachs, K.; Purkal, J.J.; Isidro, I.A.; Felix, A.; Boghaert, E.R.; et al. Application of Ldh Assay for Therapeutic Efficacy Evaluation of Ex Vivo Tumor Models. Sci. Rep. 2021, 11, 18571. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.C.; Rebersek, M.; Dermol, J.; Rems, L.; Miklavcic, D.; Davalos, R.V. Quantification of Cell Membrane Permeability Induced by Monopolar and High-Frequency Bipolar Bursts of Electrical Pulses. Biochim. Biophys. Acta 2016, 1858, 2689–2698. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.C.; Weaver, J.C.; Davalos, R.V. Characterization of Cell Membrane Permeability in Vitro Part I: Transport Behavior Induced by Single-Pulse Electric Fields. Technol. Cancer Res. Treat. 2018, 17, 1533033818792491. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Okabe, R.; Masayama, R.; Watanabe, N.M.; Umakoshi, H.; Kasahara, K.; Matubayasi, N. A Methodology of Quantifying Membrane Permeability Based on Returning Probability Theory and Molecular Dynamics Simulation. J. Chem. Phys. 2024, 161, 024108. [Google Scholar] [CrossRef] [PubMed]
- Filice, F.P.; Henderson, J.D.; Li, M.S.M.; Ding, Z. Correlating Live Cell Viability with Membrane Permeability Disruption Induced by Trivalent Chromium. ACS Omega 2019, 4, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Fishman, H.; Monin, R.; Dor-On, E.; Kinzel, A.; Haber, A.; Giladi, M.; Weinberg, U.; Palti, Y. Tumor Treating Fields (Ttfields) Increase the Effectiveness of Temozolomide and Lomustine in Glioblastoma Cell Lines. J. Neurooncol. 2023, 163, 83–94. [Google Scholar] [CrossRef]
- Clark, P.A.; Gaal, J.T.; Strebe, J.K.; Pasch, C.A.; Deming, D.A.; Kuo, J.S.; Robins, H.I. The Effects of Tumor Treating Fields and Temozolomide in Mgmt Expressing and Non-Expressing Patient-Derived Glioblastoma Cells. J. Clin. Neurosci. 2017, 36, 120–124. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. Mgmt Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Smith, R.F. Exertional Rhabdomyolysis in Naval Officer Candidates. Arch. Intern. Med. 1968, 121, 313–319. [Google Scholar] [CrossRef]
- Hamolsky, M.W.; Kaplan, N.O. Measurements of Enzymes in the Diagnosis of Acute Myocardial Infarction. Circulation 1961, 23, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Draaisma, K.; Snijders, T.J.; Senders, J.T.; Berendsen, S.; Seute, T.; Schiffelers, R.M.; van Solinge, W.W.; Berg, M.J.T.; Robe, P.A.; et al. Routine Blood Tests Do Not Predict Survival in Patients with Glioblastoma-Multivariable Analysis of 497 Patients. World Neurosurg. 2019, 126, e1081–e1091. [Google Scholar] [CrossRef] [PubMed]
- Tini, P.; Cinelli, E.; Yavorska, M.; Donnini, F.; Marampon, F.; Pastina, P.; Rubino, G.; Chibbaro, S.; Cerase, A.; Mazzei, M.A.; et al. Serum Lactate Dehydrogenase as a Prognostic Marker for Treatment Response in Idh Wild-Type Glioblastoma Patients Undergoing Stupp Protocol. J. Neurooncol. 2025, 171, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, R. In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods Protoc. 2022, 5, 17. [Google Scholar] [CrossRef] [PubMed]


| Method | Advantages | Requirements | Cost | Direct vs. Indirect Measurement |
|---|---|---|---|---|
| FC | Sensitive Requires a lower concentration of probes than plate-based readout methods | FC machine and software for data analysis | Less expensive than the plate-based readout method (5.6-fold less for 4 kDa and 20 kDa FITC-dextran probes) | Indirect measurement of size-based probe uptake into the cells |
| LDH release | Very sensitive Requires a very low volume (10 µL) of sample or media High throughput and suitable for temporal measurements Samples can be frozen for later analysis | Plate reader or luminometer Reagent stored at −80 °C | Relatively costly | Indirect measurement of cell membrane permeability via LDH release |
| Cell viability (CTG) | Very sensitive Fast assay Easy to use Scalable to any plate format | Opaque white culture plates Plate reader or luminometer | Relatively costly, particularly for 6- or 12-well plates with larger well surface areas | Direct ATP-based measurement of the number of viable cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Paniagua, M.; Khan, S.; Henning, N.W.; Konagalla, S.V.; Patel, C.B. Optimized Methods to Quantify Tumor Treating Fields (TTFields)-Induced Permeabilization of Glioblastoma Cell Membranes. Methods Protoc. 2025, 8, 10. https://doi.org/10.3390/mps8010010
Martinez-Paniagua M, Khan S, Henning NW, Konagalla SV, Patel CB. Optimized Methods to Quantify Tumor Treating Fields (TTFields)-Induced Permeabilization of Glioblastoma Cell Membranes. Methods and Protocols. 2025; 8(1):10. https://doi.org/10.3390/mps8010010
Chicago/Turabian StyleMartinez-Paniagua, Melisa, Sabbir Khan, Nikita W. Henning, Sri Vaishnavi Konagalla, and Chirag B. Patel. 2025. "Optimized Methods to Quantify Tumor Treating Fields (TTFields)-Induced Permeabilization of Glioblastoma Cell Membranes" Methods and Protocols 8, no. 1: 10. https://doi.org/10.3390/mps8010010
APA StyleMartinez-Paniagua, M., Khan, S., Henning, N. W., Konagalla, S. V., & Patel, C. B. (2025). Optimized Methods to Quantify Tumor Treating Fields (TTFields)-Induced Permeabilization of Glioblastoma Cell Membranes. Methods and Protocols, 8(1), 10. https://doi.org/10.3390/mps8010010







