Real-Time Polymerase Chain Reaction Systems for Detection and Differentiation of Unclassified Viruses of the Phenuiviridae Family
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus-Containing Ticks
2.2. RNA Isolation, PCR, and Sequencing
2.3. Phylogenetic Analysis
2.4. Primers Selection for Phenuiviruses Differentiating Test Systems
2.5. Test System Validation
3. Results
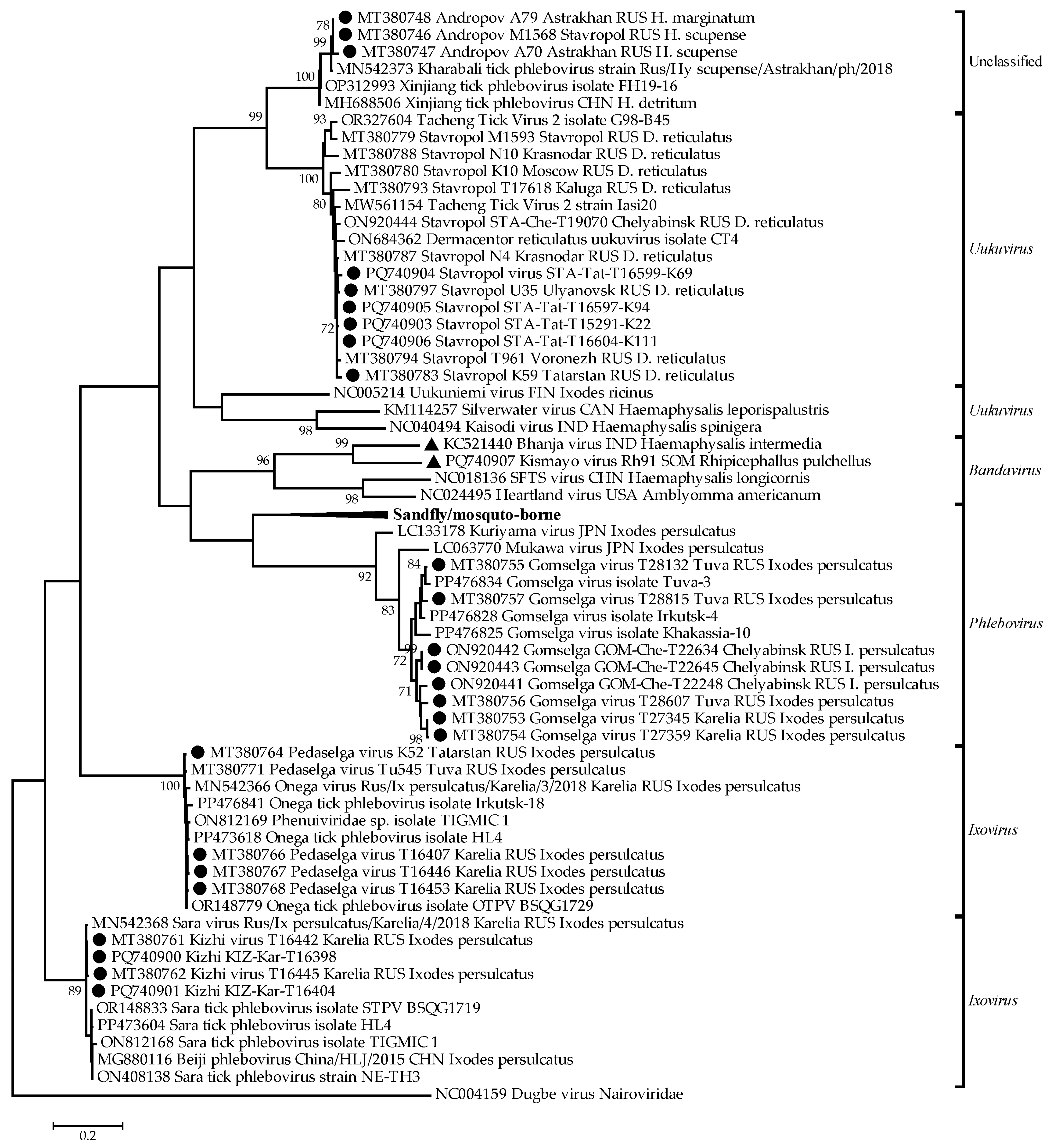
3.1. Phylogenetic Relationships Within the Phenuiviridae Family
3.2. Primer Selection for Phenuiviruses Differentiating Test Systems
- Three for detecting the Stavropol virus;
- Two, for the Pedaselga virus;
- Two, for the Andropov virus;
- Two, for the Kizhi virus;
- Three, for the Gomselga virus.
- St-rt4 for Stavropol;
- And-rt2 for Andropov;
- Pd-rt1 for Pedaselga;
- Kz-rt2 for Kizhi;
- Gom-rt3 for Gomselga.
3.3. Evaluation of Specificity for Uniplex Test Systems
3.4. Sensitivity Evaluation of the Test Systems
- Stavropol virus: 21.3;
- Andropov virus: 23.4;
- Pedaselga virus: 23.3;
- Kizhi virus—unable to detect due to overload;
- Gomselga virus—unable to detect due to overload.
3.5. Evaluation of Duplex Test Systems
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Junglen, S. Evolutionary Origin of Pathogenic Arthropod-Borne Viruses—A Case Study in the Family Bunyaviridae. Curr. Opin. Insect Sci. 2016, 16, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.-D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef]
- Dilcher, M.; Alves, M.J.; Finkeisen, D.; Hufert, F.; Weidmann, M. Genetic Characterization of Bhanja Virus and Palma Virus, Two Tick-Borne Phleboviruses. Virus Genes 2012, 45, 311–315. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimojima, M.; Fukushi, S.; Tani, H.; Fukuma, A.; Taniguchi, S.; Singh, H.; Suda, Y.; Shirabe, K.; Toda, S.; et al. Phylogenetic and Geographic Relationships of Severe Fever with Thrombocytopenia Syndrome Virus in China, South Korea, and Japan. J. Infect. Dis. 2015, 212, 889–898. [Google Scholar] [CrossRef]
- McMullan, L.K.; Folk, S.M.; Kelly, A.J.; MacNeil, A.; Goldsmith, C.S.; Metcalfe, M.G.; Batten, B.C.; Albariño, C.G.; Zaki, S.R.; Rollin, P.E.; et al. A New Phlebovirus Associated with Severe Febrile Illness in Missouri. N. Engl. J. Med. 2012, 367, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, S.S.; Wilson, D.; Feldmann, H.; Hawman, D.W. A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins. Viruses 2021, 13, 314. [Google Scholar] [CrossRef]
- Terasaki, K.; Won, S.; Makino, S. The C-Terminal Region of Rift Valley Fever Virus NSm Protein Targets the Protein to the Mitochondrial Outer Membrane and Exerts Antiapoptotic Function. J. Virol. 2013, 87, 676–682. [Google Scholar] [CrossRef]
- Smith, D.R.; Johnston, S.C.; Piper, A.; Botto, M.; Donnelly, G.; Shamblin, J.; Albariño, C.G.; Hensley, L.E.; Schmaljohn, C.; Nichol, S.T.; et al. Attenuation and Efficacy of Live-Attenuated Rift Valley Fever Virus Vaccine Candidates in Non-Human Primates. PLoS Neglected Trop. Dis. 2018, 12, e0006474. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kuwata, R.; Kimura, T.; Faizah, A.N.; Higa, Y.; Hayashi, T.; Sawabe, K.; Isawa, H. Toyo Virus, a Novel Member of the Kaisodi Group in the Genus Uukuvirus (Family Phenuiviridae) Found in Haemaphysalis formosensis ticks in Japan. Arch. Virol. 2021, 166, 2751–2762. [Google Scholar] [CrossRef]
- Torii, S.; Matsuno, K.; Qiu, Y.; Mori-Kajihara, A.; Kajihara, M.; Nakao, R.; Nao, N.; Okazaki, K.; Sashika, M.; Hiono, T.; et al. Infection of Newly Identified Phleboviruses in Ticks and Wild Animals in Hokkaido, Japan Indicating Tick-Borne Life Cycles. Ticks Tick-Borne Dis. 2019, 10, 328–335. [Google Scholar] [CrossRef]
- Baba, M.; Masiga, D.K.; Sang, R.; Villinger, J. Has Rift Valley Fever Virus Evolved with Increasing Severity in Human Populations in East Africa? Emerg. Microbes. Infect. 2016, 5, e58. [Google Scholar] [CrossRef]
- Marklewitz, M.; Tchouassi, D.P.; Hieke, C.; Heyde, V.; Torto, B.; Sang, R.; Junglen, S. Insights into the Evolutionary Origin of Mediterranean Sandfly Fever Viruses. mSphere 2020, 5, e00598-20. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Kajihara, M.; Nakao, R.; Nao, N.; Mori-Kajihara, A.; Muramatsu, M.; Qiu, Y.; Torii, S.; Igarashi, M.; Kasajima, N.; et al. The Unique Phylogenetic Position of a Novel Tick-Borne Phlebovirus Ensures an Ixodid Origin of the Genus Phlebovirus. mSphere 2018, 3, e00239-18. [Google Scholar] [CrossRef]
- Matsuu, A.; Momoi, Y.; Nishiguchi, A.; Noguchi, K.; Yabuki, M.; Hamakubo, E.; Take, M.; Maeda, K. Natural Severe Fever with Thrombocytopenia Syndrome Virus Infection in Domestic Cats in Japan. Veter. Microbiol. 2019, 236, 108346. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Pastula, D.M.; Panella, A.J.; Rabe, I.B.; Kosoy, O.I.; Walker, W.L.; Velez, J.O.; Lambert, A.J.; Fischer, M. Investigation of Heartland Virus Disease Throughout the United States, 2013–2017. Open Forum Infect. Dis. 2020, 7, ofaa125. [Google Scholar] [CrossRef] [PubMed]
- Klimentov, A.S.; Gmyl, A.P.; Butenko, A.M.; Gmyl, L.V.; Isaeva, O.V.; Larichev, V.F.; Khutoretskaya, N.V.; Karganova, G.G. Taxonomic Status of Bhanja and Kismayo Viruses (Family Bunyaviridae). Epidemiol. Infect. Dis. 2012, 17, 4–8. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I. Tick-Borne Viruses in Europe. Parasitol. Res. 2012, 111, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Tsioka, K.; Saratsis, A.; Pappa, S.; Papa, A. Pathogens Detected in Questing Ixodes ricinus Ticks in a Mountainous Area in Greece. Pathogens 2024, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Shi, M.; Zhang, M.; Liu, Z.; Feng, H.; Sun, Y. Diversity of RNA Viruses of Three Dominant Tick Species in North China. Front. Veter. Sci. 2023, 9, 1057977. [Google Scholar] [CrossRef]
- Crabtree, M.B.; Kent Crockett, R.J.; Bird, B.H.; Nichol, S.T.; Erickson, B.R.; Biggerstaff, B.J.; Horiuchi, K.; Miller, B.R. Infection and Transmission of Rift Valley Fever Viruses Lacking the NSs and/or NSm Genes in Mosquitoes: Potential Role for NSm in Mosquito Infection. PLoS Neglected Trop. Dis. 2012, 6, e1639. [Google Scholar] [CrossRef]
- Welch, S.R.; Scholte, F.E.M.; Spengler, J.R.; Ritter, J.M.; Coleman-McCray, J.D.; Harmon, J.R.; Nichol, S.T.; Zaki, S.R.; Spiropoulou, C.F.; Bergeron, E. The Crimean-Congo Hemorrhagic Fever Virus NSm Protein Is Dispensable for Growth In Vitro and Disease in Ifnar−/− Mice. Microorganisms 2020, 8, 775. [Google Scholar] [CrossRef]
- Merchant, M.; Mata, C.P.; Liu, Y.; Zhai, H.; Protasio, A.V.; Modis, Y. A Bioactive Phlebovirus-Like Envelope Protein in a Hookworm Endogenous Virus. Sci. Adv. 2022, 8, eabj6894. [Google Scholar] [CrossRef]
- Li, C.-X.; Shi, M.; Tian, J.-H.; Lin, X.-D.; Kang, Y.-J.; Chen, L.-J.; Qin, X.-C.; Xu, J.; Holmes, E.C.; Zhang, Y.-Z. Unprecedented Genomic Diversity of RNA Viruses in Arthropods Reveals the Ancestry of Negative-Sense RNA Viruses. eLife 2015, 4, e05378. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the Invertebrate RNA Virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Klimentov, A.S.; Belova, O.A.; Kholodilov, I.S.; Butenko, A.M.; Bespyatova, L.A.; Bugmyrin, S.V.; Chernetsov, N.; Ivannikova, A.Y.; Kovalchuk, I.V.; Nafeev, A.A.; et al. Phlebovirus Sequences Detected in Ticks Collected in Russia: Novel Phleboviruses, Distinguishing Criteria and High Tick Specificity. Infect. Genet. Evol. 2020, 85, 104524. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, H.; Li, Z.; Huang, M.; Si, N.; Zhao, H.; Wei, X.; Sun, B.; Gao, G.F.; Xu, Z.; et al. First Discovery of Phenuiviruses within Diverse RNA Viromes of Asiatic Toad (Bufo gargarizans) by Metagenomics Sequencing. Viruses 2023, 15, 750. [Google Scholar] [CrossRef]
- Sun, M.; Ji, Y.; Li, G.; Shao, J.; Chen, R.; Gong, H.; Chen, S.; Chen, J. Highly Adaptive Phenuiviridae with Biomedical Importance in Multiple Fields. J. Med. Virol. 2022, 94, 2388–2401. [Google Scholar] [CrossRef]
- Gilbert, C.; Feschotte, C. Horizontal Acquisition of Transposable Elements and Viral Sequences: Patterns and Consequences. Curr. Opin. Genet. Dev. 2018, 49, 15–24. [Google Scholar] [CrossRef]
- Kapuscinski, M.L.; Bergren, N.A.; Russell, B.J.; Lee, J.S.; Borland, E.M.; Hartman, D.A.; King, D.C.; Hughes, H.R.; Burkhalter, K.L.; Kading, R.C.; et al. Genomic Characterization of 99 Viruses from the Bunyavirus Families Nairoviridae, Peribunyaviridae, and Phenuiviridae, Including 35 Previously Unsequenced Viruses. PLoS Pathog. 2021, 17, e1009315. [Google Scholar] [CrossRef] [PubMed]
- Kholodilov, I.; Belova, O.; Ivannikova, A.; Gadzhikurbanov, M.; Makenov, M.; Yakovlev, A.; Polienko, A.; Dereventsova, A.; Litov, A.; Gmyl, L.; et al. Distribution and Characterisation of Tick-Borne Flavi-, Flavi-like, and Phenuiviruses in the Chelyabinsk Region of Russia. Viruses 2022, 14, 2699. [Google Scholar] [CrossRef] [PubMed]
- Klimentov, A.S.; Butenko, A.M.; Khutoretskaya, N.V.; Shustova, E.Y.; Larichev, V.F.; Isaeva, O.V.; Karganova, G.G.; Lukashev, A.N.; Gmyl, A.P. Development of Pan-Phlebovirus RT-PCR Assay. J. Virol. Methods 2016, 232, 29–32. [Google Scholar] [CrossRef]
- Watzinger, F.; Ebner, K.; Lion, T. Detection and Monitoring of Virus Infections by Real-Time PCR. Mol. Asp. Med. 2006, 27, 254–298. [Google Scholar] [CrossRef] [PubMed]
- Shishova, A.; Dyugay, I.; Fominykh, K.; Baryshnikova, V.; Dereventsova, A.; Turchenko, Y.; Slavokhotova, A.A.; Ivin, Y.; Dmitriev, S.E.; Gmyl, A. Enteroviruses Manipulate the Unfolded Protein Response through Multifaceted Deregulation of the Ire1-Xbp1 Pathway. Viruses 2022, 14, 2486. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Bratuleanu, B.E.; Temmam, S.; Chrétien, D.; Regnault, B.; Pérot, P.; Bouchier, C.; Bigot, T.; Savuța, G.; Eloit, M. The Virome of Rhipicephalus, Dermacentor and Haemaphysalis Ticks from Eastern Romania Includes Novel Viruses with Potential Relevance for Public Health. Transbounding Emerg. Dis. 2022, 69, 1387–1403. [Google Scholar] [CrossRef]
- Ergunay, K.; Bourke, B.P.; Reinbold-Wasson, D.D.; Nikolich, M.P.; Nelson, S.P.; Caicedo-Quiroga, L.; Vaydayko, N.; Kirkitadze, G.; Chunashvili, T.; Long, L.S.; et al. The Expanding Range of Emerging Tick-Borne Viruses in Eastern Europe and the Black Sea Region. Sci. Rep. 2023, 13, 19824. [Google Scholar] [CrossRef]
- Sameroff, S.; Tokarz, R.; Vucelja, M.; Jain, K.; Oleynik, A.; Boljfetić, M.; Bjedov, L.; Yates, R.A.; Margaletić, J.; Oura, C.A.L.; et al. Virome of Ixodes ricinus, Dermacentor reticulatus, and Haemaphysalis concinna Ticks from Croatia. Viruses 2022, 14, 929. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Sun, H.; Xu, C.; Wen, Y.; Wu, X.; Zhang, W.; Nie, K.; Li, F.; Fu, S.; et al. Metatranscriptomics Reveals the RNA Virome of Ixodes persulcatus in the China–North Korea Border, 2017. Viruses 2023, 16, 62. [Google Scholar] [CrossRef]
- Sahoo, S.; Mandal, S.; Das, P.; Bhattacharya, S.; Chandy, M. An Analysis of the Standard Curve Parameters of Cytomegalovirus, BK Virus and Hepatitis B Virus Quantitative Polymerase Chain Reaction from a Clinical Virology Laboratory in Eastern India. Indian J. Med. Microbiol. 2022, 40, 81–85. [Google Scholar] [CrossRef] [PubMed]
| Isolate | Collection Site and Date | Tick Species | Number of Pools | Number of Ticks | NCBI Number |
|---|---|---|---|---|---|
| Stavropol | Republic of Tatarstan, 2012 | Dermacentor reticulatus | 5 | 12 | MT380783, PQ740903–PQ740906 |
| Ulyanovsk Region, 2014 | Dermacentor reticulatus | 1 | 5 | MT380797 | |
| Andropov | Stavropol Krai, 2011–2012 | Hyalomma scupense | 1 | 22 | MT380746 |
| Astrakhan Region, 2015 | Hyalomma scupense Hyalomma marginatum | 2 | 20 | MT380747, MT380748 | |
| Pedaselga | Republic of Karelia, 2012 | Ixodes persulcatus | 3 | 3 | MT380766–MT380768 |
| Republic of Tatarstan, 2012 | Ixodes persulcatus | 1 | 1 | MT380764 | |
| Kizhi | Republic of Karelia, 2012 | Ixodes persulcatus | 5 | 5 | MT380761, MT380762, PQ740900–PQ740902 |
| Gomselga | Chelyabinsk Region, 2015 | Ixodes persulcatus | 3 | 17 | ON920441– ON920443 |
| Republic of Karelia, 2018 | Ixodes persulcatus | 2 | 10 | MT380753, MT380754 | |
| Republic of Tuva, 2018 | Ixodes persulcatus | 3 | 13 | MT380755–MT380757 |
| Virus | Location | Oligonucleotide | Sequence |
|---|---|---|---|
| Stavropol | L segment | St-rtF1 | 5′-GGCTATGGYGAYCCHCCIYT-3′ |
| St-rtR1 | 5′-TGAGHGTRGAYACTCTATCC-3′ | ||
| St-rtPr1 | (6-FAM)-TCTGCAACYTGKCCCTRATCATGYT-(BHQ-1) | ||
| St-rtF2 | 5′-CAACYTGKCCCTRATCATGY-3′ | ||
| St-rtR2 | 5′-TYTCCACCACCTTCYTCCKR-3′ | ||
| St-rtPr2 | (6-FAM)-RCCYTCWAACACGCAYGTGCAYCA-(BHQ-1) | ||
| St-rtF4 | 5′-TAGAGTRTCYACICTMAAGG-3′ | ||
| St-rtR4 | 5′-CTGYYTCCAGGCATTGCTTY-3′ | ||
| St-rtPr4 | (6-FAM)-RCCYTCWAACACGCAYGTGCAYCA-(BHQ-1) | ||
| Andropov | L segment | And-rtF1 | 5′-GAGTGATGGCTYGAAACAGT-3′ |
| And-rtR1 | 5′-TCGCTTCTCAATGGTCTCCA-3′ | ||
| And-rtPr1 | (6-FAM)-ACACCCATGTGCACCACGTTYTG-(BHQ-1) | ||
| And-rtF2 | 5′-ACTCAACTCCAGCACTCATCA-3′ | ||
| And-rtR2 | 5′-TGGGTTCTCATGTCACCCAAW-3′ | ||
| And-rtPr2 | (ROX)-ACTGGAGGACCARCACCCTGG-(BHQ-2) | ||
| Pedaselga | L segment | Pd-rtF1 | 5′-TCTCTGGGTGGCTACTCTCT-3′ |
| Pd-rtR1 | 5′-GGTRGSACYTCTTRGGATCG-3′ | ||
| Pd-rtPr1 | (6-FAM)-AGCCTTTAGCGTTCCGAGCTGC-(BHQ-1) | ||
| Pd-rtF2 | 5′-CRCTAAAGGCWTCCTCAACC-3′ | ||
| Pd-rtR2 | 5′-TCATGCACATGAGTGTGCTC-3′ | ||
| Pd-rtRt2 | (6-FAM)-ACGATCCYAAGAWGTSCYACCACAGG-(BHQ-1) | ||
| Kizhi | L segment | Kz-rtF1 | 5′-ATCAGCCACGTACGATCAGA-3′ |
| Kz-rtR1 | 5′-AGTCCCTCCAAACACTCCTG-3′ | ||
| Kz-rtPr1 | (ROX)-TCACAGAGCAACCCACATYCATGAGCT-(BHQ-2) | ||
| Kz-rtF2 | 5′-TACTGTGCTGACTCTGCCAy-3′ | ||
| Kz-rtR2 | 5′-GTGGCTGATGCTTTCAGTGT-3′ | ||
| Kz-rtPr2 | (ROX)-TGCCATTGGCAACTACGACCTCG-(BHQ-2) | ||
| Gomselga | L segment | Gm-rtF2 | 5′-CCACCCTHAAGGCCACATCR |
| Gm-rtR2 | 5′-AGYCTCTTTCCTGCYCTYGT | ||
| Gm-rtPr2 | (Cy5)-TGTRCACTCYGARAACAAGGGAMGA-(BHQ-2) | ||
| Gm-rtF3 | 5′-TRCACTCYGARAACAAGGGA-3′ | ||
| Gm-rtR3 | 5′-GCTCTCCTGRAATGCCTCTA-3′ | ||
| Gm-rtPr3 | (Cy5)-AGTTGCmCCrYTGACrAGrGCAGG-(BHQ-2) | ||
| Gm-rtF5 | 5′-RGGTAyCTAGGGAArCAGGA-3′ | ||
| Gm-rtR5 | 5′-CTCAAGrGTGAGGTGGCAGA-3′ | ||
| Gm-rtPr5 | (Cy5)-YCCTsTrAAGCCYACYATGCATGAGT-(BHQ-2) |
| Virus | Sample | Test System St-rt4 * | Test System And-rt2 * | Test System Pd-rt1 * | Test System Kz-rt2 * | Test System Gom-rt3 * |
|---|---|---|---|---|---|---|
| Stavropol | PQ740903 | 22.8 ± 1.3 | - | - | - | - |
| MT380783 | 18.4 ± 0.4 | - | - | - | - | |
| PQ740904 | 23.7 ± 0.9 | - | - | - | - | |
| PQ740905 | 16.2 ± 0.5 | - | - | - | - | |
| PQ740906 | 20.9 ± 0.5 | - | - | - | - | |
| MT380797 | 22.4 ± 0.5 | - | - | - | - | |
| Andropov | MT380746 | - | 24.1 ± 0.8 | - | - | - |
| MT380747 | - | 22.7 ± 0.9 | - | - | - | |
| MT380748 | - | 24.9 ± 0.6 | - | - | - | |
| Pedaselga | MT380766 | - | - | 28.1 ± 0.8 | - | - |
| MT380767 | - | - | 23.4 ± 1.1 | - | - | |
| MT380768 | - | - | 26.1 ± 1.2 | - | - | |
| MT380764 | - | - | 21.8 ± 0.9 | - | - | |
| Kizhi | MT380761 | - | - | - | 4.9 ± 1.2 | - |
| MT380762 | - | - | - | 6.2 ± 0.6 | - | |
| PQ740902 | - | - | - | 12.7 ± 1.2 | - | |
| PQ740900 | - | - | - | 18.8 ± 0.9 | - | |
| PQ740901 | - | - | - | 21.6 ± 1.3 | - | |
| Gomselga | ON920441 | - | - | - | - | 21.8 ± 0.6 |
| ON920442 | - | - | - | - | 16.5 ± 1.8 | |
| ON920443 | - | - | - | - | 17.8 ± 0.7 | |
| MT380753 | - | - | - | - | 28.4 ± 0.4 | |
| MT380754 | - | - | - | - | 6.8 ± 1.1 | |
| MT380755 | - | - | - | - | 20.2 ± 1.3 | |
| MT380756 | - | - | - | - | 22.0 ± 0.8 | |
| MT380757 | - | - | - | - | 19.7 ± 1.7 | |
| Tick-borne encephalitis virus | OQ673267 | - | - | - | - | - |
| Bandavirus bhanjanagarense | KC521440 | - | - | - | - | - |
| Bandavirus kismaayoense | PQ740907 | - | - | - | - | - |
| Uukuvirus uukuniemiense | NC005214 | - | - | - | - | - |
| Unclassified tick suspensions | ||||||
| Dermacentor reticulatus | - | - | - | - | - | |
| Hyalomma scupense | - | - | - | - | - | |
| Hyalomma marginatum | - | - | - | - | - | |
| Ixodes persulcatus | - | - | - | - | - | |
| Dilution of the Control Sample | Ct Stavropol | Ct Andropov | Ct Pedaselga | Ct Kizhi | Ct Gomselga |
|---|---|---|---|---|---|
| no dilution | 21.3 | 23.4 | 23.3 | - | - |
| 10−1 | 29.3 | 30.3 | 27.5 | - | 4.8 |
| 10−2 | 31.9 | 32.5 | 30.6 | 4.2 | 9.1 |
| 10−3 | 35.9 | 35.9 | 33.7 | 11.6 | 12.6 |
| 10−4 | 38.9 | 39.3 | 36.2 | 13.4 | 14.5 |
| 10−5 | N/A | N/A | 37.3 | 17.1 | 17.6 |
| 10−6 | N/A | N/A | N/A | 20.9 | 21.6 |
| 10−7 | - | - | - | 24.2 | 24.3 |
| 10−8 | - | - | - | 27.2 | 29.1 |
| 10−9 | - | - | - | 31.8 | 30.1 |
| 10−10 | - | - | - | 34.8 | N/A |
| 10−11 | - | - | - | N/A | N/A |
| N/C | N/A | N/A | N/A | N/A | N/A |
| Parameters of the Real-Time PCR | Test System St-rt4 | Test System And-rt2 | Test System Pd-rt1 | Test System Kz-rt2 | Test System Gom-rt3 |
|---|---|---|---|---|---|
| R2 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 |
| Slope | −3.54 | −3.48 | −3.13 | −3.57 | −3.19 |
| Efficiency * | 91.64 | 93.80 | 108.61 | 90.43 | 105.58 |
| Sample | Ct *, FAM Channel | Ct *, ROX Channel | Ct *, Uniplex |
|---|---|---|---|
| Pedaselga (MT380766) | 30.1 ± 0.7 | - | 28.1 ± 0.8 |
| Kizhi (PQ740900) | - | 19.7 ± 0.6 | 18.8 ± 0.9 |
| Pedaselga (MT380767) | 25.7 ± 0.8 | - | 23.4 ± 1.1 |
| Kizhi (MT380762) | - | 7.5 ± 0.5 | 6.2 ± 0.6 |
| Pedaselga (MT380768) | 27.5 ± 0.9 | - | 26.1 ± 1.2 |
| Kizhi (PQ740902) | - | 20.5 ± 0.6 | 12.7 ± 1.2 |
| Sample | Ct *, ROX Channel | Ct *, Cy5 Channel | Ct *, Uniplex |
|---|---|---|---|
| Kizhi (PQ740900) | 20.4 ± 0.9 | - | 18.8 ± 0.9 |
| Gomselga (ON920441) | - | 25.9 ± 0.7 | 21.8 ± 0.9 |
| Kizhi (MT380762) | 8.5 ± 0.9 | - | 6.2 ± 0.6 |
| Gomselga (ON920442) | - | 18.9 ± 0.7 | 16.5 ± 1.8 |
| Kizhi (PQ740902) | 14.7 ± 0.8 | - | 12.7 ± 1.2 |
| Gomselga (ON920443) | - | 18.5 ± 0.9 | 17.8 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dereventsova, A.V.; Klimentov, A.S.; Kholodilov, I.S.; Belova, O.A.; Butenko, A.M.; Karganova, G.G. Real-Time Polymerase Chain Reaction Systems for Detection and Differentiation of Unclassified Viruses of the Phenuiviridae Family. Methods Protoc. 2025, 8, 20. https://doi.org/10.3390/mps8010020
Dereventsova AV, Klimentov AS, Kholodilov IS, Belova OA, Butenko AM, Karganova GG. Real-Time Polymerase Chain Reaction Systems for Detection and Differentiation of Unclassified Viruses of the Phenuiviridae Family. Methods and Protocols. 2025; 8(1):20. https://doi.org/10.3390/mps8010020
Chicago/Turabian StyleDereventsova, Alena V., Alexander S. Klimentov, Ivan S. Kholodilov, Oxana A. Belova, Alexander M. Butenko, and Galina G. Karganova. 2025. "Real-Time Polymerase Chain Reaction Systems for Detection and Differentiation of Unclassified Viruses of the Phenuiviridae Family" Methods and Protocols 8, no. 1: 20. https://doi.org/10.3390/mps8010020
APA StyleDereventsova, A. V., Klimentov, A. S., Kholodilov, I. S., Belova, O. A., Butenko, A. M., & Karganova, G. G. (2025). Real-Time Polymerase Chain Reaction Systems for Detection and Differentiation of Unclassified Viruses of the Phenuiviridae Family. Methods and Protocols, 8(1), 20. https://doi.org/10.3390/mps8010020





