Any Sugar with That? Assessment of Dissolved Sucrose as Supplementary Feed in Nursery Rearing of Juvenile Bivalves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spat Source
2.2. Experimental Design
2.3. Growth and Survival
2.4. Biochemical Analysis
2.4.1. Ash-Free Dry Weight
2.4.2. Calorific Content
2.4.3. Protein, Lipid and Carbohydrate Contents
2.5. Statistical Analyses
3. Results
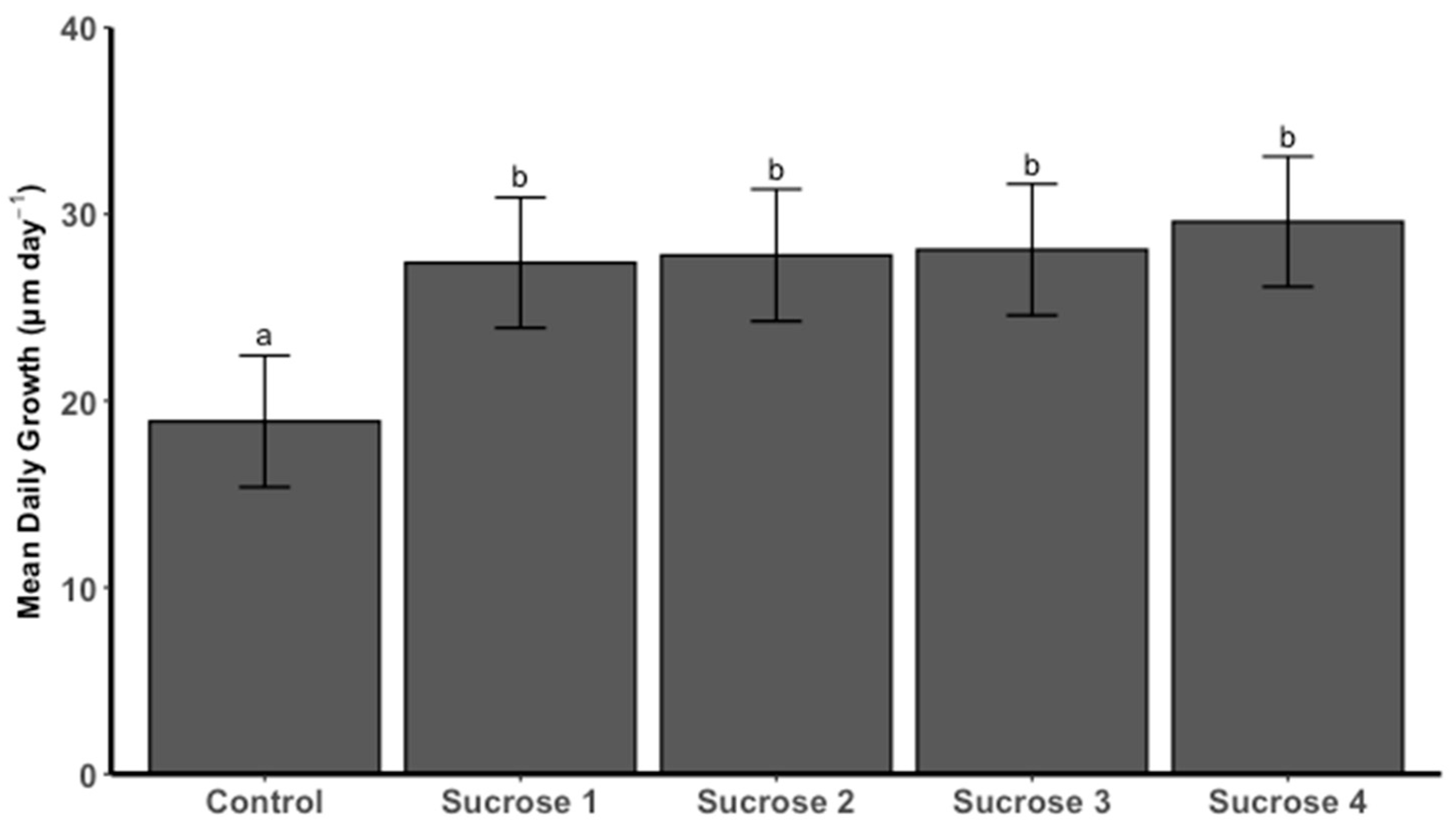
3.1. Spat Growth
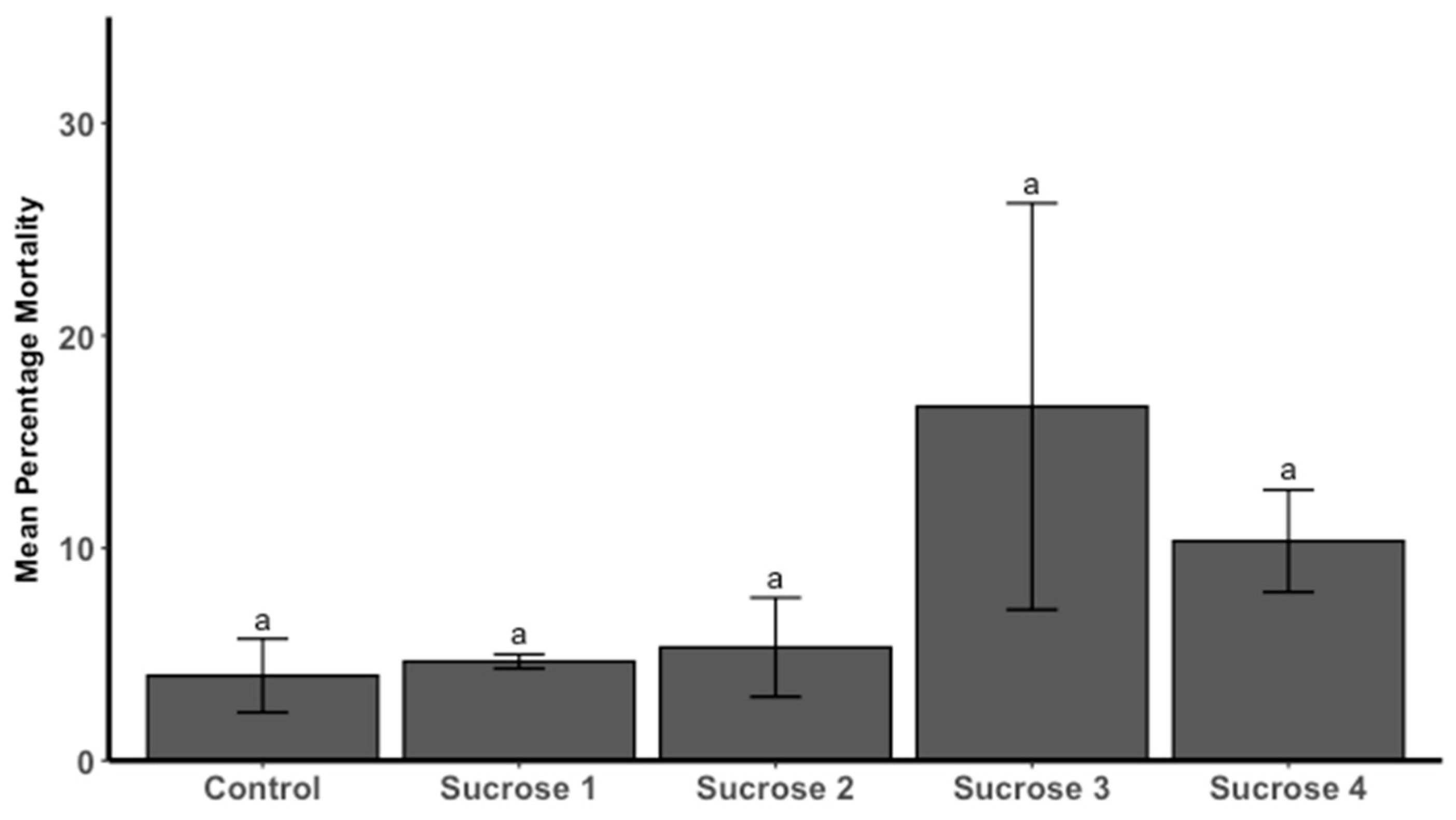
3.2. Spat Mortality
3.3. Biochemical Analysis
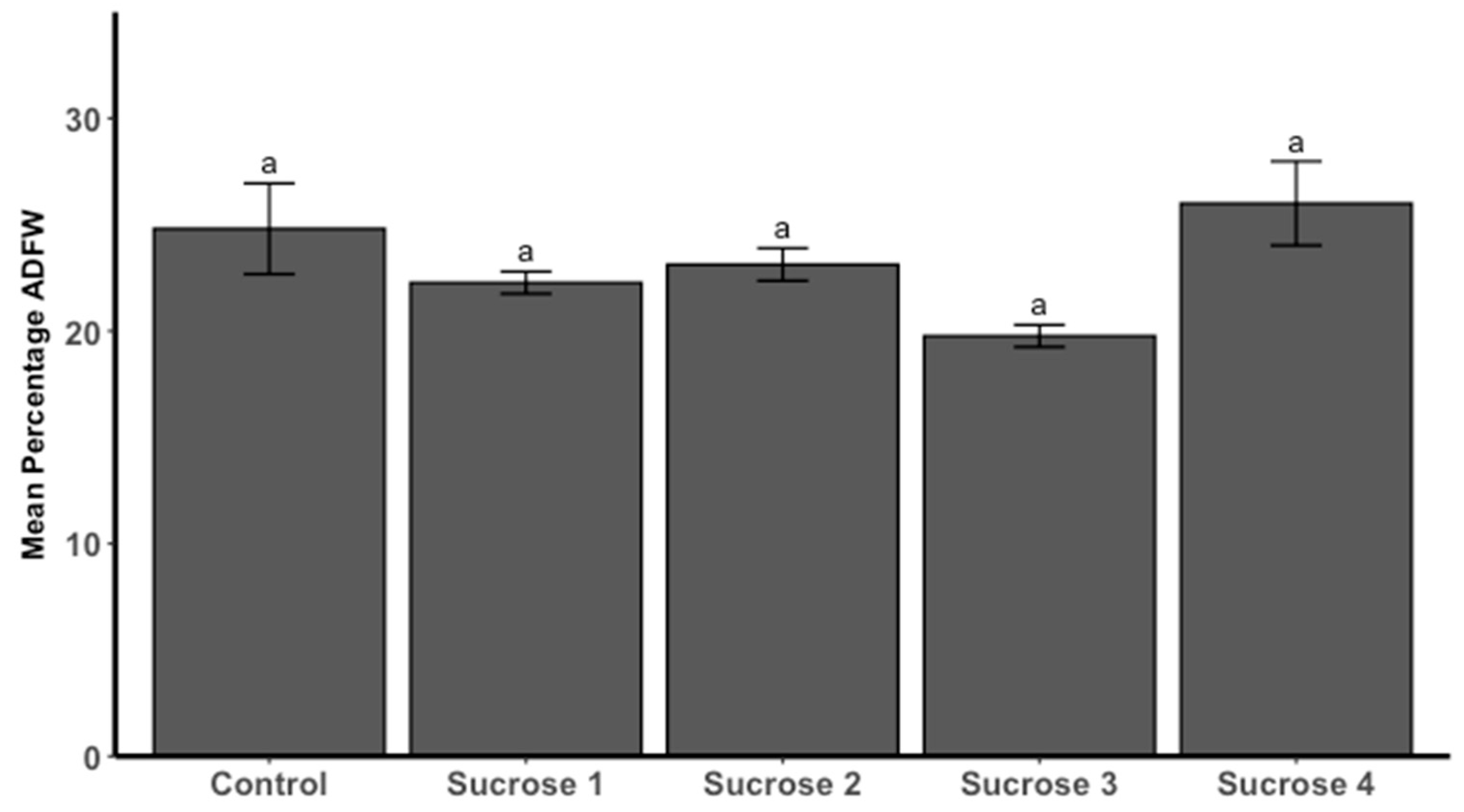
3.3.1. Ash-Free Dry Weight
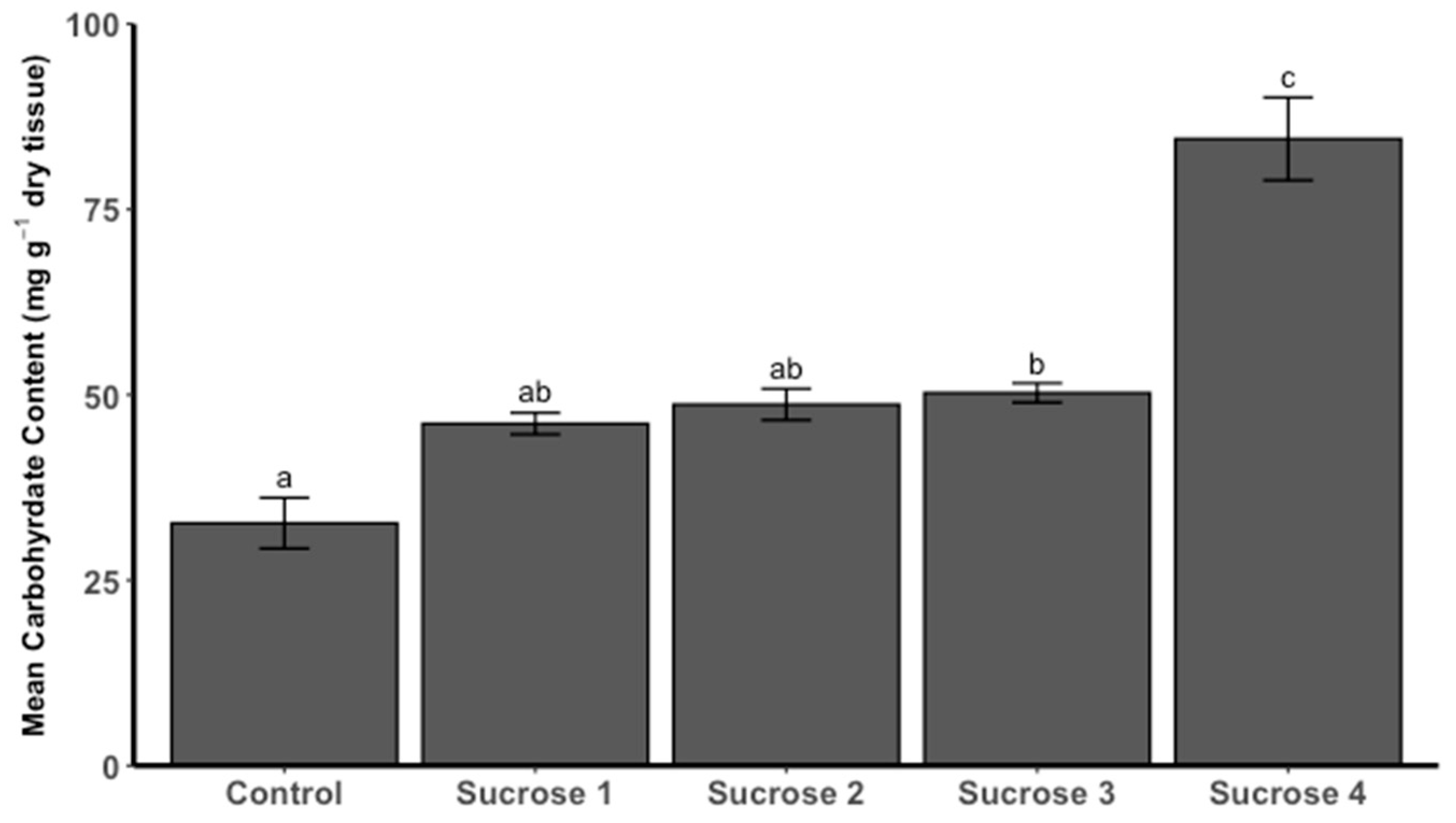
3.3.2. Carbohydrate Content
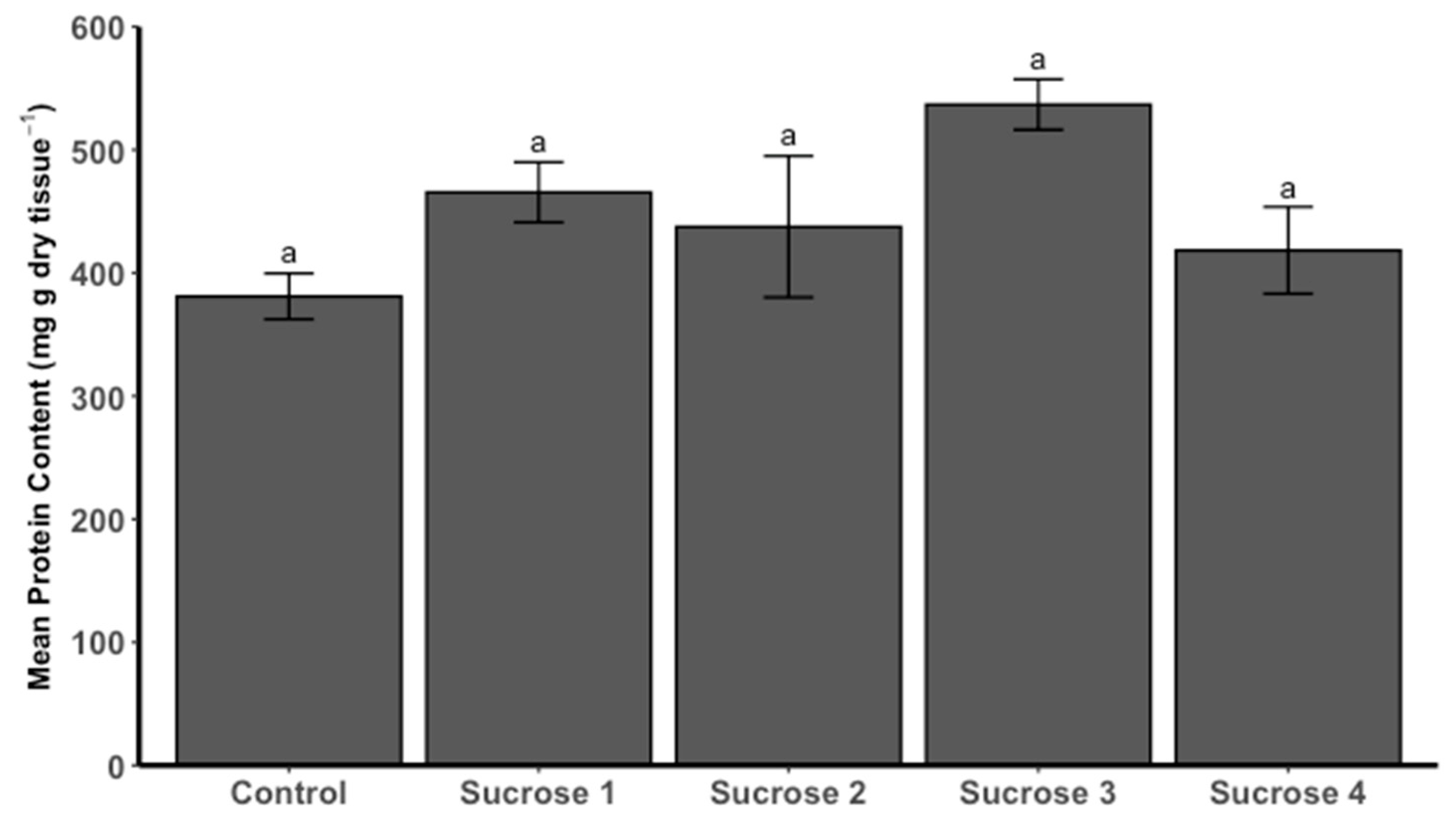
3.3.3. Protein Content
3.3.4. Lipid Content
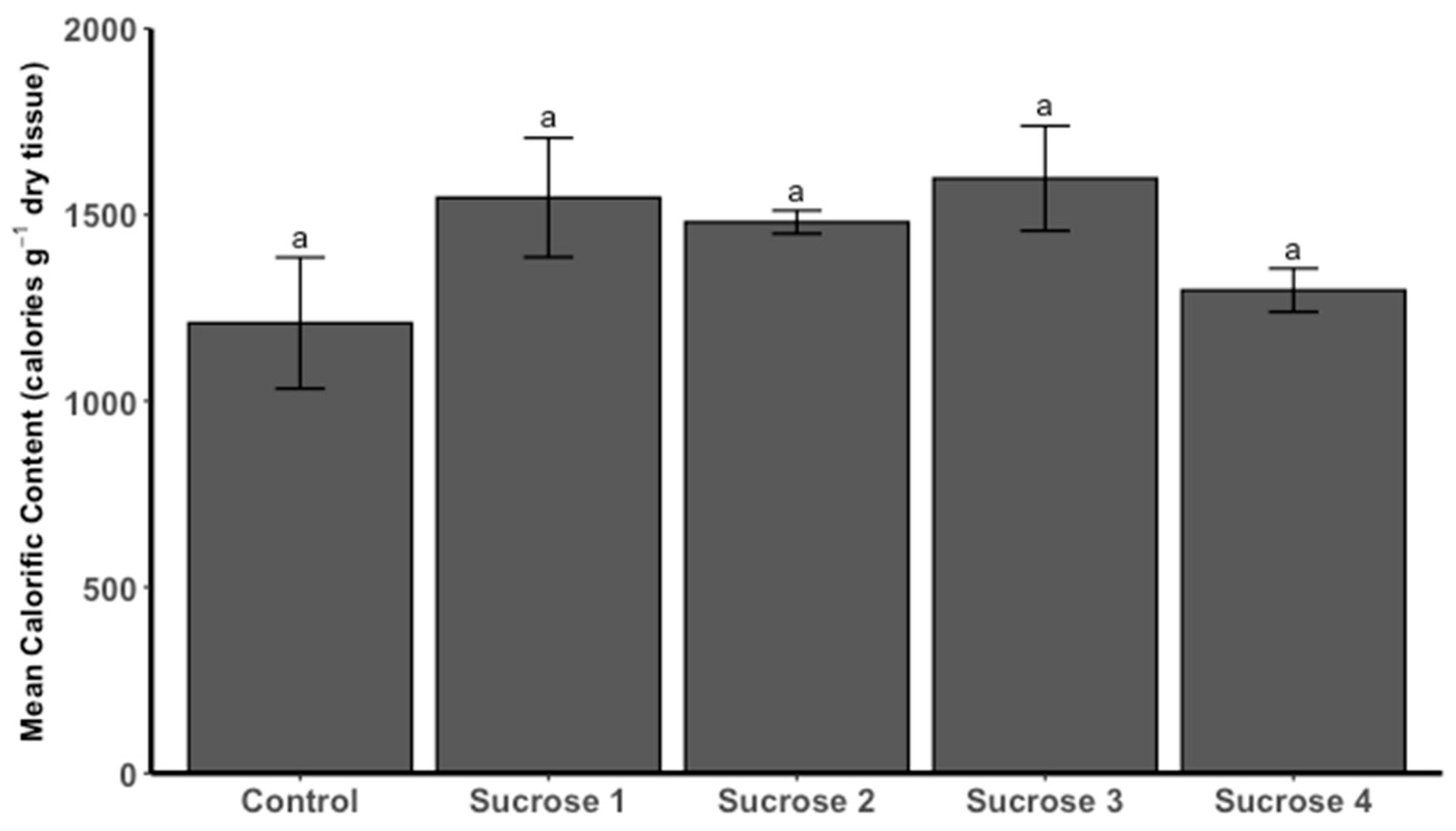
3.3.5. Calorific Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tacon, A.G. Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 2020, 28, 43–56. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2024. [Google Scholar]
- Kamermans, P.; Capelle, J. Provisioning of mussel seed and its efficient use in culture. In Goods and Services of Marine Bivalves; Springer: Cham, Switzerland, 2019; pp. 27–49. [Google Scholar]
- van der Schatte Olivier, A.; Jones, L.; Le Vay, L.; Christie, M.; Wilson, J.; Malham, S.K. A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquac. 2020, 12, 3–25. [Google Scholar] [CrossRef]
- Barrett, L.T.; Theuerkauf, S.J.; Rose, J.M.; Alleway, H.K.; Bricker, S.B.; Parker, M.; Petrolia, D.R.; Jones, R.C. Sustainable growth of non-fed aquaculture can generate valuable ecosystem benefits. Ecosyst. Serv. 2022, 53, 101396. [Google Scholar] [CrossRef]
- Gentry, R.R.; Alleway, H.K.; Bishop, M.J.; Gillies, C.L.; Waters, T.; Jones, R. Exploring the potential for marine aquaculture to contribute to ecosystem services. Rev. Aquac. 2020, 12, 499–512. [Google Scholar] [CrossRef]
- Avdelas, L.; Avdic-Mravlje, E.; Marques, A.C.B.; Cano, S.; Capelle, J.J.; Carvalho, N.; Cozzolino, M.; Dennis, J.; Ellis, T.; Polanco, J.M.F.; et al. The decline of mussel aquaculture in the European Union: Causes, economic impacts and opportunities. Rev. Aquac. 2021, 13, 91–118. [Google Scholar] [CrossRef]
- Skelton, B.M.; South, P.M.; Jeffs, A.G. Inefficiency of conversion of seed into market-ready mussels in New Zealand's Greenshell’ mussel (Perna canaliculus) industry. Aquaculture 2022, 560, 738584. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Hatchery Culture of Bivalves: A Practical Manual; FAO: Rome, Italy, 2004. [Google Scholar]
- Aquaculture New Zealand. Aquaculture for New Zealand, A Sector Overview with Key Facts and Statistics; Aquaculture New Zealand: Nelson, New Zealand, 2022. [Google Scholar]
- Stenton-Dozey, J.M.; Heath, P.; Ren, J.S.; Zamora, L.N. New Zealand aquaculture industry: Research, opportunities and constraints for integrative multitrophic farming. N. Z. J. Mar. Freshw. Res. 2021, 55, 265–285. [Google Scholar] [CrossRef]
- Skelton, B.M.; Jeffs, A.G. The loss of spat following seeding onto coastal Greenshell™ mussel (Perna canaliculus) farms. Aquaculture 2021, 544, 737115. [Google Scholar] [CrossRef]
- Supono, S.; Dunphy, B.; Jeffs, A. Retention of green-lipped mussel spat: The roles of body size and nutritional condition. Aquaculture 2020, 520, 735017. [Google Scholar] [CrossRef]
- Supono, S.; Dunphy, B.; Jeffs, A. Role of nutritional history in the attachment and retention of green-lipped mussel spat. Aquaculture 2021, 533, 736094. [Google Scholar] [CrossRef]
- Buchanan, S.; Babcock, R. Primary and secondary settlement by the greenshell mussel Perna canaliculus. J. Shellfish. Res. 1997, 16, 71–76. [Google Scholar]
- Carton, A.; Jeffs, A.G.; Foote, G.; Palmer, H.; Bilton, J. Evaluation of methods for assessing the retention of seed mussels (Perna canaliculus) prior to seeding for grow-out. Aquaculture 2007, 262, 521–527. [Google Scholar] [CrossRef]
- Supono, S.; Yu, X.; Skelton, B.M.; McKay, W.J.G.; Jeffs, A. Effect of starvation on the nutritional condition of juvenile green-lipped mussels of different sizes. Aquaculture 2022, 560, 738580. [Google Scholar] [CrossRef]
- Alfaro, A.C.; McArdle, B.; Jeffs, A.G. Temporal patterns of arrival of beachcast green-lipped mussel (Perna canaliculus) spat harvested for aquaculture in New Zealand and its relationship with hydrodynamic and meteorological conditions. Aquaculture 2010, 302, 208–218. [Google Scholar] [CrossRef]
- Skelton, B.M.; Múgica, M.; Zamora, L.N.; Delorme, N.J.; Stanley, J.A.; Jeffs, A.G. Nutritional condition of wild and hatchery-reared, green-lipped mussel (Perna canaliculus) spat used for aquaculture. Aquac. Fish Fish. 2024, 4, e145. [Google Scholar] [CrossRef]
- Delorme, N.; Biessy, L.; South, P.M.; Zamora, L.N.; Ragg, N.L.C.; Burritt, D.J. Stress-on-stress responses of a marine mussel, Perna canaliculus: Food limitation reduces the ability to cope with heat stress in juveniles. Mar. Ecol. Prog. Ser. 2020, 644, 105–117. [Google Scholar] [CrossRef]
- Babarro, J.M.; Reiriz, M.J.F.; Labarta, U. Secretion of byssal threads and attachment strength of Mytilus galloprovincialis: The influence of size and food availability. J. Mar. Biol. Assoc. UK 2008, 88, 783–791. [Google Scholar] [CrossRef]
- Coutteau, P.; Sorgeloos, P. The use of artificial diets in the hatchery rearing of bivalve mollusks. Meded.-Fac. Landbouwwet. Rijksuniv. Gent 1993, 57, 2111. [Google Scholar]
- Mckinnon, D.; Rimmer, M.; Kolkovski, S. Hatchery Feeds: Research and Development Plan 2000–2005; Report for the Fisheries Research and Development Corporation: Canberra, Australia, 2004; p. 88. [Google Scholar]
- Willer, D.F.; Aldridge, D.C. Microencapsulated diets to improve growth and survivorship in juvenile European flat oysters (Ostrea edulis). Aquaculture 2019, 505, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Campanati, C.; Arantzamendi, L.; Zorita, I.; Juez, A.; Aldridge, D.C. Microencapsulated diets using thraustochytrids and macroalgae side streams for nursery rearing of Mytilus galloprovincialis spat. J. World Aquac. Soc. 2022, 54, 994–1012. [Google Scholar] [CrossRef]
- Gui, Y.; Zamora, L.; Dunphy, B.J.; Jeffs, A.G. Evaluation of the formulated diet MySpat for feeding hatchery-reared spat of the green-lipped mussel, Perna canaliculus (Gmelin, 1791). Aquac. Res. 2016, 47, 3907–3912. [Google Scholar] [CrossRef]
- Skelton, B.M.; McKay, W.J.G.; Yang, K.; Wu, Z.; Jeffs, A.G. Investigation of the potential of liposome and microparticulate feeds to partially replace microalgae in the nursery rearing of green-lipped mussels (Perna canaliculus). Aquac. Nutr. 2021, 27, 1730–1737. [Google Scholar] [CrossRef]
- Manahan, D.T. The uptake and metabolism of dissolved amino acids by bivalve larvae. Biol. Bull. 1983, 164, 236–250. [Google Scholar] [CrossRef]
- Tantanasarit, C.; Babel, S.; Englande, A.J.; Meksumpun, S. Influence of size and density on filtration rate modeling and nutrient uptake by green mussel (Perna viridis). Mar. Pollut. Bull. 2013, 68, 38–45. [Google Scholar] [CrossRef] [PubMed]
- van Broekhoven, W.; Troost, K.; Jansen, H.; Smaal, A. Nutrient regeneration by mussel Mytilus edulis spat assemblages in a macrotidal system. J. Sea Res. 2014, 88, 36–46. [Google Scholar] [CrossRef]
- Bunde, T.A.; Fried, M. The uptake of dissolved free fatty acids from seawater by a marine filter feeder, Crassostrea virginica. Comp. Biochem. Physiol. Part A Physiol. 1978, 60, 139–144. [Google Scholar] [CrossRef]
- Nell, J.; Skeel, M.; Dunkley, P. Uptake of some dissolved organic nutrients by the Sydney rock oyster Saccostrea commercialis. Mar. Biol. 1983, 74, 313–318. [Google Scholar] [CrossRef]
- Jordan, A.; Skelton, B.; Mugica, M.; Jeffs, A. The uptake of dissolved glucose by juvenile green-lipped mussels (Perna canaliculus). Aquac. Fish Fish. 2024, 4, e70001. [Google Scholar] [CrossRef]
- Avigad, G. Sucrose and other disaccharides. In Plant Carbohydrates I: Intracellular Carbohydrates; Tanner, F.A.L.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 217–347. [Google Scholar]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Arnold, W. The selection of sucrose as the translocate of higher plants. J. Theor. Biol. 1968, 21, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.E.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; O’Rorke, R.; Nodder, S.D.; Jeffs, A.G. Nutritional composition of potential zooplankton prey of the spiny lobster phyllosoma (Jasus edwardsii). Mar. Freshw. Res. 2013, 65, 337–349. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Yuan, C. Lee Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.; Levine, D.; Jones, D. Microparticulate feeds for marine suspension-feeders. J. Microencapsul. 1985, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Boeing, P.; Escondido, C. Use of spray-dried Schizochytrium sas a partial algal replacement for juvenile bivalves. J. Shellfish Res. 1997, 16, 284. [Google Scholar]
- Swift, M.L.; Conger, K.; Exler, J.; Lakshmanan, S. Uptake of glucose and orthophosphate by the American oyster. Life Sci. 1975, 17, 1679–1684. [Google Scholar] [CrossRef]
- Stephens, G.C.; Schinske, R.A. Uptake of amino acids by marine invertebrates 1. Limnol. Oceanogr. 1961, 6, 175–181. [Google Scholar] [CrossRef]
- Séguineau, C.; Soudant, P.; Moal, J.; Delaporte, M.; Miner, P.; Quéré, C.; Samain, J.F. Techniques for delivery of arachidonic acid to Pacific oyster, Crassostrea gigas, spat. Lipids 2005, 40, 931–939. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, K.; Liu, Y.; Huang, H.; Ma, Y.; Chen, D.; Ma, B.; Xu, J. Effects of dietary carbohydrate/lipid ratios on growth, body composition, amylase activity, oxidative status, and mTOR/autophagy pathway in juvenile clam, Sinonovacula constricta. Aquaculture 2024, 578, 740119. [Google Scholar] [CrossRef]
- Enright, C.; Newkirk, G.F.; Craigie, J.S.; Castell, J.D. Growth of juvenile Ostrea edulis L. fed Chaetoceros gracilis Schütt of varied chemical composition. J. Exp. Mar. Biol. Ecol. 1986, 96, 15–26. [Google Scholar] [CrossRef]
- Guo, J.; Fu, Y.; Wu, Z.; Yu, X.; Guo, Y.; Liu, J.; Zhang, W.; Mai, K. Effects of dietary carbohydrate levels on growth performance, body composition, glucose/lipid metabolism and insulin signaling pathway in abalone Haliotis discus hannai. Aquaculture 2022, 557, 738284. [Google Scholar] [CrossRef]
- Sim-Smith, C.J.; Jeffs, A.G. A novel method for determining the nutritional condition of seed green-lipped mussels, Perna canaliculus. J. Shellfish. Res. 2011, 30, 7–11. [Google Scholar] [CrossRef]
- Bennett, R., Jr.; Nakada, H.I. Comparative carbohydrate metabolism of marine molluscs—I. The intermediary metabolism of Mytilus californianus and Haliotus rufescens. Comp. Biochem. Physiol. 1968, 24, 787–797. [Google Scholar] [CrossRef]
- Waldock, M.; Holland, D. Fatty acid metabolism in young oysters, Crassostrea gigas: Polyunsaturated fatty acids. Lipids 1984, 19, 332–336. [Google Scholar] [CrossRef]
- Gallager, S.M.; Mann, R. Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture 1986, 56, 105–121. [Google Scholar] [CrossRef]
- Chu, F.L.E.; Webb, K.L. Polyunsaturated fatty acids and neutral lipids in developing larvae of the oyster, Crassostrea virginica. Lipids 1984, 19, 815. [Google Scholar] [CrossRef]
- Gabbott, P.; Bayne, B. Biochemical effects of temperature and nutritive stress on Mytilus edulis L. J. Mar. Biol. Assoc. UK 1973, 53, 269–286. [Google Scholar] [CrossRef]
- Laing, I. The response of Manila clam, Tapes philippinarum, juveniles to nutritive stress. J. Exp. Mar. Biol. Ecol. 1993, 173, 111–121. [Google Scholar] [CrossRef]
- FAO. Food Energy–Methods of Analysis and Conversion Factors; Food and Agriculture Organization of the United Nations Technical Workshop Report: Rome, Italy, 2003; Volume 77, pp. 8–9. [Google Scholar]
- Pequignat, E. A kinetic and autoradiographic study of the direct assimilation of amino acids and glucose by organs of the mussel Mytilus edulis. Mar. Biol. 1973, 19, 227–244. [Google Scholar] [CrossRef]
- Jørgensen, C.B. Bivalve Filter Feeding: Hydrodynamics, Bioenergetics, Physiology and Ecology; Olsen & Olsen: Fredensborg, Denmark, 1990. [Google Scholar]
- Fankboner, P.; De Burgh, M. Comparative rates of dissolved organic carbon accumulation by juveniles and pediveligers of the Japanese oyster Crassostrea gigas Thunberg. Aquaculture 1978, 13, 205–212. [Google Scholar] [CrossRef]
- Martínez-Quintana, J.A.; Yepiz-Plascencia, G. Glucose and other hexoses transporters in marine invertebrates: A mini review. Electron. J. Biotechnol. 2012, 15, 16. [Google Scholar]
- Wilson-O'Brien, A.L.; Patron, N.; Rogers, S. Evolutionary ancestry and novel functions of the mammalian glucose transporter (GLUT) family. BMC Evol. Biol. 2010, 10, 152. [Google Scholar] [CrossRef]
- Hylleberg Kristensen, J. Carbohydrases of some marine invertebrates with notes on their food and on the natural occurrence of the carbohydrates studied. Mar. Biol. 1972, 14, 130–142. [Google Scholar] [CrossRef]
- Albentosa, M.; Beiras, R.; Camacho, A.P. Determination of optimal thermal conditions for growth of clam (Venerupis pullastra) seed. Aquaculture 1994, 126, 315–328. [Google Scholar] [CrossRef]
- Almada-Villela, P.C.; Davenport, J.; Gruffydd, L.D. The effects of temperature on the shell growth of young Mytilus edulis L. J. Exp. Mar. Biol. Ecol. 1982, 59, 275–288. [Google Scholar] [CrossRef]
- Buchanan, S.J. Spat Production of the Greenshell™ Mussel Perna Canaliculus in New Zealand. Ph.D. Thesis, The University of Auckland, Auckland, New Zealand, 1999. [Google Scholar]
- Supono, S.; Mugica, M.; Spreitzenbarth, S.; Jeffs, A. Potential for Concentrated Microalgae as Replacement Diets for Juvenile Green-Lipped Mussels, Perna canaliculus. Aquac. Res. 2023, 2023, 1–9. [Google Scholar] [CrossRef]
- Pacheco-Vega, J.M.; Sánchez-Saavedra, M.D.P. The biochemical composition of Chaetoceros muelleri (Lemmermann Grown) with an agricultural fertilizer. J. World Aquac. Soc. 2009, 40, 556–560. [Google Scholar] [CrossRef]
- Bartlett, B.R. Biochemical Changes in the Pacific Oyster, Crassostrea Gigas (Thunberg, 1795), During Larval Development and Metamorphosis. Ph.D. Thesis, ScholarsArchive at Oregon State University, Corvallis, OR, USA, 1978. [Google Scholar]
- Whyte, J.; Bourne, N.; Ginther, N.G.; Hodgson, C.A. Compositional changes in the larva to juvenile development of the scallop Crassadoma gigantea (Gray). J. Exp. Mar. Biol. Ecol. 1992, 163, 13–29. [Google Scholar] [CrossRef]
- Aquaculture New Zealand. New Zealand Greenshell Mussel Spat Strategy; Report Aquaculture New Zealand: Nelson, New Zealand, 2020; p. 23. [Google Scholar]
- De Zwaan, A.; Wijsman, T. Anaerobic metabolism in bivalvia (Mollusca) characteristics of anaerobic metabolism. Comp. Biochem. Physiol. Part B Comp. Biochem. 1976, 54, 313–323. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordan, A.; Skelton, B.; Mugica, M.; Jeffs, A. Any Sugar with That? Assessment of Dissolved Sucrose as Supplementary Feed in Nursery Rearing of Juvenile Bivalves. Fishes 2025, 10, 27. https://doi.org/10.3390/fishes10010027
Jordan A, Skelton B, Mugica M, Jeffs A. Any Sugar with That? Assessment of Dissolved Sucrose as Supplementary Feed in Nursery Rearing of Juvenile Bivalves. Fishes. 2025; 10(1):27. https://doi.org/10.3390/fishes10010027
Chicago/Turabian StyleJordan, Andy, Bradley Skelton, Maria Mugica, and Andrew Jeffs. 2025. "Any Sugar with That? Assessment of Dissolved Sucrose as Supplementary Feed in Nursery Rearing of Juvenile Bivalves" Fishes 10, no. 1: 27. https://doi.org/10.3390/fishes10010027
APA StyleJordan, A., Skelton, B., Mugica, M., & Jeffs, A. (2025). Any Sugar with That? Assessment of Dissolved Sucrose as Supplementary Feed in Nursery Rearing of Juvenile Bivalves. Fishes, 10(1), 27. https://doi.org/10.3390/fishes10010027






