Effects of Maternal Stress on the Development of the Somatotropic Axis During the Larval and Juvenile Stages in Zebrafish (Danio rerio)
Abstract
1. Introduction
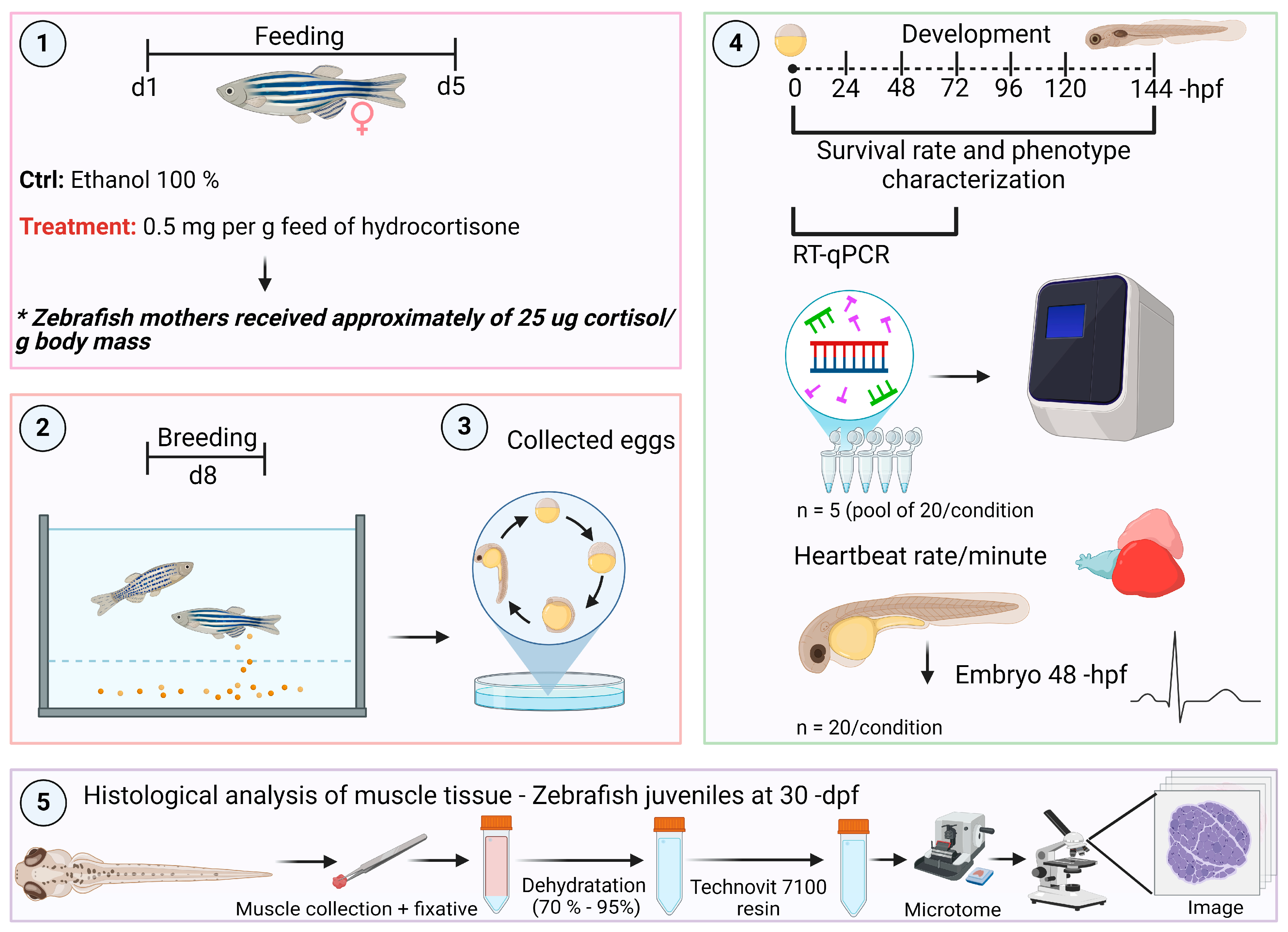
2. Materials and Methods
2.1. Animal Husbandry
2.2. Maternal Stress-Associated Cortisol
2.3. Breeding
2.4. Transcript Levels of the Somatotropic Axis by Quantitative Real-Time PCR (qPCR)
2.5. Embryo and Larvae Phenotypes
2.6. Heart Rate Measurement in Embryos at 48 Hpf
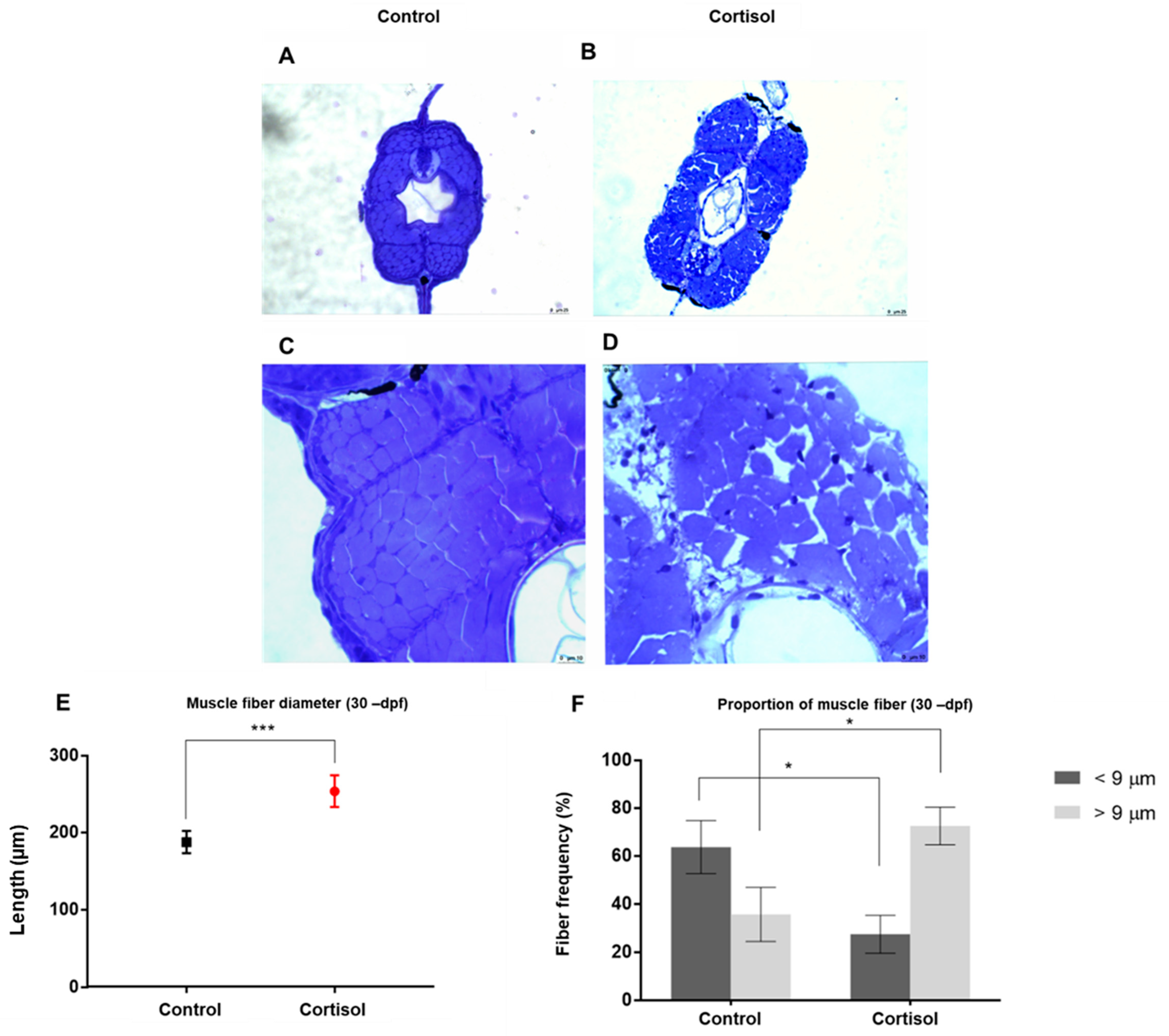
2.7. Histological Analysis of Muscle Tissue
2.8. Statistical Analysis
3. Results
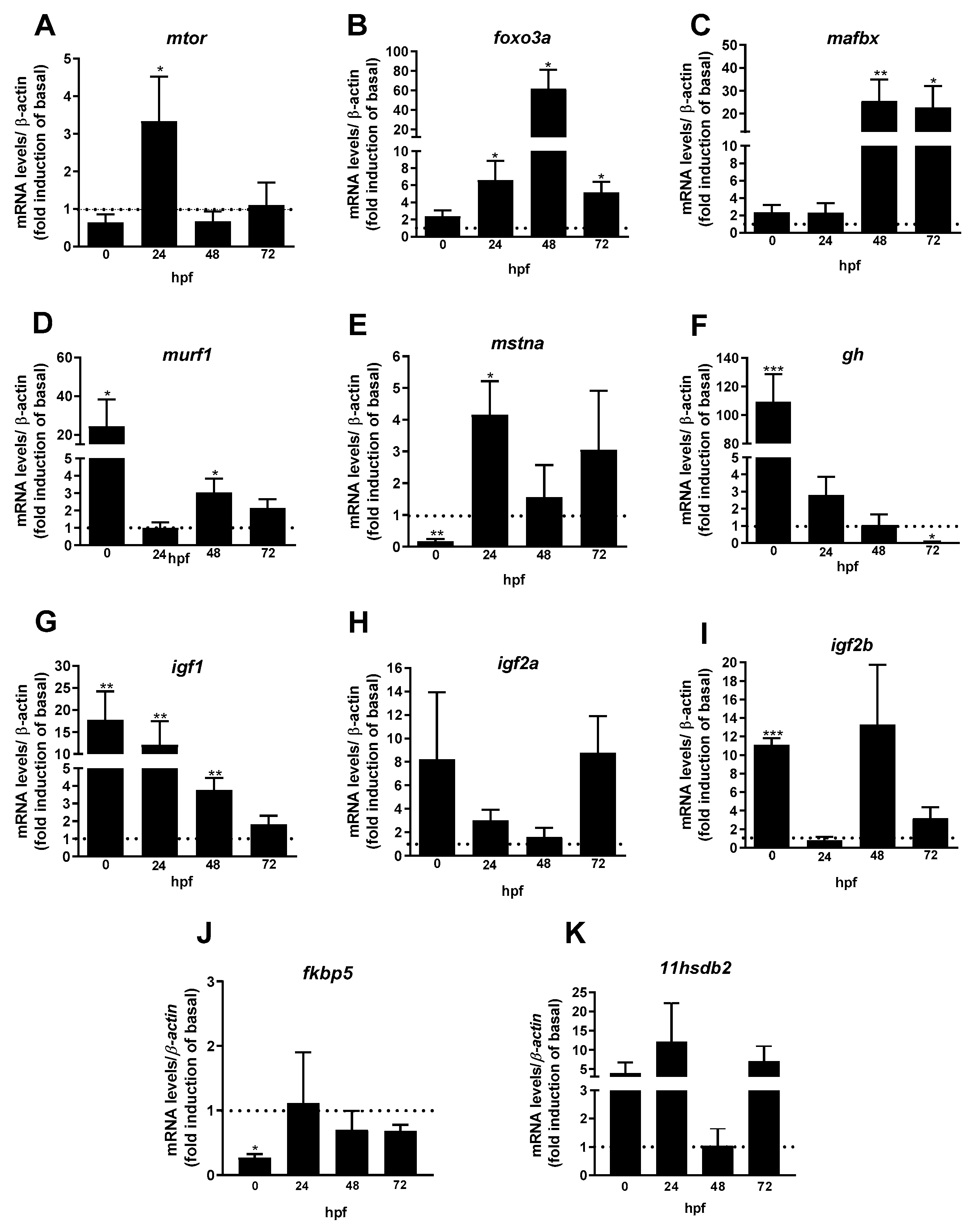
3.1. Maternal Cortisol Influences the mRNA Expression Levels of Genes Associated with the Somatotropic and HPI Axes in Zebrafish Offspring
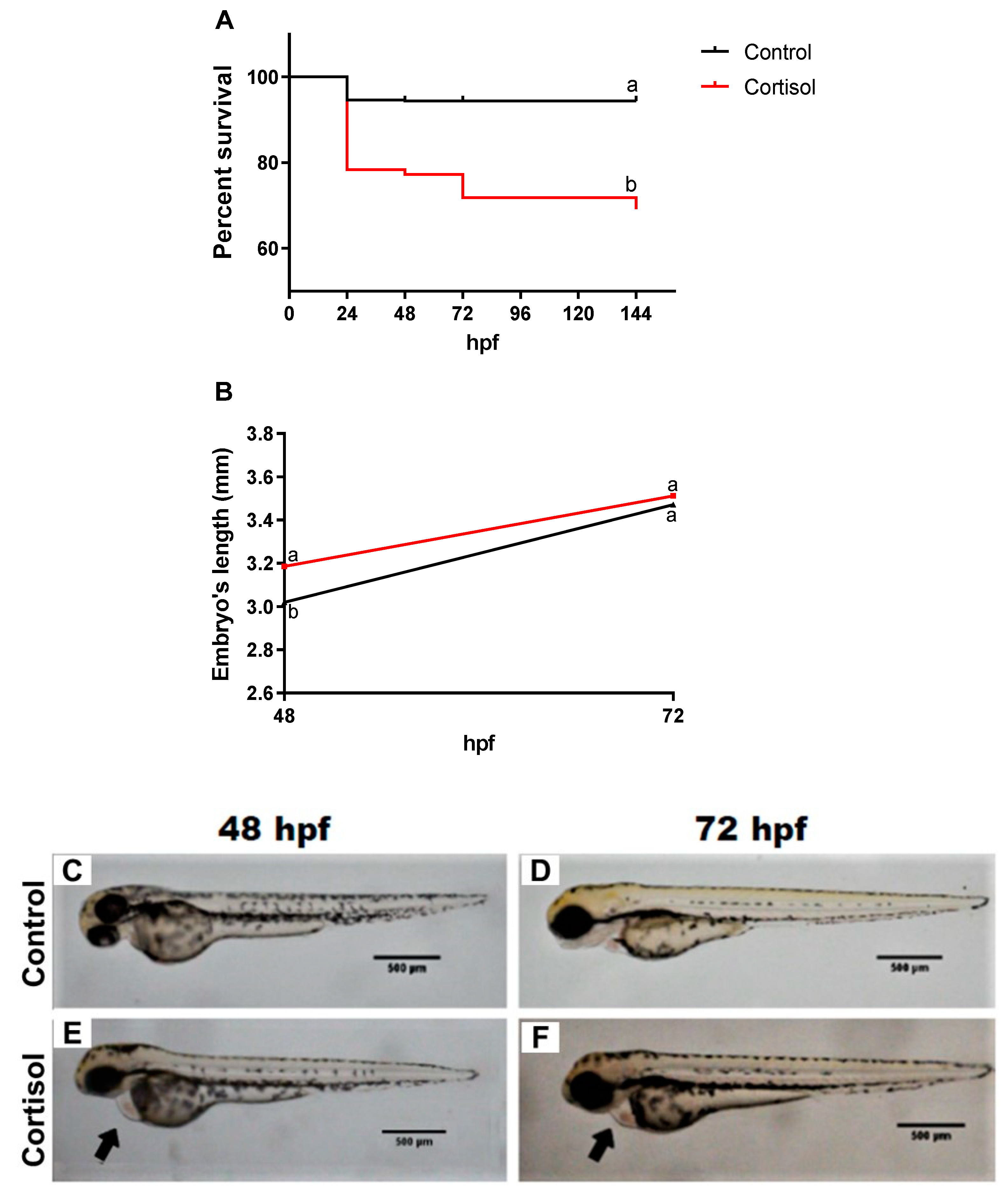
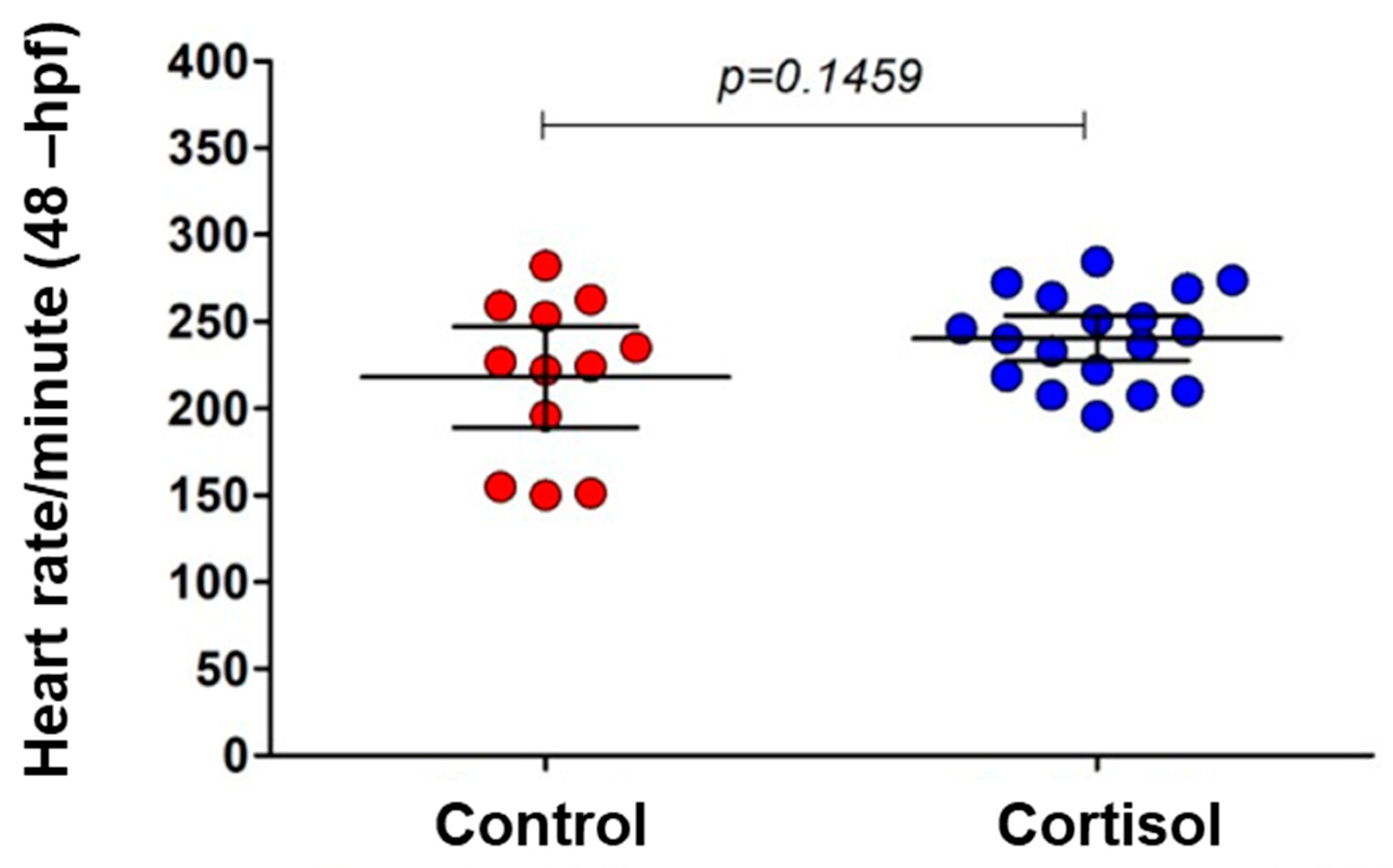
3.2. Maternal Cortisol Treatment Affected Different Stages of Offspring Development in Different Parameters of Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental programming of fetal growth and development. Vet. Clin. Food. Anim. 2019, 35, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Klibaner-Schiff, E.; Simonin, E.M.; Akdis, C.A.; Cheong, A.; Johnson, M.R.K.; Kirsh, S.; Kline, O.; Mazumdar, M.; Oken, E.; Sampath, V.; et al. Environmental exposures influence multigenerational epigenetic transmission. Clin. Epigenet. 2024, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef] [PubMed]
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar]
- Levine, S. Infantile experience and resistance to physiological stress. Science 1957, 126, 405. [Google Scholar] [CrossRef]
- Joseph, D.; Whirledge, S. Stress and the HPA axis: Balancing homeostasis and fertility. Int. J. Mol. Sci. 2017, 18, 2224. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A. Prenatal social stress in the rat programs neuroendocrine and behavioral responses to stress in the adult offspring: Sex-specific effects. J. Neuroendocrinol. 2010, 22, 258–271. [Google Scholar] [CrossRef]
- Dreiling, M.; Schiffner, R.; Bischoff, S.; Rupprecht, S.; Kroegel, N.; Schubert, H.; Rakers, F. Impact of chronic maternal stress during early gestation on maternal-fetal stress transfer and fetal stress sensitivity in sheep. Stress 2017, 21, 1–10. [Google Scholar] [CrossRef]
- D’Agostino, S.; Testa, M.; Aliperti, V.; Venditti, M.; Minucci, S.; Aniello, F.; Donizetti, A. Expression pattern dysregulation of stress- and neuronal activity-related genes in response to prenatal stress paradigm in zebrafish larvae. Cell Stress Chaperones 2019, 24, 1005–1012. [Google Scholar] [CrossRef]
- Entringer, S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 320–327. [Google Scholar] [CrossRef]
- Coulon, M.; Wellman, C.L.; Marjara, I.S.; Janczak, A.M.; Zanella, A.J. Early adverse experience alters dendritic spine density and gene expression in prefrontal cortex and hippocampus in lambs. Psychoneuroendocrinology 2013, 38, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Babenko, O.; Kovalchuk, I.; Metz, G.A.S. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015, 48, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.J.; Cumberland, A.L.; Shaw, J.C.; Bennett, G.A.; Kelleher, M.A.; Walker, D.W.; Palliser, H.K. Loss of neurosteroid-mediated protection following stress during fetal life. J. Steroid Biochem. Mol. Biol. 2016, 160, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Veru, F.; Laplante, D.P.; Luheshi, G.; King, S. Prenatal maternal stress exposure and immune function in the offspring. Stress 2014, 17, 133–148. [Google Scholar] [CrossRef]
- Marques, A.H.; Bjørke-Monsen, A.-L.; Teixeira, A.L.; Silverman, M.N. Maternal stress, nutrition and physical activity: Impact on immune function, CNS development, and psychopathology. Brain Res. 2015, 1617, 28–46. [Google Scholar] [CrossRef]
- Nesan, D.; Vijayan, M.M. Embryo exposure to elevated cortisol level leads to cardiac performance dysfunction in zebrafish. Mol. Cell. Endocrinol. 2012, 363, 85–91. [Google Scholar] [CrossRef]
- Haussmann, M.F.; Longenecker, A.S.; Marchetto, N.M.; Juliano, S.A.; Bowden, R.M. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. Biol. Sci. 2011, 279, 1447–1456. [Google Scholar] [CrossRef]
- De Fraipont, M.; Clobert, J.; John-Alder, H.; Meylan, S. Increased pre-natal maternal corticosterone promotes philopatry of offspring in common lizards Lacerta Vivipara. J. Anim. Ecol. 2000, 69, 404–4013. [Google Scholar] [CrossRef]
- Tissier, M.L.; Williams, T.D.; Criscuolo, F. Maternal effects underlie ageing costs of growth in the zebra finch (Taeniopygia guttata). PLoS ONE 2014, 9, e97705. [Google Scholar] [CrossRef][Green Version]
- Egbuniwe, I.C.; Akogwu, M.S.; Obetta, T.U. Mechanisms underlying reproductive responses of Japanese quails to heat stress conditions. Int. J. Biometeorol. 2024, 68, 2173–2184. [Google Scholar] [CrossRef]
- Faught, E.; Best, C.; Vijayan, M.M. Maternal stress-associated cortisol stimulation may protect embryos from cortisol excess in zebrafish. R. Soc. Open Sci. 2016, 24, 160032. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Vijayan, M.M. The mineralocorticoid receptor is essential for stress axis regulation in zebrafish larvae. Sci. Rep. 2018, 8, 18081. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.T.O.; Mariano, W.S.; Santos, L.R.B. Estresse em peixes cultivados: Agravantes e atenuantes para o manejo rentável. In Manejo e Sanidade de Peixes em Cultivo, 1st ed.; Tavares-Dias, M., Ed.; Embrapa Amapá: Macapá, Brasil, 2009; pp. 226–247. [Google Scholar]
- Leatherland, J.F.; Li, M.; Barkataki, S. Stressors, glucocorticoids and ovarian function in teleosts. J. Fish. Biol. 2010, 76, 86–111. [Google Scholar] [CrossRef] [PubMed]
- Nesan, D.; Vijayan, M.M. Role of glucocorticoid in developmental programming: Evidence from zebrafish. Gen. Comp. Endocrinol. 2013, 181, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef]
- Nesan, D.; Vijayan, M.M. Maternal cortisol mediates hypothalamus-pituitary-interrenal axis development in zebrafish. Sci. Rep. 2016, 6, 22582. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotome muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Gurevich, D.B.; Sonntag, C.; Hersey, L.; Alaei, S.; Nim, H.T.; Siegel, A.; Hall, T.E.; Rossello, F.J.; Boyd, S.E. Muscle stem cells undergo extensive clonal drift during tissue growth via meox1-mediated induction of G2 cell-cycle arrest. Cell Stem Cell 2017, 21, 107–119. [Google Scholar] [CrossRef]
- Keenan, S.; Currie, P. The developmental phases of zebrafish myogenesis. J. Dev. Biol. 2019, 7, 12. [Google Scholar] [CrossRef]
- Hinits, Y.; Osborn, D.P.S.; Hughes, S.M. Differential requirements for myogenic regulatory factors distinguish medial and lateral somitic, cranial and fin muscle fibre populations. Development 2009, 136, 403–414. [Google Scholar] [CrossRef]
- Pikulkaew, S.; Benato, F.; Celeghin, A.; Zucal, C.; Skobo, T.; Colombo, L.; Valle, L.D. The knockdown of maternal glucocorticoid receptor mRNA alters embryo development in zebrafish. Dev. Dyn. 2011, 240, 874–889. [Google Scholar] [CrossRef] [PubMed]
- Alderman, S.L.; Mcguire, A.; Bernier, N.J.; Vijayan, M.M. Central and peripheral glucocorticoid receptors are involved in the plasma cortisol response to an acute stressor in rainbow trout. Gen. Comp. Endocrinol. 2012, 176, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.; Zahid, H.; Ansari, R.; Lee, R.C.; Partap, A.; Gerlai, R. Breeding zebrafish: A review of different methods and a discussion on standardization. Zebrafish 2017, 14, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, R.H.; Greebe, C.D.; Van De Kant, H.; Bogerd, J.; França, L.R.; Schulz, R.W. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar] [CrossRef]
- Tovo-Neto, A.; Martinez, E.R.M.; Melo, A.G.; Doretto, L.B.; Butzge, A.J.; Rodrigues, M.S.; Nakajima, R.T.; Habibi, H.R.; Nóbrega, R.H. Cortisol directly stimulates spermatogonial differentiation, meiosis, and spermiogenesis in zebrafish (Danio rerio) testicular explants. Biomolecules 2020, 10, 429. [Google Scholar] [CrossRef]
- Figueiredo, M.A.; Mareco, E.A.; Silva, M.D.P.; Marins, L.F. Muscle-specific growth hormone receptor (GHR) overexpression induces hyperplasia but not hypertrophy in transgenic zebrafish. Transgenic Res. 2012, 21, 457–469. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Capelle, P.M.; Semeniuk, C.A.D.; Love, O.P. Glucocorticoids in fish eggs: Variation, interactions with the environment, and the potential to shape offspring fitness. Physiol. Biochem. Zool. 2017, 90, 15–33. [Google Scholar] [CrossRef]
- Glickman, N.; Yelon, D. Cardiac development in zebrafish: Coordination of form and function. Semin. Cell Dev. Biol. 2002, 13, 507–513. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, C.; Bern, H.A. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 2005, 243, 215–285. [Google Scholar]
- Nóbrega, R.H.; Morais, R.D.V.S.; Crespo, D.; De Waal, P.P.; França, L.R.; Schulz, R.W.; Bogerd, J. Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology 2015, 156, 3804–3817. [Google Scholar] [CrossRef]
- Wilson, K.S.; Matrone, G.; Livingstone, D.E.W.; Al-Dujaili, E.A.S.; Mullins, J.J.; Tucker, C.S.; Hadoke, P.W.F.; Kenyon, C.J.; Denvir, M.A. Physiological roles of glucocorticoids during early embryonic development of the zebrafish (Danio rerio). J. Physiol. 2013, 591, 6209–6220. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Weber, G.M.; Blemings, K.P.; Silverstein, J.T. Insulin-like growth factor-I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1332–R1342. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Carpio, Y.; Borroto, I.; Gonzálezo, O.; Estradam, P. Myostatin gene silenced by RNAi show a zebrafish giant phenotype. J. Biotechnol. 2005, 119, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hu, S.; Gong, H.; Chen, M.; Lu, J.; Wu, J. Suppression of myostatin with vector-based RNA interference causes a double-muscle effect in transgenic zebrafish. Biochem. Biophys. Res. Commun. 2009, 387, 766–771. [Google Scholar] [CrossRef]
- Maccatrozzo, L.; Bargelloni, L.; Cardazzo, B.; Rizzo, G.; Patarnello, T. A novel second myostatin gene is present in teleost fish. FEBS Lett. 2001, 509, 36–40. [Google Scholar] [CrossRef]
- Amali, A.A.; Lin, C.J.-F.; Chen, Y.-H.; Wang, W.-L.; Gong, H.-Y.; Lee, C.-Y.; Wu, J.-L. Up-regulation of muscle-specific transcription factors during embryonic somitogenesis of zebrafish (Danio rerio) by knock-down of myostatin-1. Dev. Dyn. 2004, 229, 847–856. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, Z.; Shi, C.; Zhai, G.; Jin, X.; He, J.; Lou, Q.; Yin, Z. Depletion of myostatin b promotes somatic growth and lipid metabolism in zebrafish. Front. Endocrinol. 2016, 7, 88. [Google Scholar] [CrossRef]
- Oakes, J.A.; Li, N.; Wistow, B.R.C.; Griffin, A.; Barnard, L.; Storbeck, K.-H.; Cunliffe, V.T.; Krone, N.P. Ferredoxin 1b deficiency leads to testis disorganization, impaired spermatogenesis, and feminization in zebrafish. Endocrinology 2019, 160, 2401–2416. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.d.S.; Toledo, V.P.B.; Nóbrega, R.H. Effects of Maternal Stress on the Development of the Somatotropic Axis During the Larval and Juvenile Stages in Zebrafish (Danio rerio). Fishes 2025, 10, 37. https://doi.org/10.3390/fishes10020037
Rodrigues MdS, Toledo VPB, Nóbrega RH. Effects of Maternal Stress on the Development of the Somatotropic Axis During the Larval and Juvenile Stages in Zebrafish (Danio rerio). Fishes. 2025; 10(2):37. https://doi.org/10.3390/fishes10020037
Chicago/Turabian StyleRodrigues, Maira da Silva, Vinícius Prazeres Barbosa Toledo, and Rafael Henrique Nóbrega. 2025. "Effects of Maternal Stress on the Development of the Somatotropic Axis During the Larval and Juvenile Stages in Zebrafish (Danio rerio)" Fishes 10, no. 2: 37. https://doi.org/10.3390/fishes10020037
APA StyleRodrigues, M. d. S., Toledo, V. P. B., & Nóbrega, R. H. (2025). Effects of Maternal Stress on the Development of the Somatotropic Axis During the Larval and Juvenile Stages in Zebrafish (Danio rerio). Fishes, 10(2), 37. https://doi.org/10.3390/fishes10020037








