Genetics of Physiological Variation Within and Between Larval Wild-Type AB and Backcrossed NHGRI-1 Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Husbandry
2.2. Experimental Design
- control group in normoxia (~7.8 mg L−1 O2) at 28 °C
- low temperature of 23 °C in normoxia
- high temperature of 33 °C in normoxia
- moderate hypoxia of ~4.9 mg L−1 O2 at 28 °C
- severe hypoxia of ~3.7 mg L−1 O2 at 28 °C
2.3. Physiological Variables
2.4. Statistics
3. Results
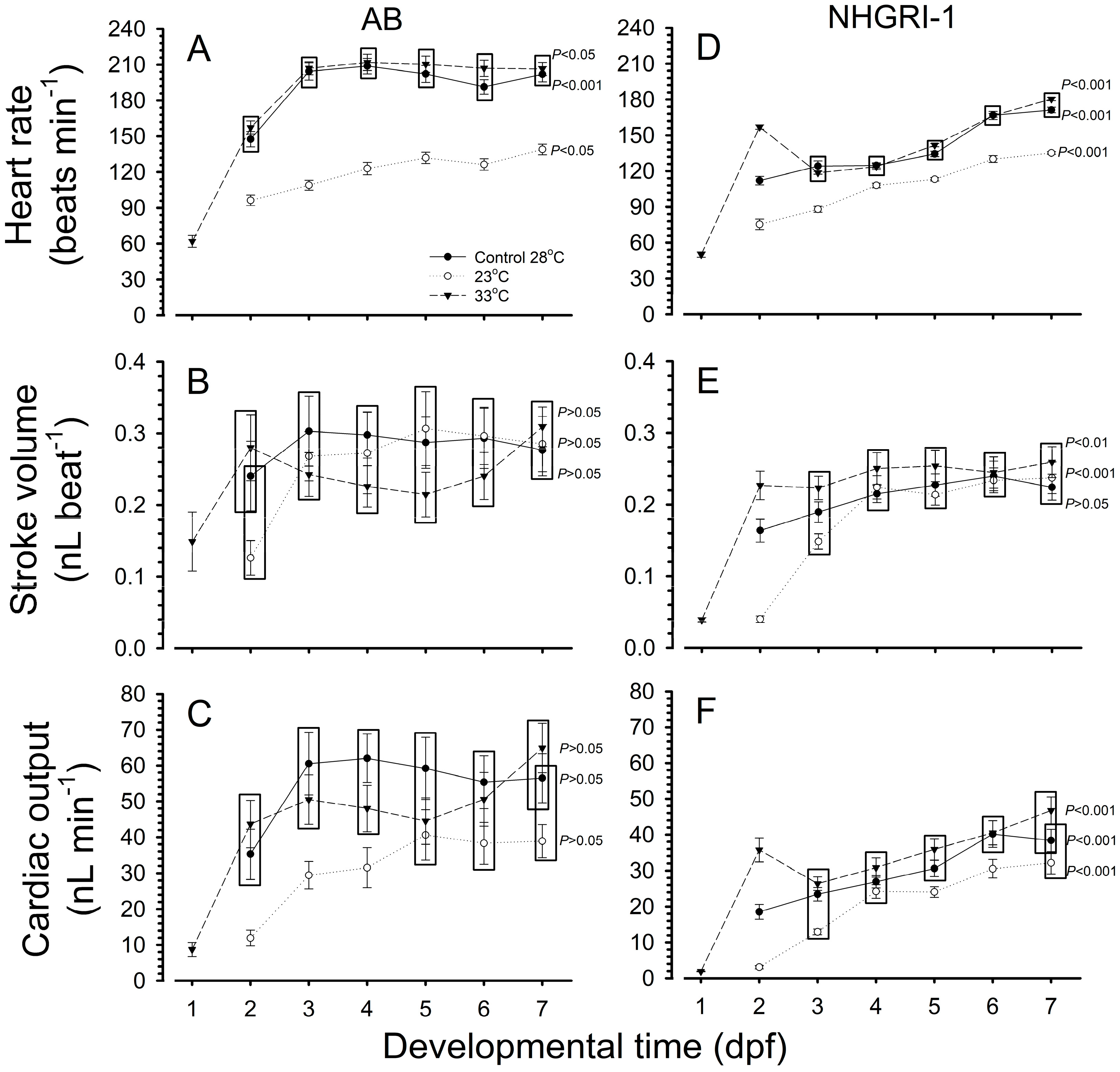
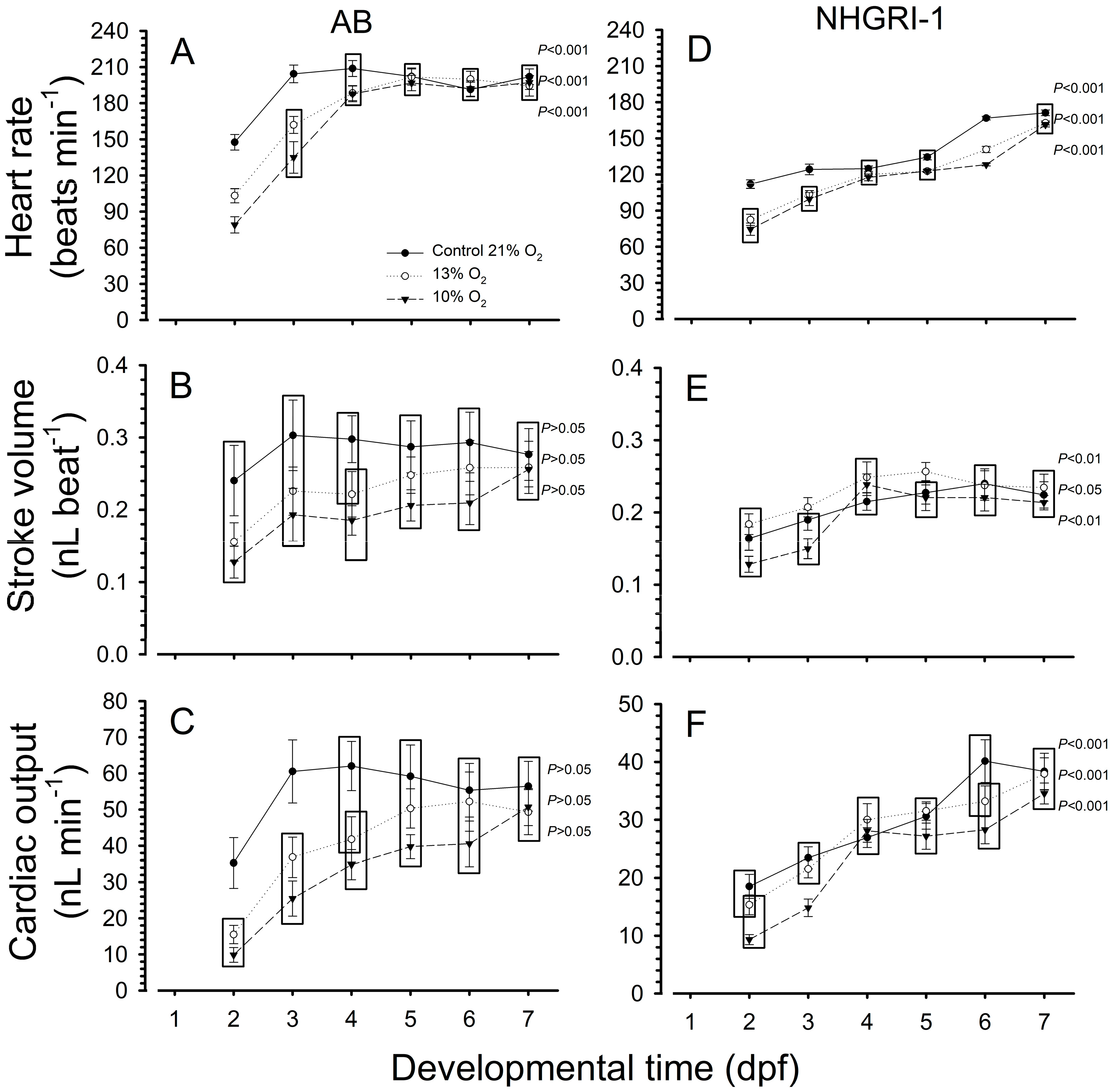
3.1. Heart Rate
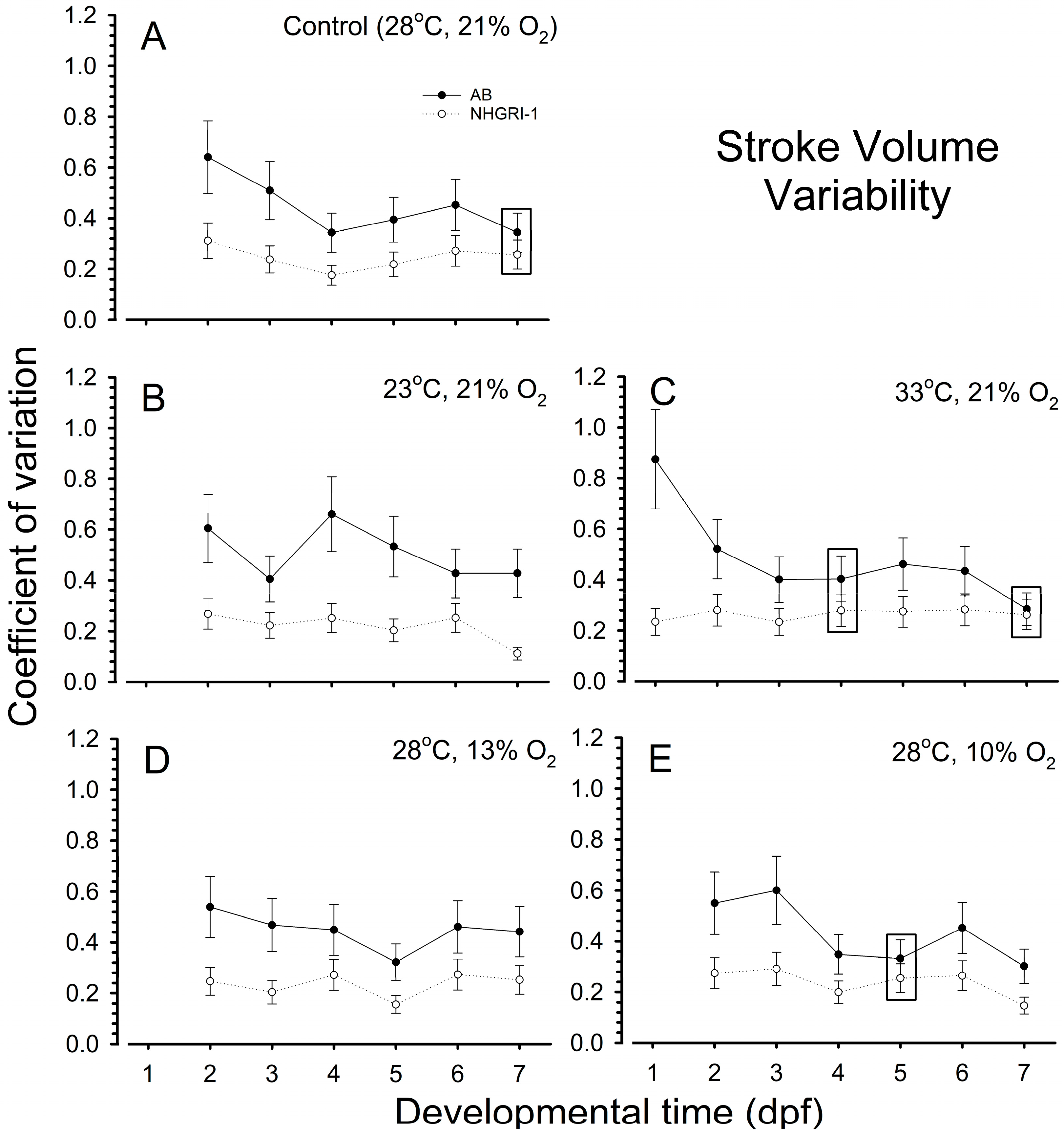
3.2. Stroke Volume
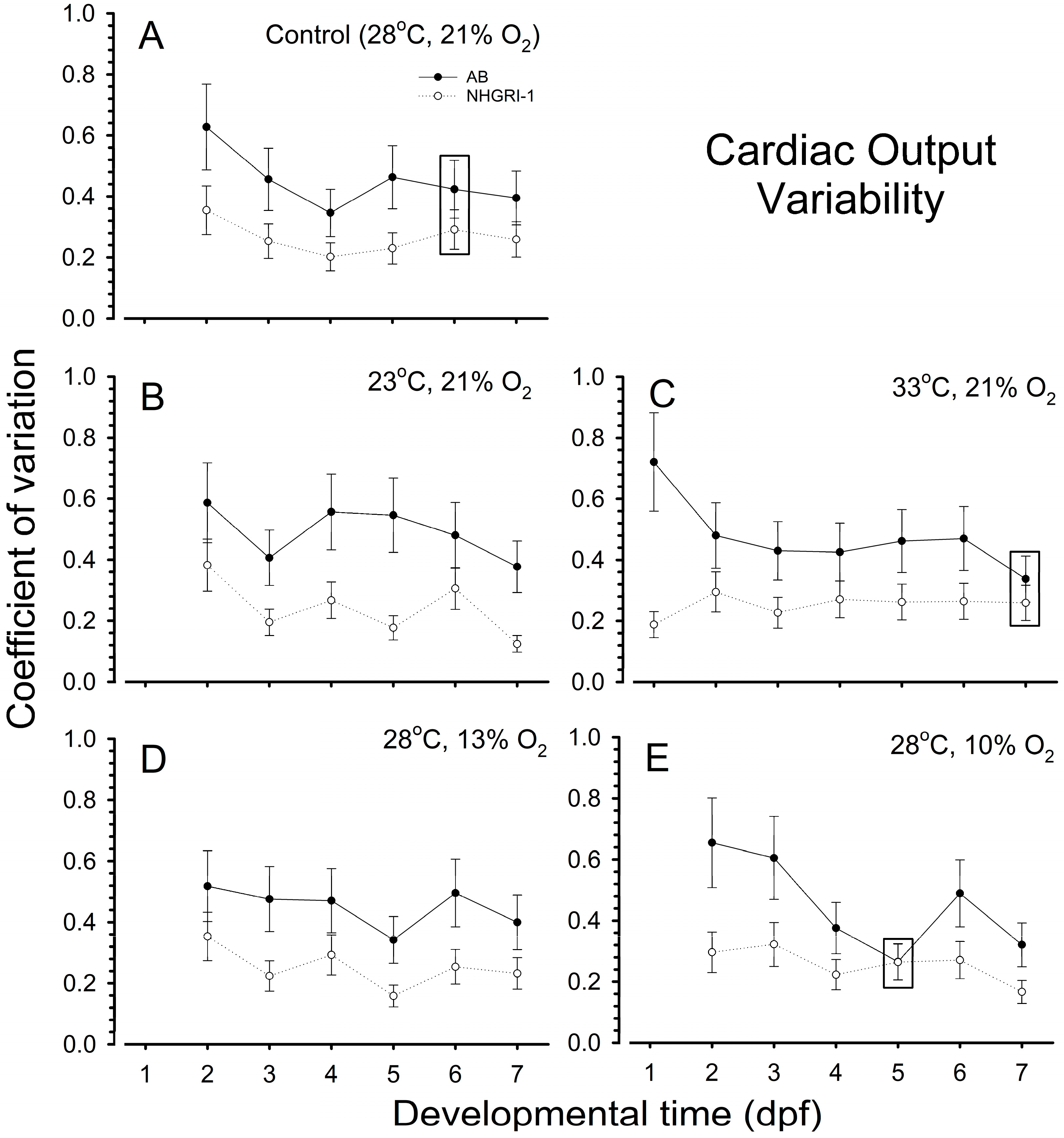
3.3. Cardiac Output
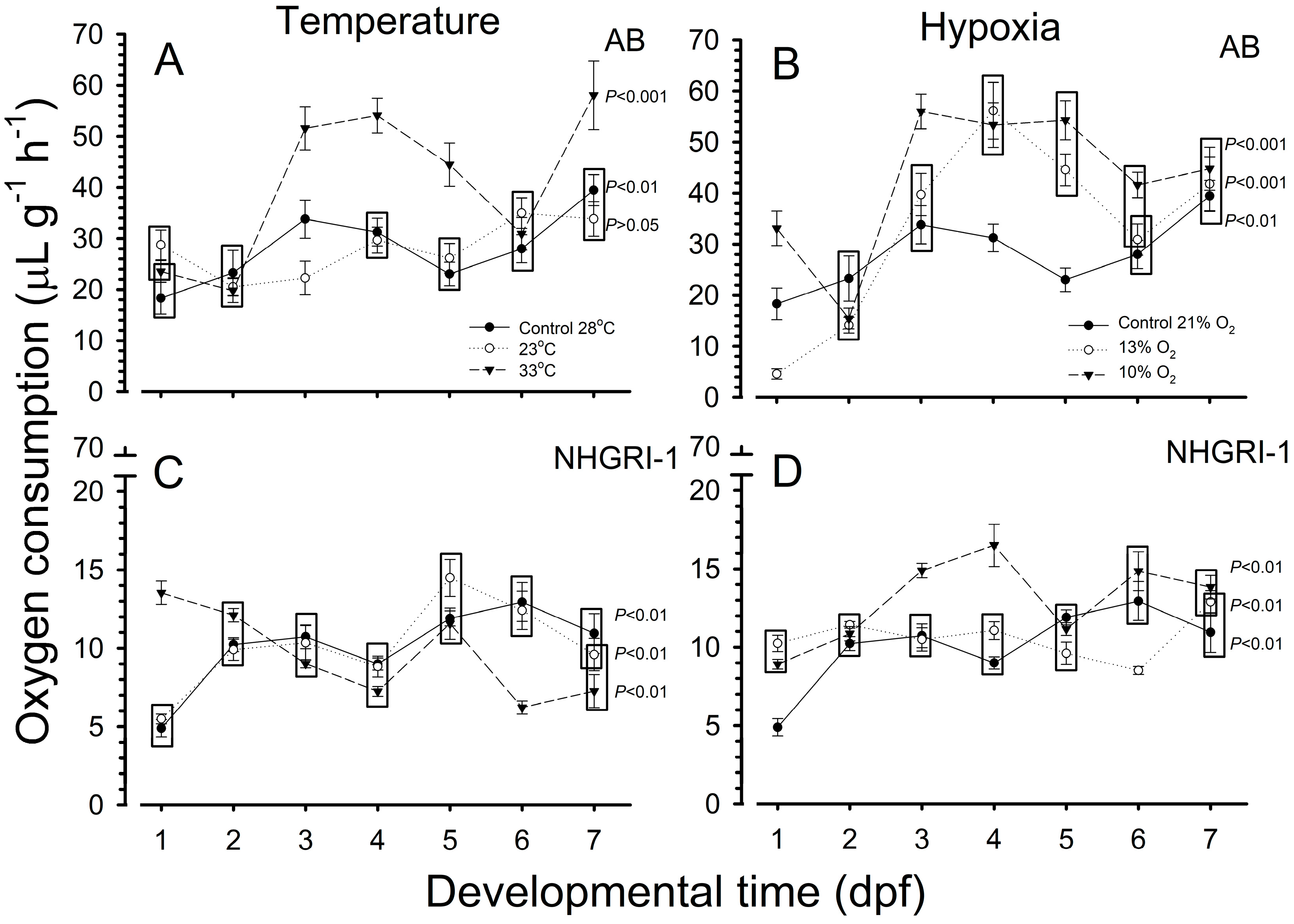
3.4. Oxygen Consumption
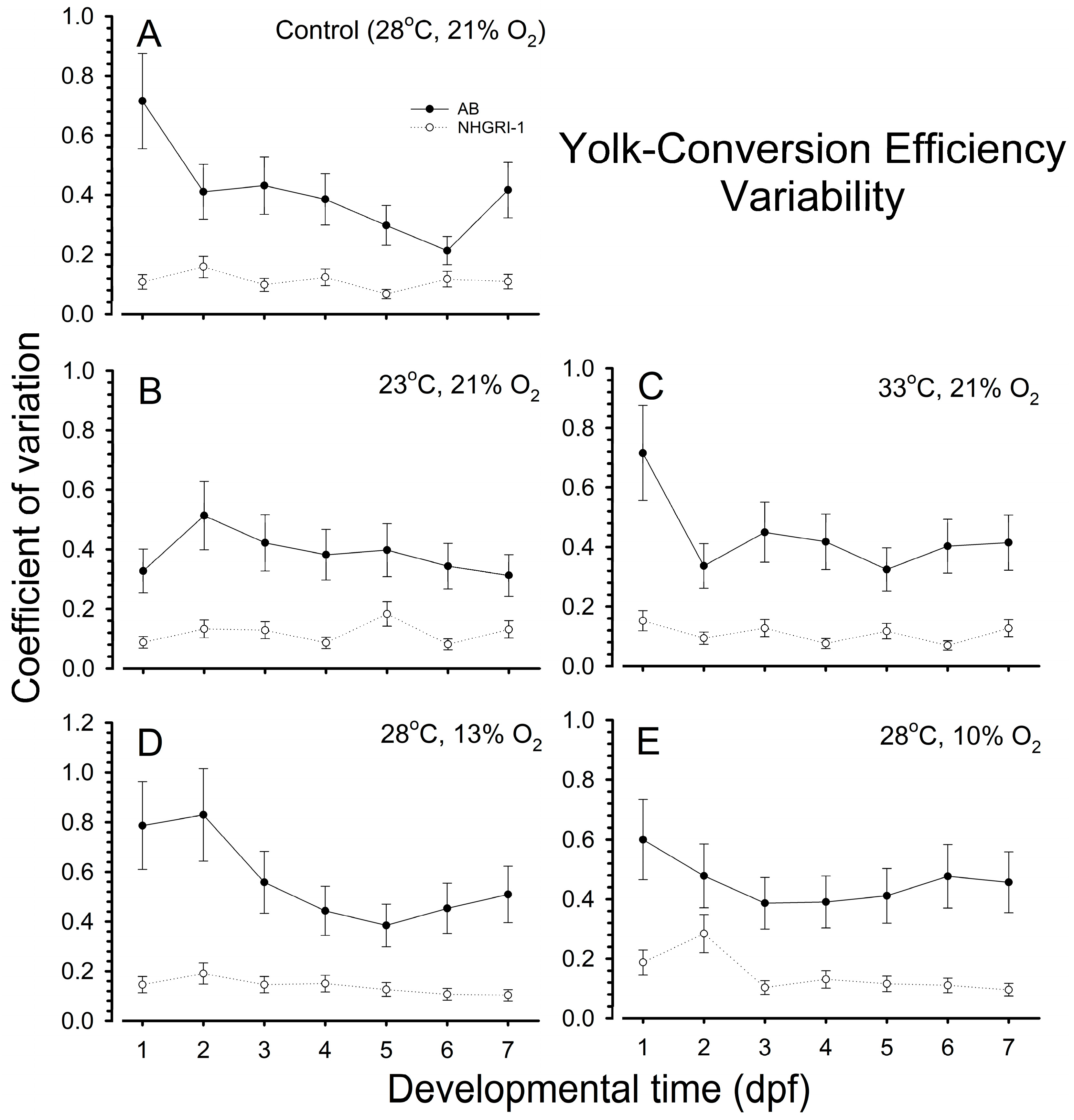
3.5. Yolk Conversion Efficiency
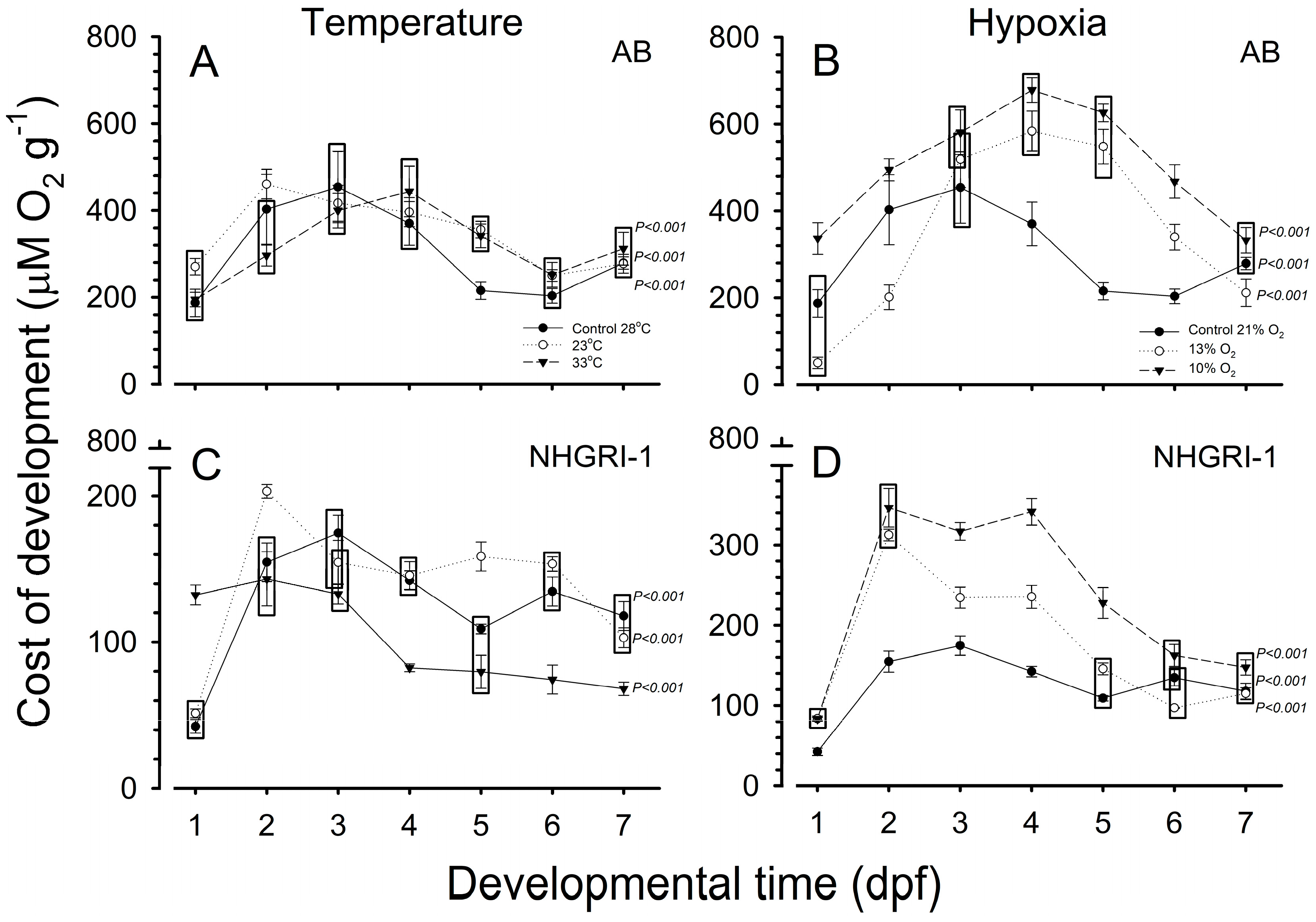
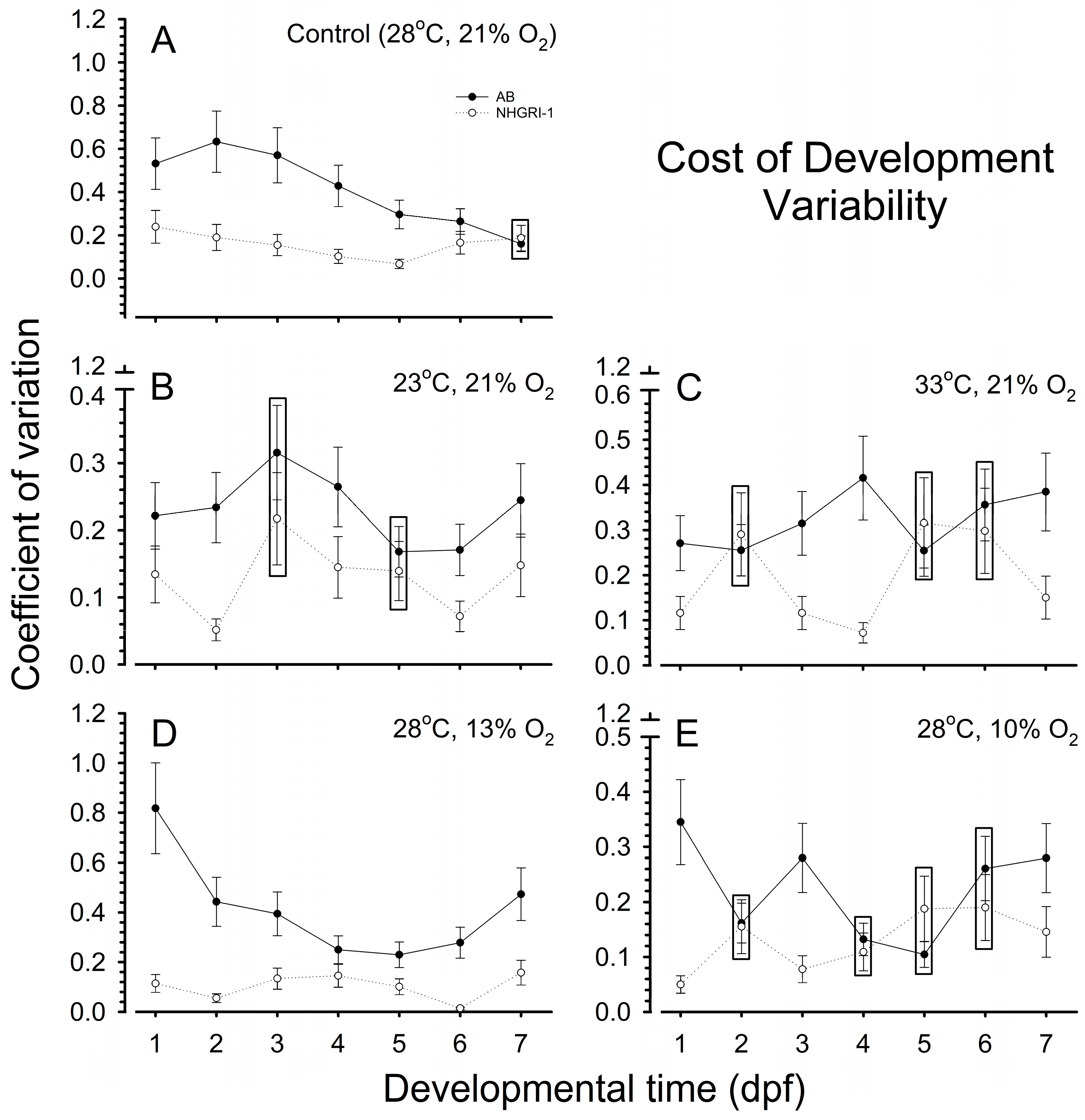
3.6. Cost of Development
3.7. Coefficients of Variation
4. Discussion
4.1. Physiological Variation Across Taxa
4.2. Physiological Variation Among Zebrafish Strains/Lines
4.3. Zebrafish as a Model for Studying Mechanisms of Physiological Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coskun, A.; Zarepour, A.; Zarrabi, A. Physiological Rhythms and Biological Variation of Biomolecules: The Road to Personalized Laboratory Medicine. Int. J. Mol. Sci. 2023, 24, 6275. [Google Scholar] [CrossRef] [PubMed]
- Prosser, C.L. Physiological variation in animals. Biol. Rev. 1955, 30, 229–261. [Google Scholar] [CrossRef]
- Tanner, R.L.; Dowd, W.W. Inter-individual physiological variation in responses to environmental variation and environmental change: Integrating across traits and time. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 238, 110577. [Google Scholar] [CrossRef] [PubMed]
- Hollins, J.; Thambithurai, D.; Koeck, B.; Crespel, A.; Bailey, D.M.; Cooke, S.J.; Lindström, J.; Parsons, K.J.; Killen, S.S. A physiological perspective on fisheries-induced evolution. Evol. Appl. 2018, 11, 561–576. [Google Scholar] [CrossRef]
- Kasihmuddin, S.M.; Cob, Z.C.; Noor, N.M.; Das, S.K. Effect of different temperature variations on the physiological state of catfish species: A systematic review. Fish. Physiol. Biochem. 2024, 50, 413–434. [Google Scholar] [CrossRef]
- Lindmark, M.; Audzijonyte, A.; Blanchard, J.L.; Gårdmark, A. Temperature impacts on fish physiology and resource abundance lead to faster growth but smaller fish sizes and yields under warming. Glob. Change Biol. 2022, 28, 6239–6253. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Axelsson, M.; Chabot, D.; Claireaux, G.; Cooke, S.J.; Corner, R.A.; De Boeck, G.; Domenici, P.; Guerreiro, P.M.; Hamer, B.; et al. Conservation physiology of marine fishes: State of the art and prospects for policy. Conserv. Physiol. 2016, 4, cow046. [Google Scholar] [CrossRef]
- Mukherjee, J.; Moniruzzaman, M.; Chakraborty, S.B.; Lek, S.; Ray, S. Towards a physiological response of fishes under variable environmental conditions: An approach through neural network. Ecol. Indic. 2017, 78, 381–394. [Google Scholar] [CrossRef]
- Payne, N.L.; Morley, S.A.; Halsey, L.G.; Smith, J.A.; Stuart-Smith, R.; Waldock, C.; Bates, A.E. Fish heating tolerance scales similarly across individual physiology and populations. Commun. Biol. 2021, 4, 264. [Google Scholar] [CrossRef]
- Göpel, T.; Burggren, W.W. Insufficient reporting of experimental variables as a cause for nonreproducibility in animal physiology? A case study. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2022, 323, R363–R374. [Google Scholar] [CrossRef]
- Chan, J.C.; Houghton, A.B.; Bale, T.L. Strained in Planning Your Mouse Background? Using the HPA Stress Axis as a Biological Readout for Backcrossing Strategies. Neuropsychopharmacology 2017, 42, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Crim, M.J.; Lawrence, C. A fish is not a mouse: Understanding differences in background genetics is critical for reproducibility. Lab Anim. 2021, 50, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.L.; Ericsson, A.C. Microbiota and reproducibility of rodent models. Lab Anim. 2017, 46, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mandillo, S.; Tucci, V.; Hölter, S.M.; Meziane, H.; Banchaabouchi, M.A.; Kallnik, M.; Lad, H.V.; Nolan, P.M.; Ouagazzal, A.M.; Coghill, E.L.; et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: A cross-laboratory study. Physiol. Genom. 2008, 34, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.A.; Hill, D.R. Reproducibility, Replicability, and Repeatability: A survey of reproducible research with a focus on high performance computing. arXiv 2024, arXiv:2402.07530. [Google Scholar] [CrossRef]
- Sukoff Rizzo, S.J.; McTighe, S.; McKinzie, D.L. Genetic Background and Sex: Impact on Generalizability of Research Findings in Pharmacology Studies. In Good Research Practice in Non-Clinical Pharmacology and Biomedicine; Bespalov, A., Michel, M.C., Steckler, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 147–162. [Google Scholar]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Terenina, E.E.; Cavigelli, S.; Mormede, P.; Zhao, W.; Parks, C.; Lu, L.; Jones, B.C.; Mulligan, M.K. Genetic factors mediate the impact of chronic stress and subsequent response to novel acute stress. Front. Neurosci. 2019, 13, 438. [Google Scholar] [CrossRef]
- Stark, G. Large and expensive brain comes with a short lifespan: The relationship between brain size and longevity among fish taxa. J. Fish. Biol. 2022, 101, 92–99. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; Nadler, L.; Domenici, P. The role of physiological traits in assortment among and within fish shoals. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Alfonso, S.; Houdelet, C.; Bessa, E.; Geffroy, B.; Sadoul, B. Water temperature explains part of the variation in basal plasma cortisol level within and between fish species. J. Fish Biol. 2023, 103, 828–838. [Google Scholar] [CrossRef]
- Shuai, F.; Yu, S.; Lek, S.; Li, X. Habitat effects on intra-species variation in functional morphology: Evidence from freshwater fish. Ecol. Evol. 2018, 8, 10902–10913. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.P.; Da Silva, J.C.; Baumgartner, D.; Baumgartner, G.; Delariva, R.L. Is resource partitioning the key? The role of intra-interspecific variation in coexistence among five small endemic fish species (Characidae) in subtropical rivers. J. Fish Biol. 2018, 93, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, F. An Account of the Fishes Found in the River Ganges and Its Branches; Archibald Constable: Fife, UK, 1822; Volume 1. [Google Scholar]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, L.; Richard, E.M.; Sourbron, J.; Lagae, L.; Maurice, T.; Delprat, B. Use of Zebrafish Models to Boost Research in Rare Genetic Diseases. Int. J. Mol. Sci. 2021, 22, 13356. [Google Scholar] [CrossRef]
- Miller, C.L.; Dugand, R.J.; McGuigan, K. Variability of morphology–performance relationships under acute exposure to different temperatures in 3 strains of zebrafish. Curr. Zool. 2024, zoae032. [Google Scholar] [CrossRef]
- Daane, J.M.; Rohner, N.; Konstantinidis, P.; Djuranovic, S.; Harris, M.P. Parallelism and Epistasis in Skeletal Evolution Identified through Use of Phylogenomic Mapping Strategies. Mol. Biol. Evol. 2016, 33, 162–173. [Google Scholar] [CrossRef]
- LaFave, M.C.; Varshney, G.K.; Vemulapalli, M.; Mullikin, J.C.; Burgess, S.M. A defined zebrafish line for high-throughput genetics and genomics: NHGRI-1. Genetics 2014, 198, 167–170. [Google Scholar] [CrossRef]
- Martinez-Bautista, G.; Padilla, P.; Burggren, W.W. Genetic Basis for Morphological Variation in the Zebrafish Danio rerio: Insights from a Low-Heterozygosity Line. Fishes 2024, 9, 164. [Google Scholar] [CrossRef]
- Moore, F.B.; Hosey, M.; Bagatto, B. Cardiovascular system in larval zebrafish responds to developmental hypoxia in a family specific manner. Front. Zool. 2006, 3, 4. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Wood, C.M.; Harter, T.S.; Dal Pont, G.; Val, A.L.; Matthews, P.G.D. Metabolic fuel use after feeding in the zebrafish (Danio rerio): A respirometric analysis. J. Exp. Biol. 2019, 222, jeb194217. [Google Scholar] [CrossRef]
- Burggren, W.W.; Bautista, G.M.; Coop, S.C.; Couturier, G.M.; Delgadillo, S.P.; García, R.M.; González, C.A.A. Developmental cardiorespiratory physiology of the air-breathing tropical gar, Atractosteus tropicus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R689–R701. [Google Scholar] [CrossRef] [PubMed]
- Perrichon, P.; Grosell, M.; Burggren, W.W. Heart performance determination by visualization in larval fishes: Influence of alternative models for heart shape and volume. Front. Physiol. 2017, 8, 464. [Google Scholar] [CrossRef] [PubMed]
- Jevtov, I.; Samuelsson, T.; Yao, G.; Amsterdam, A.; Ribbeck, K. Zebrafish as a model to study live mucus physiology. Sci. Rep. 2014, 4, 6653. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Seok, S.H.; Lee, H.Y. Development of a Zebrafish Larvae Model for Diabetic Heart Failure With Reduced Ejection Fraction. Korean Circ. J. 2023, 53, 34–46. [Google Scholar] [CrossRef]
- Mueller, C.A.; Eme, J.; Manzon, R.G.; Somers, C.M.; Boreham, D.R.; Wilson, J.Y. Embryonic critical windows: Changes in incubation temperature alter survival, hatchling phenotype, and cost of development in lake whitefish (Coregonus clupeaformis). J. Comp. Physiol. B 2015, 185, 315–331. [Google Scholar] [CrossRef]
- Rombough, P.J. Growth, aerobic metabolism, and dissolved oxygen requirements of embryos and alevins of steelhead, Salmo gairdneri. Can. J. Zool. 1988, 66, 651–660. [Google Scholar] [CrossRef]
- Irvin, H. A Report on the Statistical Properties of the Coefficient of Variation and Some Applications. Master’s Thesis, Utah State University, Logan, UT, USA, 1970. [Google Scholar]
- De Luca, E.; Zaccaria, G.M.; Hadhoud, M.; Rizzo, G.; Ponzini, R.; Morbiducci, U.; Santoro, M.M. ZebraBeat: A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci. Rep. 2014, 4, 4898. [Google Scholar] [CrossRef]
- Krylov, V.; Machikhin, A.; Sizov, D.; Guryleva, A.; Sizova, A.; Zhdanova, S.; Tchougounov, V.; Burlakov, A. Influence of hypomagnetic field on the heartbeat in zebrafish embryos. Front. Physiol. 2022, 13, 1040083. [Google Scholar] [CrossRef]
- Burggren, W.; Abramova, R.; Bautista, N.M.; Fritsche Danielson, R.; Dubansky, B.; Gupta, A.; Hansson, K.; Iyer, N.; Jagadeeswaran, P.; Jennbacken, K.; et al. A larval zebrafish model of cardiac physiological recovery following cardiac arrest and myocardial hypoxic damage. Biol. Open 2024, 13, bio060230. [Google Scholar] [CrossRef]
- Vazquez Roman, K.N.; Burggren, W.W. Metabolic responses to crude oil during early life stages reveal critical developmental windows in the zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109274. [Google Scholar] [CrossRef]
- Strecker, R.; Seiler, T.-B.; Hollert, H.; Braunbeck, T. Oxygen requirements of zebrafish (Danio rerio) embryos in embryo toxicity tests with environmental samples. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Barrionuevo, W.R.; Fernandes, M.N.; Rocha, O. Aerobic and anaerobic metabolism for the zebrafish, Danio rerio, reared under normoxic and hypoxic conditions and exposed to acute hypoxia during development. Braz. J. Biol. 2010, 70, 425–434. [Google Scholar] [CrossRef]
- Bagatto, B.; Pelster, B.; Burggren, W.W. Growth and metabolism of larval zebrafish: Effects of swim training. J. Exp. Biol. 2001, 204, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, V.H.; Farber, S.A. Lipid Uptake, Metabolism, and Transport in the Larval Zebrafish. Front. Endocrinol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Sant, K.E.; Navarrete, J.; George, U.Z. Mathematical modeling of the interaction between yolk utilization and fish growth in zebrafish, Danio rerio. Development 2021, 148, dev193508. [Google Scholar] [CrossRef]
- Anton, S.; Gadenne, C.; Marion-Poll, F. Frontiers in Invertebrate Physiology—An Update to the Grand Challenge. Front. Physiol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Woodard, S.H.; Fischman, B.J.; Venkat, A.; Hudson, M.E.; Varala, K.; Cameron, S.A.; Clark, A.G.; Robinson, G.E. Genes involved in convergent evolution of eusociality in bees. Proc. Natl. Acad. Sci. USA 2011, 108, 7472–7477. [Google Scholar] [CrossRef]
- Pyza, E.M. Plasticity in invertebrate sensory systems. Front. Physiol. 2013, 4, 226. [Google Scholar] [CrossRef]
- Vafopoulou, X. The coming of age of insulin-signaling in insects. Front. Physiol. 2014, 5, 216. [Google Scholar] [CrossRef]
- Ho, D.H.; Burggren, W.W. Parental hypoxic exposure confers offspring hypoxia resistance in zebrafish (Danio rerio). J. Exp. Biol. 2012, 215, 4208–4216. [Google Scholar] [CrossRef]
- Swanson, D.L.; Vézina, F.; McKechnie, A.E.; Nord, A. Avian behavioral and physiological responses to challenging thermal environments and extreme weather events. Front. Ecol. Evol. 2022, 10, 1034659. [Google Scholar] [CrossRef]
- von May, R.; Catenazzi, A.; Santa-Cruz, R.; Gutierrez, A.S.; Moritz, C.; Rabosky, D.L. Thermal physiological traits in tropical lowland amphibians: Vulnerability to climate warming and cooling. PLoS ONE 2019, 14, e0219759. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.C.; Klemz, M.; Wada, H.; Darling, R.; Dias, T.; O’Brien, B.K.; Probst, C.M.; Zhang, M.; Zimova, M. Temperature, size and developmental plasticity in birds. Biol. Lett. 2022, 18, 20220357. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Seebacher, F. Physiology can predict animal activity, exploration, and dispersal. Commun. Biol. 2022, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Wild, K.H.; Pottier, P.; Carrasco, M.I.; Nakagawa, S.; Noble, D.W.A. Developmental environments do not affect thermal physiological traits in reptiles: An experimental test and meta-analysis. Biol. Lett. 2023, 19, 20230019. [Google Scholar] [CrossRef]
- Park, J.K.; Do, Y. Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters. Animals 2023, 13, 3162. [Google Scholar] [CrossRef]
- Brosset, P.; Cooke, S.J.; Schull, Q.; Trenkel, V.M.; Soudant, P.; Lebigre, C. Physiological biomarkers and fisheries management. Rev. Fish Biol. Fish. 2021, 31, 797–819. [Google Scholar] [CrossRef]
- Torday, J.S. The Emergence of Physiology and Form: Natural Selection Revisited. Biology 2016, 5, 15. [Google Scholar] [CrossRef]
- Milo, R.; Hou, J.H.; Springer, M.; Brenner, M.P.; Kirschner, M.W. The relationship between evolutionary and physiological variation in hemoglobin. Proc. Natl. Acad. Sci. 2007, 104, 16998–17003. [Google Scholar] [CrossRef]
- Suurväli, J.; Whiteley, A.R.; Zheng, Y.; Gharbi, K.; Leptin, M.; Wiehe, T. The Laboratory Domestication of Zebrafish: From Diverse Populations to Inbred Substrains. Mol. Biol. Evol. 2019, 37, 1056–1069. [Google Scholar] [CrossRef]
- Whiteley, A.R.; Bhat, A.; Martins, E.P.; Mayden, R.L.; Arunachalam, M.; Uusi-Heikkilä, S.; Ahmed, A.T.; Shrestha, J.; Clark, M.; Stemple, D.; et al. Population genomics of wild and laboratory zebrafish (Danio rerio). Mol. Ecol. 2011, 20, 4259–4276. [Google Scholar] [CrossRef]
- Audira, G.; Siregar, P.; Strungaru, S.A.; Huang, J.C.; Hsiao, C.D. Which Zebrafish Strains Are More Suitable to Perform Behavioral Studies? A Comprehensive Comparison by Phenomic Approach. Biology 2020, 9, 200. [Google Scholar] [CrossRef]
- Liu, X.; Guo, N.; Lin, J.; Zhang, Y.; Chen, X.Q.; Li, S.; He, L.; Li, Q. Strain-dependent differential behavioral responses of zebrafish larvae to acute MK-801 treatment. Pharmacol. Biochem. Behav. 2014, 127, 82–89. [Google Scholar] [CrossRef]
- Mahabir, S.; Chatterjee, D.; Gerlai, R. Short exposure to low concentrations of alcohol during embryonic development has only subtle and strain- dependent effect on the levels of five amino acid neurotransmitters in zebrafish. Neurotoxicol Teratol. 2018, 68, 91–96. [Google Scholar] [CrossRef]
- Meyer, B.M.; Froehlich, J.M.; Galt, N.J.; Biga, P.R. Inbred strains of zebrafish exhibit variation in growth performance and myostatin expression following fasting. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 1–9. [Google Scholar] [CrossRef]
- Siregar, P.; Juniardi, S.; Audira, G.; Lai, Y.H.; Huang, J.C.; Chen, K.H.; Chen, J.R.; Hsiao, C.D. Method Standardization for Conducting Innate Color Preference Studies in Different Zebrafish Strains. Biomedicines 2020, 8, 271. [Google Scholar] [CrossRef]
- Volgin, A.D.; Yakovlev, O.A.; Demin, K.A.; de Abreu, M.S.; Alekseeva, P.A.; Friend, A.J.; Lakstygal, A.M.; Amstislavskaya, T.G.; Bao, W.; Song, C.; et al. Zebrafish models for personalized psychiatry: Insights from individual, strain and sex differences, and modeling gene x environment interactions. J. Neurosci. Res. 2019, 97, 402–413. [Google Scholar] [CrossRef]
- Wakamatsu, Y.; Ogino, K.; Hirata, H. Swimming capability of zebrafish is governed by water temperature, caudal fin length and genetic background. Sci. Rep. 2019, 9, 16307. [Google Scholar] [CrossRef]
- van den Bos, R.; Althuizen, J.; Tschigg, K.; Bomert, M.; Zethof, J.; Filk, G.; Gorissen, M. Early life exposure to cortisol in zebrafish (Danio rerio): Similarities and differences in behaviour and physiology between larvae of the AB and TL strains. Behav. Pharmacol. 2019, 30, 260–271. [Google Scholar] [CrossRef]
- The Zebrafish Informaton Network (ZFIN). Available online: https://zfin.org (accessed on 18 August 2024).
- Feugere, L.; Bates, A.; Emagbetere, T.; Chapman, E.; Malcolm, L.E.; Bulmer, K.; Hardege, J.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Heat induces multiomic and phenotypic stress propagation in zebrafish embryos. PNAS Nexus 2023, 2, pgad137. [Google Scholar] [CrossRef]
- Feugere, L.; Scott, V.F.; Rodriguez-Barucg, Q.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Thermal stress induces a positive phenotypic and molecular feedback loop in zebrafish embryos. J. Therm. Biol. 2021, 102, 103114. [Google Scholar] [CrossRef] [PubMed]
- Haapanen-Saaristo, A.-M.; Virtanen, N.; Tcarenkova, E.; Vaparanta, K.; Ampuja, M.; Vehniäinen, E.-R.; Paatero, I. Heat stress sensitizes zebrafish embryos to neurological and cardiac toxicity. Biochem. Biophys. Res. Commun. 2024, 733, 150682. [Google Scholar] [CrossRef] [PubMed]
- Mandic, M.; Best, C.; Perry, S.F. Loss of hypoxia-inducible factor 1α affects hypoxia tolerance in larval and adult zebrafish (Danio rerio). Proc. Biol. Sci. 2020, 287, 20200798. [Google Scholar] [CrossRef] [PubMed]
- Rombough, P. The functional ontogeny of the teleost gill: Which comes first, gas or ion exchange? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Jonz, M.G. Chapter 10—Respiratory System. In The Zebrafish in Biomedical Research; Cartner, S.C., Eisen, J.S., Farmer, S.C., Guillemin, K.J., Kent, M.L., Sanders, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 103–107. [Google Scholar]
- Liem, K.L. Larvae of air-breathing fishes as countercurrent flow devices in hypoxic environments. Science 1981, 211, 1177–1179. [Google Scholar] [CrossRef]
- Mandic, M.; Flear, K.; Qiu, P.; Pan, Y.K.; Perry, S.F.; Gilmour, K.M. Aquatic surface respiration improves survival during hypoxia in zebrafish (Danio rerio) lacking hypoxia-inducible factor 1-α. Proc. Biol. Sci. 2022, 289, 20211863. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, A.; Liu, H.; Wang, H. Deletion of the foxO4 Gene Increases Hypoxia Tolerance in Zebrafish. Int. J. Mol. Sci. 2023, 24, 8942. [Google Scholar] [CrossRef]
- Dinarello, A.; Betto, R.M.; Diamante, L.; Tesoriere, A.; Ghirardo, R.; Cioccarelli, C.; Meneghetti, G.; Peron, M.; Laquatra, C.; Tiso, N.; et al. STAT3 and HIF1α cooperatively mediate the transcriptional and physiological responses to hypoxia. Cell Death Discov. 2023, 9, 226. [Google Scholar] [CrossRef]
- Burggren, W.W. Epigenetics as a source of variation in comparative animal physiology—Or—Lamarck is lookin’ pretty good these days. J. Exp. Biol. 2014, 217, 682–689. [Google Scholar] [CrossRef]
- Abdallah, S.J.; Thomas, B.S.; Jonz, M.G. Aquatic surface respiration and swimming behaviour in adult and developing zebrafish exposed to hypoxia. J. Exp. Biol. 2015, 218, 1777–1786. [Google Scholar] [CrossRef]
- Jonz, M.G.; Nurse, C.A. Development of oxygen sensing in the gills of zebrafish. J. Exp. Biol. 2005, 208, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Cadiz, L.; Bundgaard, A.; Malte, H.; Fago, A. Hypoxia enhances blood O(2) affinity and depresses skeletal muscle O(2) consumption in zebrafish (Danio rerio). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 234, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, A.; Ortega-Recalde, O.; Dutoit, L.; Besson, A.A.; Chia, J.H.Z.; King, T.; Nakagawa, S.; Hickey, A.; Gemmell, N.J.; Hore, T.; et al. Paternal hypoxia exposure primes offspring for increased hypoxia resistance. BMC Biol. 2022, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Pelster, B. Developmental plasticity in the cardiovascular system of fish, with special reference to the zebrafish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 547–553. [Google Scholar] [CrossRef]
- Wu, R.S.S. Chapter 3 Effects of Hypoxia on Fish Reproduction and Development. In Fish Physiology; Richards, J.G., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 27, pp. 79–141. [Google Scholar]
- Mueller, C.A.; Joss, J.M.; Seymour, R.S. The energy cost of embryonic development in fishes and amphibians, with emphasis on new data from the Australian lungfish, Neoceratodus forsteri. J. Comp. Physiol. B 2011, 181, 43–52. [Google Scholar] [CrossRef]
- Shang, E.H.; Wu, R.S. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ. Sci. Technol. 2004, 38, 4763–4767. [Google Scholar] [CrossRef]
- Jonz, M.G.; Buck, L.T.; Perry, S.F.; Schwerte, T.; Zaccone, G. Sensing and surviving hypoxia in vertebrates. Ann. N. Y. Acad. Sci. 2016, 1365, 43–58. [Google Scholar] [CrossRef]
- Guryev, V.; Koudijs, M.J.; Berezikov, E.; Johnson, S.L.; Plasterk, R.H.; van Eeden, F.J.; Cuppen, E. Genetic variation in the zebrafish. Genome Res. 2006, 16, 491–497. [Google Scholar] [CrossRef]
- Uribe-Salazar, J.M.; Kaya, G.; Sekar, A.; Weyenberg, K.; Ingamells, C.; Dennis, M.Y. Evaluation of CRISPR gene-editing tools in zebrafish. BMC Genom. 2022, 23, 12. [Google Scholar] [CrossRef]
- Holden, L.A.; Brown, K.H. Baseline mRNA expression differs widely between common laboratory strains of zebrafish. Sci. Rep. 2018, 8, 4780. [Google Scholar] [CrossRef]
- Nonnis, S.; Angiulli, E.; Maffioli, E.; Frabetti, F.; Negri, A.; Cioni, C.; Alleva, E.; Romeo, V.; Tedeschi, G.; Toni, M. Acute environmental temperature variation affects brain protein expression, anxiety and explorative behaviour in adult zebrafish. Sci. Rep. 2021, 11, 2521. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Bautista, G.; Cartee, M.R.; Kunder, D.; Lee, C.; Tang, K.; Nagarajan, N.; Padilla, P.; Burggren, W. Genetics of Physiological Variation Within and Between Larval Wild-Type AB and Backcrossed NHGRI-1 Zebrafish (Danio rerio). Fishes 2025, 10, 59. https://doi.org/10.3390/fishes10020059
Martinez-Bautista G, Cartee MR, Kunder D, Lee C, Tang K, Nagarajan N, Padilla P, Burggren W. Genetics of Physiological Variation Within and Between Larval Wild-Type AB and Backcrossed NHGRI-1 Zebrafish (Danio rerio). Fishes. 2025; 10(2):59. https://doi.org/10.3390/fishes10020059
Chicago/Turabian StyleMartinez-Bautista, Gil, Moira Ryann Cartee, Dyuksha Kunder, Crystelle Lee, Karol Tang, Neha Nagarajan, Pamela Padilla, and Warren Burggren. 2025. "Genetics of Physiological Variation Within and Between Larval Wild-Type AB and Backcrossed NHGRI-1 Zebrafish (Danio rerio)" Fishes 10, no. 2: 59. https://doi.org/10.3390/fishes10020059
APA StyleMartinez-Bautista, G., Cartee, M. R., Kunder, D., Lee, C., Tang, K., Nagarajan, N., Padilla, P., & Burggren, W. (2025). Genetics of Physiological Variation Within and Between Larval Wild-Type AB and Backcrossed NHGRI-1 Zebrafish (Danio rerio). Fishes, 10(2), 59. https://doi.org/10.3390/fishes10020059







