Morphology and Histology of the Digestive System of Japanese Mantis Shrimp (Oratosquilla oratoria)
Abstract
:1. Introduction
2. Materials and Methods
2.1. O. oratoria Collection
2.2. Morphological Observation
2.3. Histological Observation
2.4. Statistical Analysis
3. Results
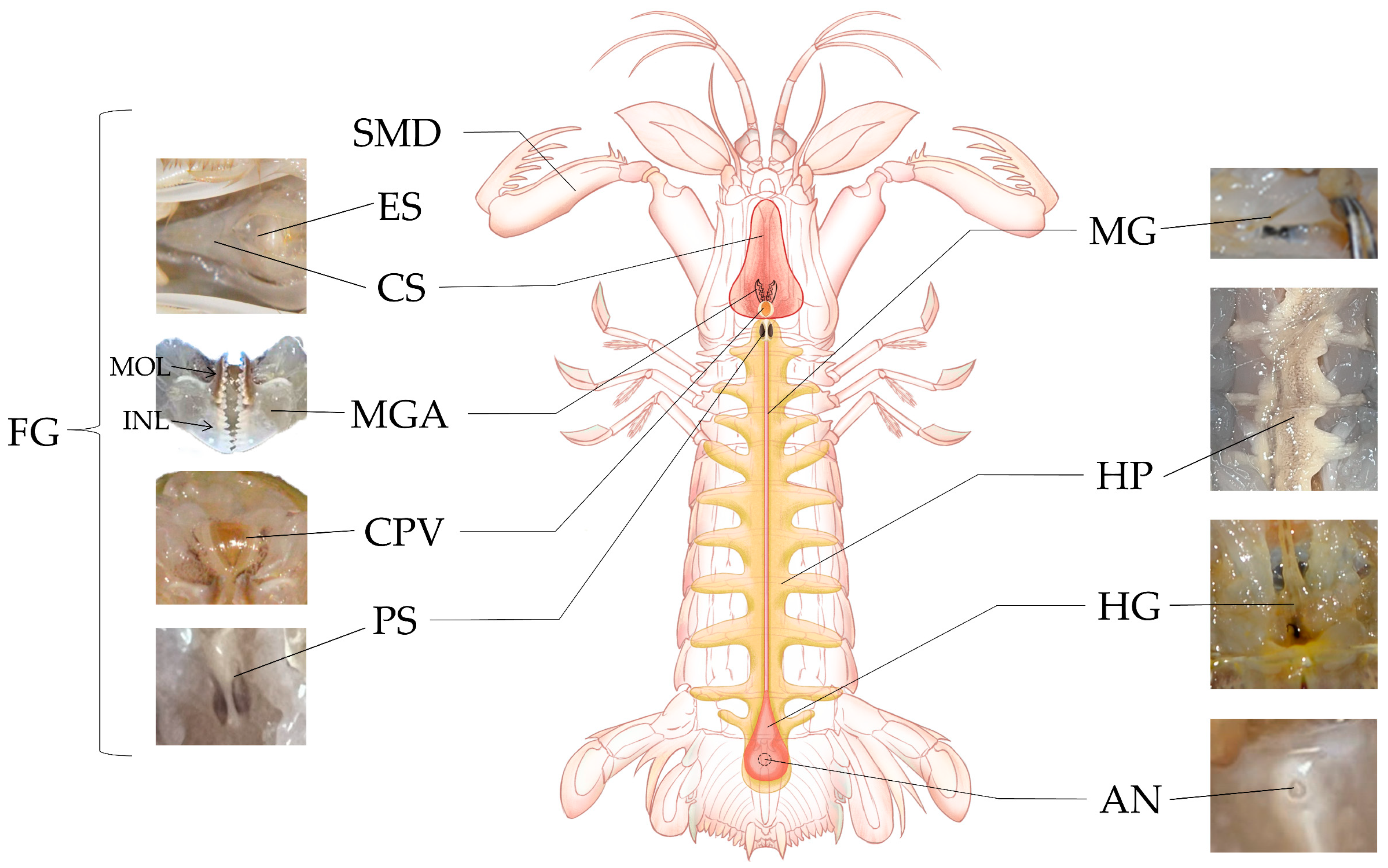
3.1. Composition of the Digestive System
3.2. Characteristics of Different Organs in the Digestive System
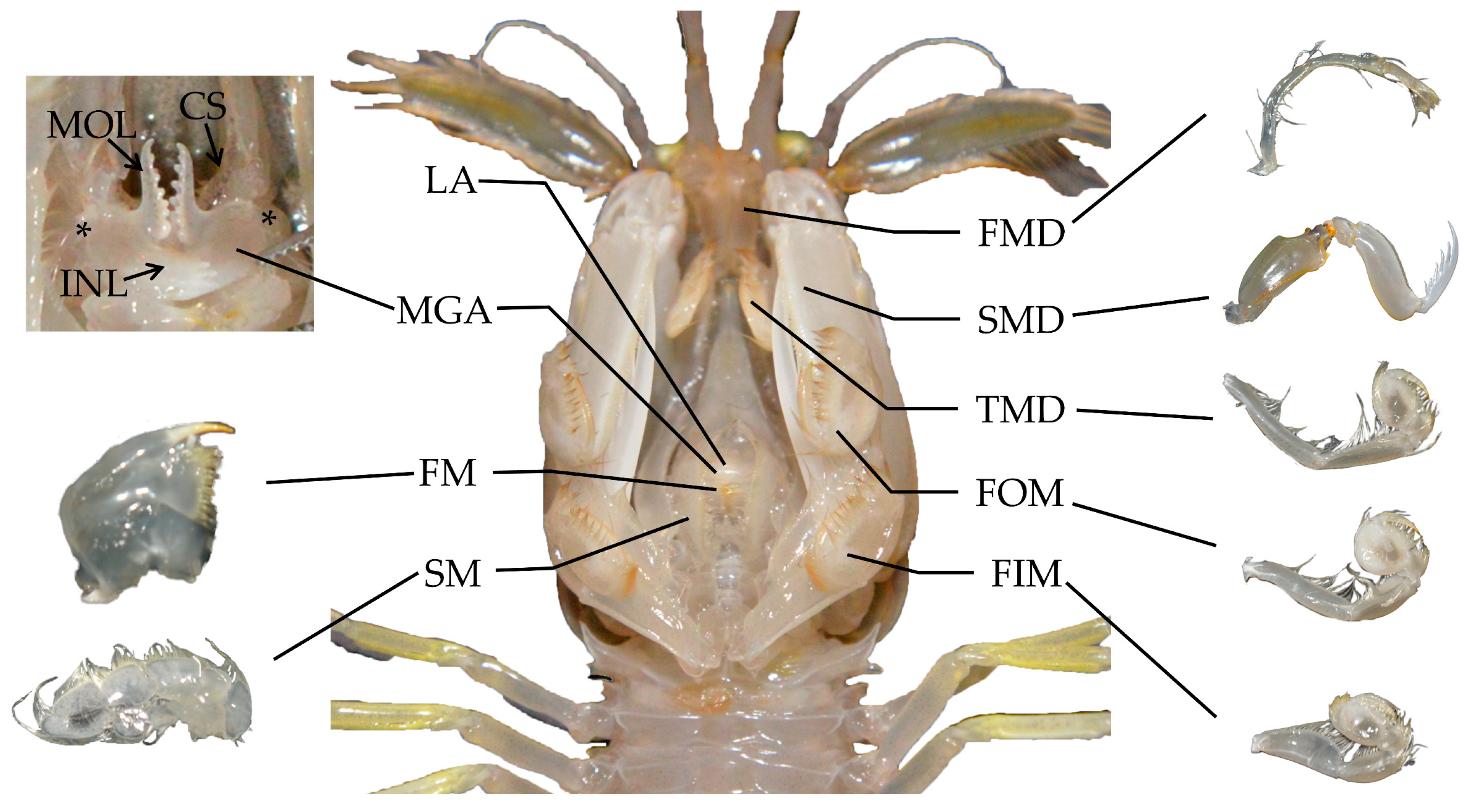
3.2.1. Mouthparts
3.2.2. Foregut
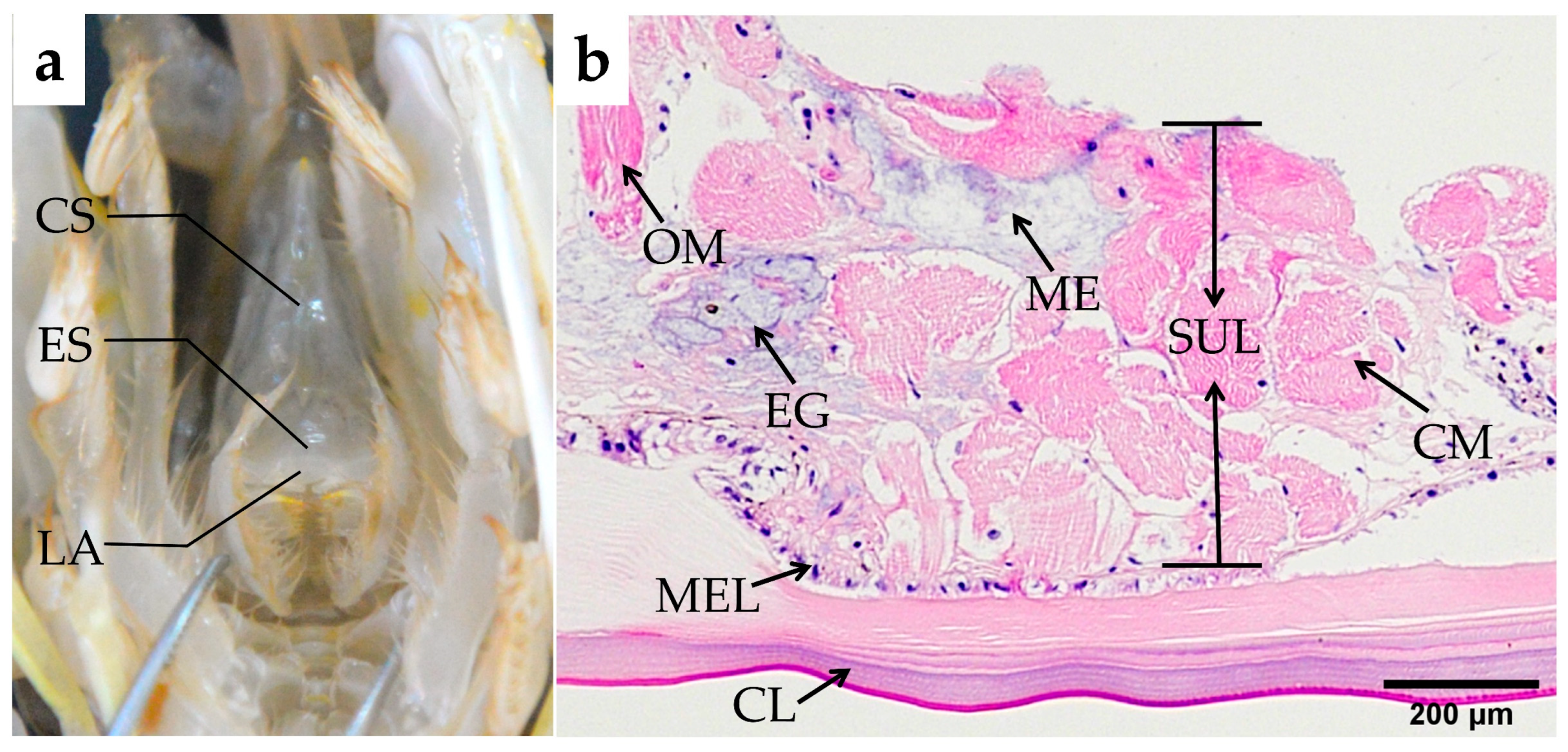
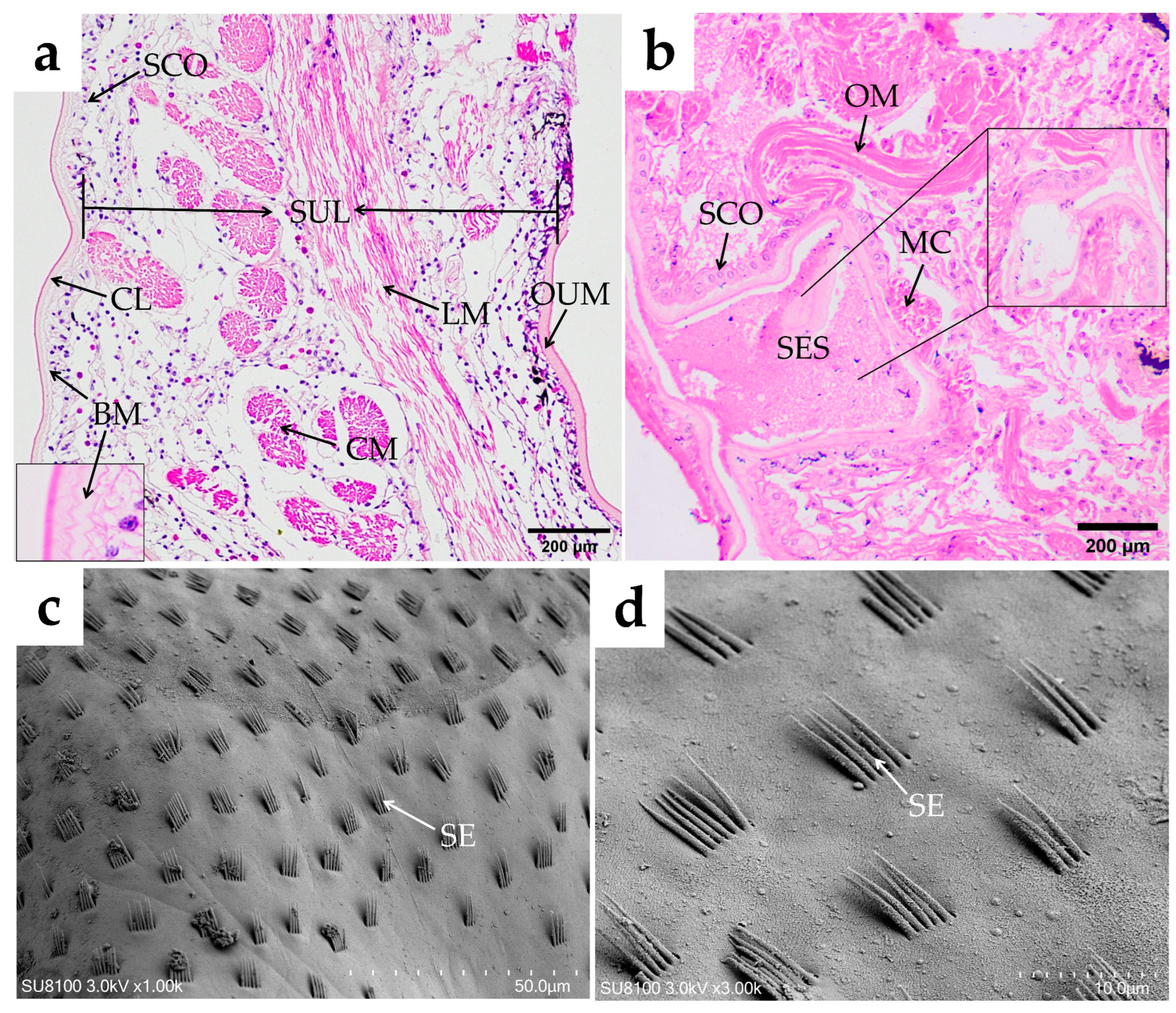
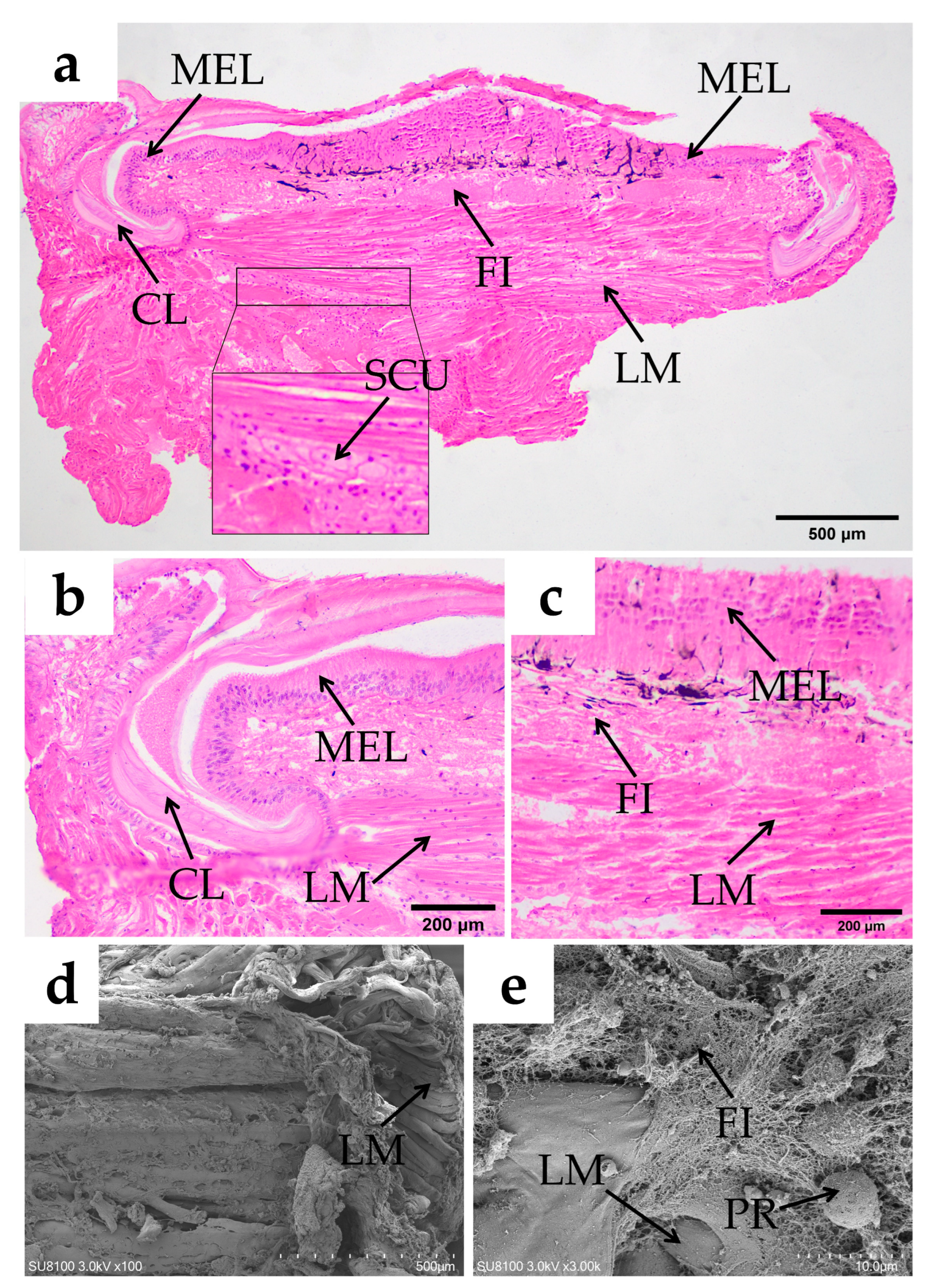
Esophagus
Cardiac Stomach
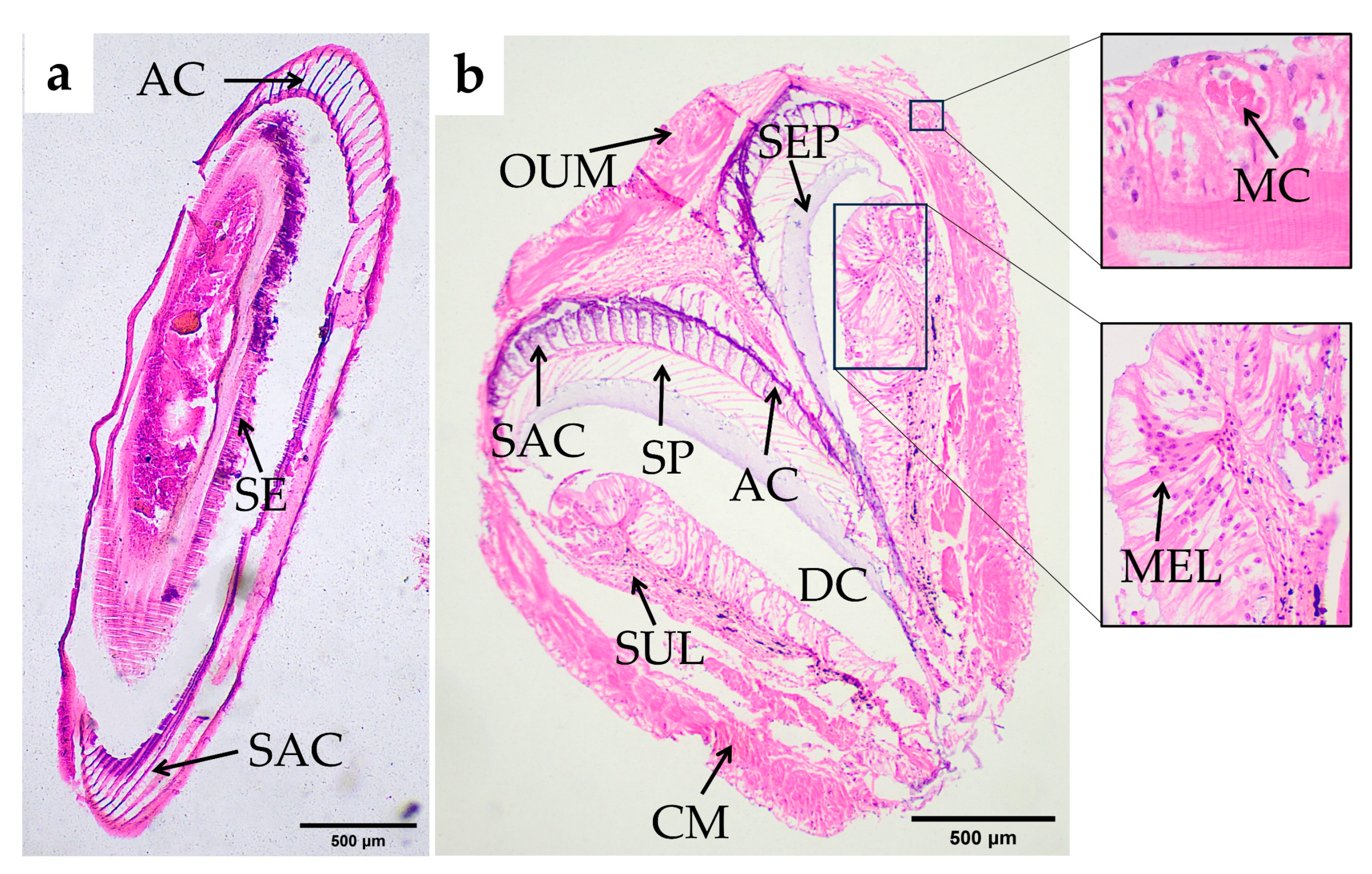
Pyloric Stomach
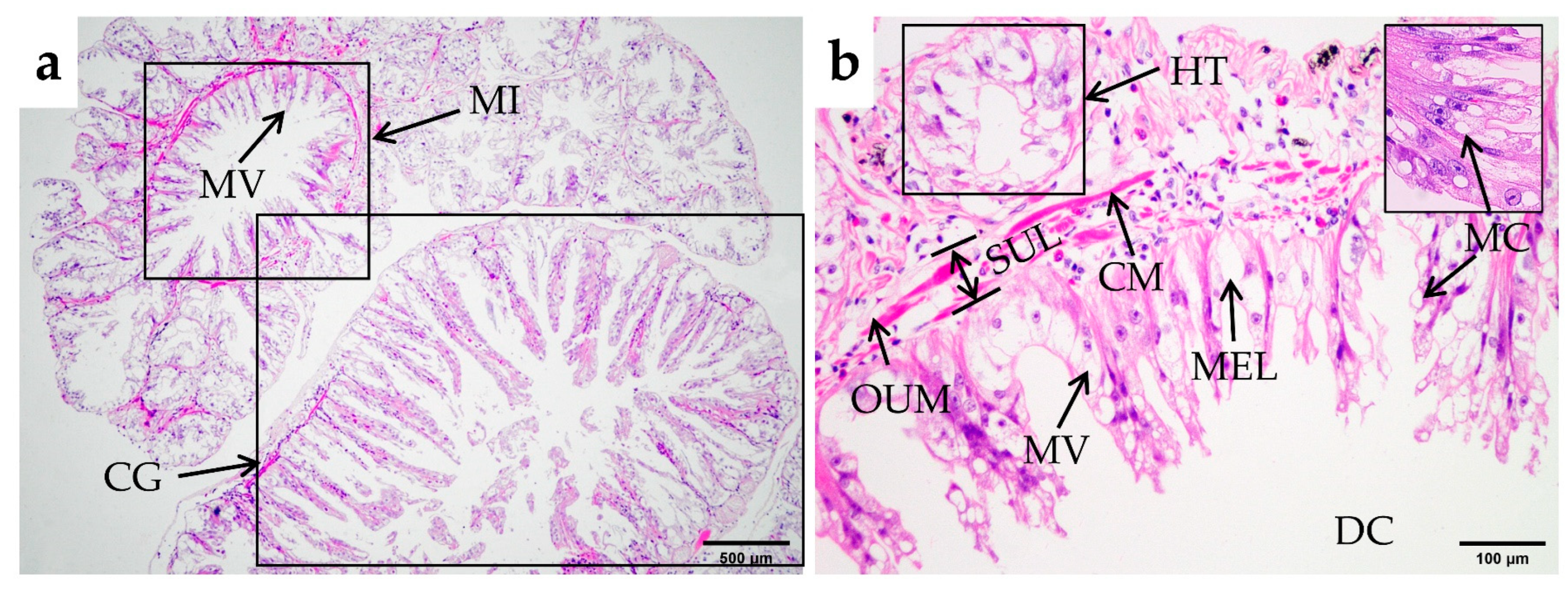
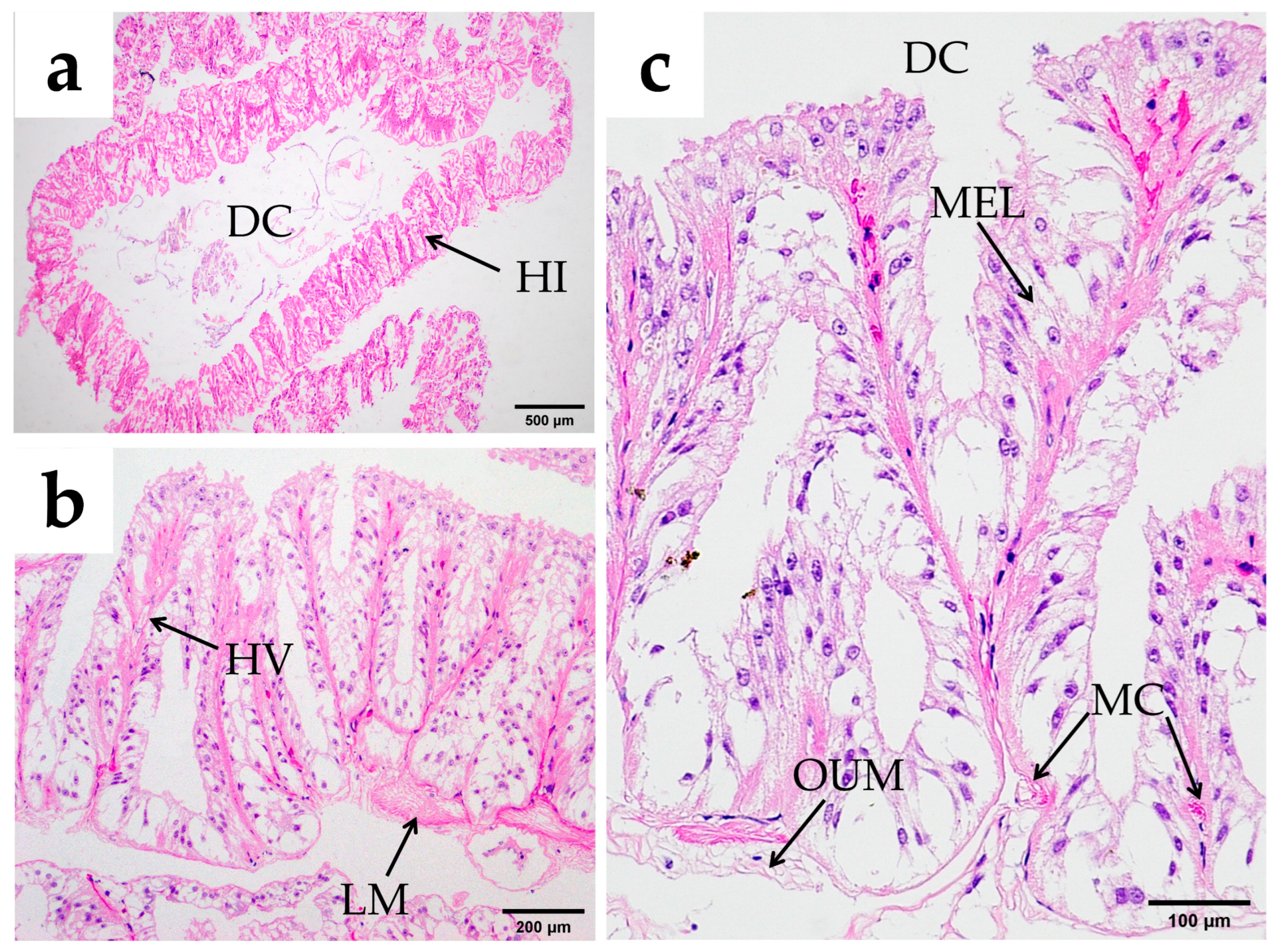
3.2.3. Midgut
3.2.4. Hindgut and Anus
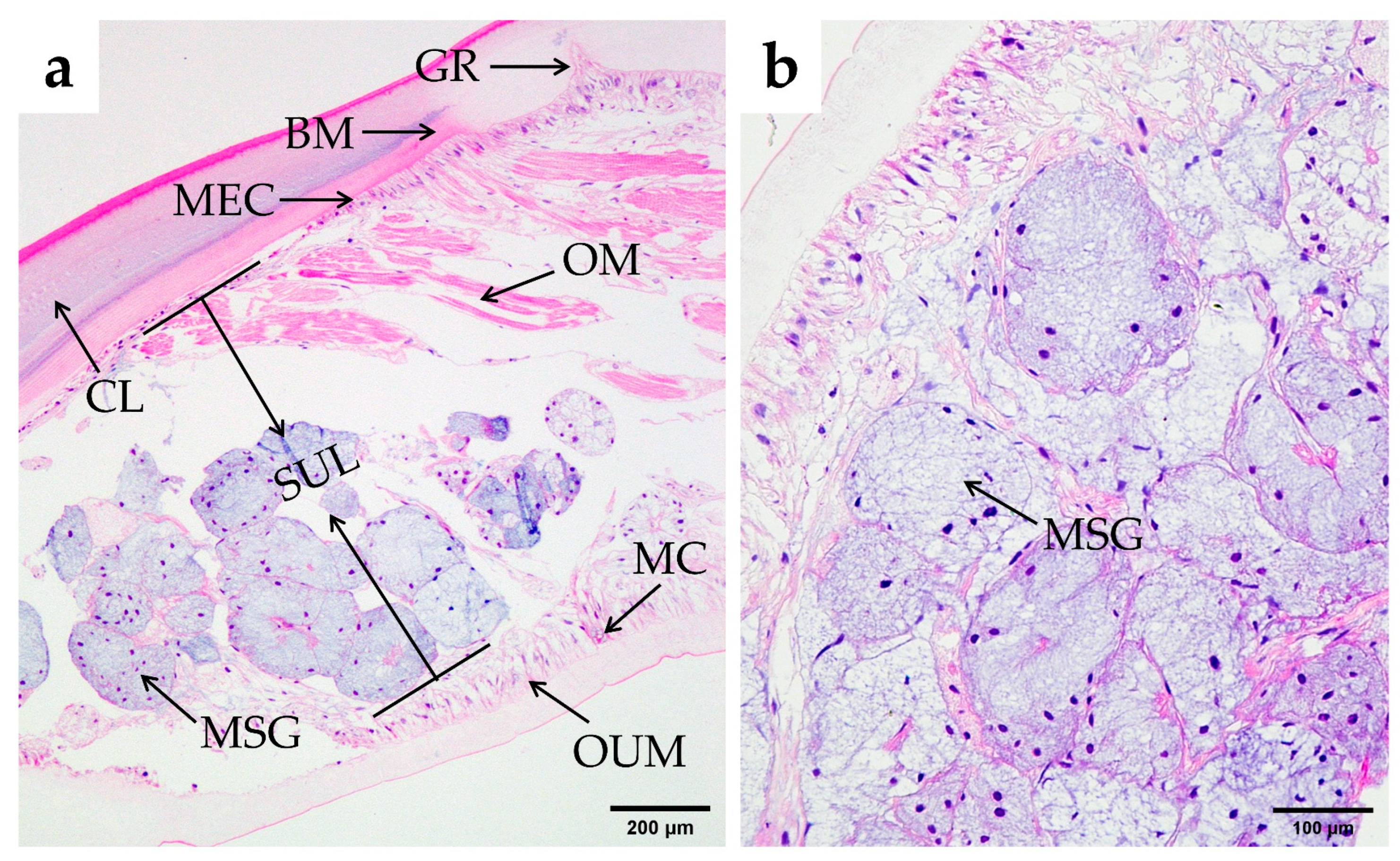
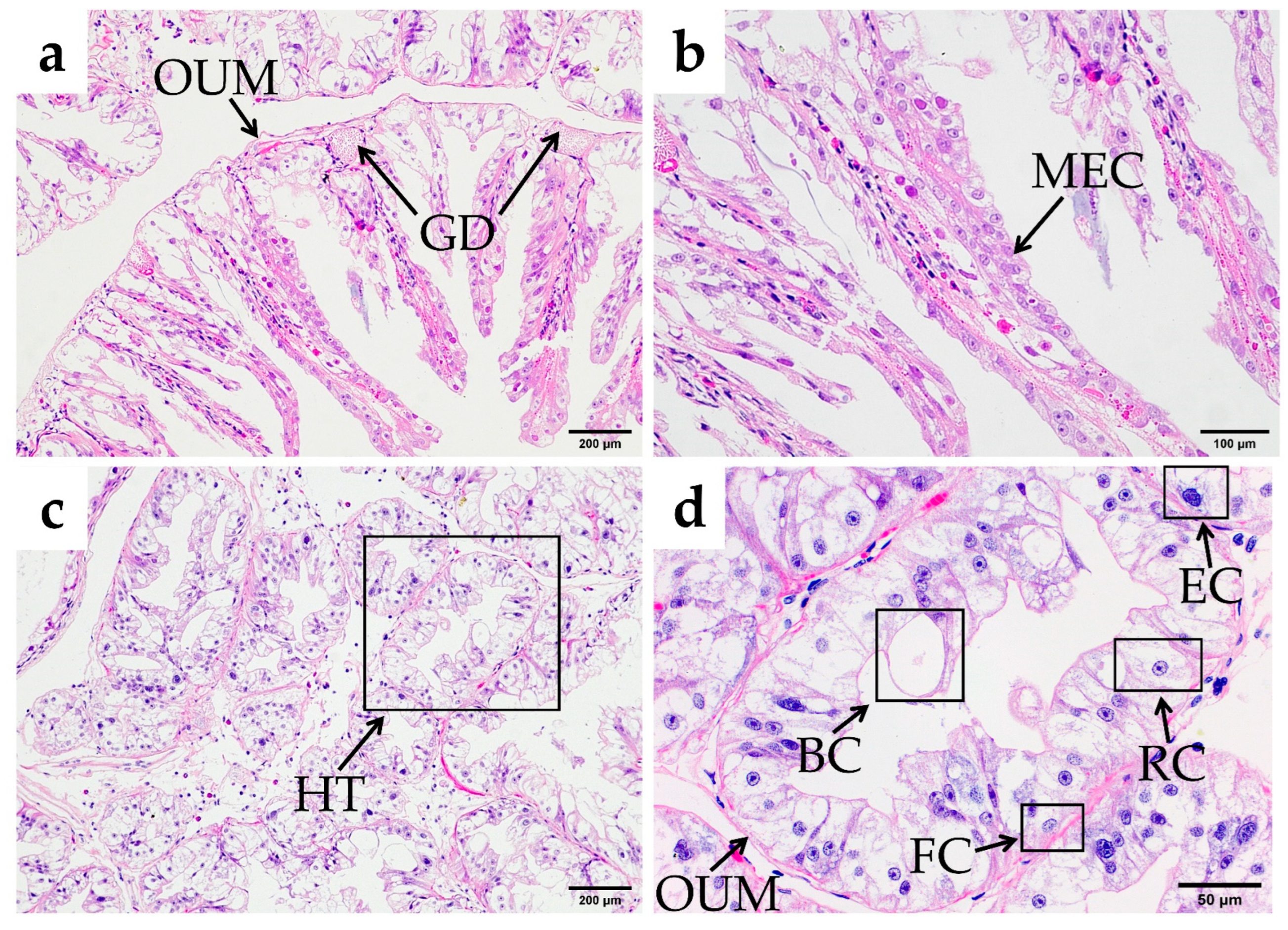
3.2.5. Hepatopancreas
3.2.6. Tissue Morphology Measurement and Analysis
4. Discussion
4.1. Structural and Functional Relationship of the Stomach in O. oratoria
4.2. Differences Between the Midgut and Hindgut of O. oratoria
4.3. Functions of the Hepatopancreas of O. oratoria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, C.; Zhong, L.S. Cryptic diversity in the Japanese mantis shrimp Oratosquilla oratoria (Crustacea: Squillidae): Allopatric diversification, secondary contact and hybridization. Sci. Rep. 2017, 7, 1972. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yu, H.; Yu, H.Q.; Xu, B.D.; Zhang, C.L.; Ren, Y.P.; Xue, Y.; Xu, L.L. Optimization of environmental variables in habitat suitability modeling for mantis shrimp Oratosquilla oratoria in the Haizhou Bay and adjacent waters. Acta Oceanol. Sin. 2020, 39, 36–47. [Google Scholar] [CrossRef]
- Lou, F.; Gao, T.; Han, Z. Transcriptome analyses reveal alterations in muscle metabolism, immune responses and reproductive behavior of Japanese mantis shrimp (Oratosquilla oratoria) at different cold temperature. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 32, 100615. [Google Scholar] [CrossRef]
- Li, F.; Cong, X.R.; Zhang, X.M. Niches of four large crustacean species in Laizhou Bay. J. Fish. China 2021, 45, 1384–1394. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, S.Y.; Liu, S.D.; Dong, X.Q. Morphological difference analysis on three different stocks of Oratosquilla oratoria in the Bohai Sea and the Yellow Sea. Mar. Fish. 2020, 42, 672–686. [Google Scholar] [CrossRef]
- Lou, F.R.; Gao, T.X.; Cai, S.S.; Han, Z.Q. De novo assembly and annotation of the whole transcriptome of Oratosquilla oratoria. Mar. Genom. 2018, 38, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Li, M.K.; Zhang, C.L.; Xu, B.D.; Xue, Y.; Ren, Y.P. A comparison of GAM and GWR in modelling spatial distribution of Japanese mantis shrimp (Oratosquilla oratoria) in coastal waters. Estuar. Coast. Shelf Sci. 2020, 244, 106928. [Google Scholar] [CrossRef]
- Yang, M.; Li, X. Population genetic structure of the mantis shrimp Oratosquilla oratoria (Crustacea: Squillidae) in the Yellow Sea and East China Sea. J. Ocean. Limnol. 2018, 36, 905–912. [Google Scholar] [CrossRef]
- Sheng, F.L.; Zeng, X.Q.; Xue, Y. Study on propagation and feeding habits of Oratosquilla oratoria in the inshore waters of Qingdao. Per. Ocean Univ. China 2009, 39, 326–332. [Google Scholar] [CrossRef]
- Ning, J.J.; Du, F.Y.; Wang, X.H.; Gu, Y.G.; Wang, L.G.; Li, Y.F. Feeding habits of mantis shrimp based on stable isotope analysis. J. Fish. China 2016, 40, 903–910. [Google Scholar] [CrossRef]
- deVries, M.S. The role of feeding morphology and competition in governing the diet breadth of sympatric stomatopod crustaceans. Biol. Lett. 2017, 13, 20170055. [Google Scholar] [CrossRef]
- Fang, Z.P.; Pan, Q.S.; Huang, F.J.; Luo, L.T.; He, J. Studies on histology and scanning electron microscopic of digestive tract of Chinese mitten-handed crab (Eriocheir sinensis). Acta Hydrobiol. Sin. 2002, 26, 136–141. [Google Scholar] [CrossRef]
- Dong, L.X.; Jiang, M.; Lu, X.; Ji, Z.H.; Wei, H.J.; Li, Q.; Sun, Y.H.; Chen, J.; Li, P.; Li, M.G.; et al. Morphological and histological observation of the digestive system in Ancherythroculter Nigrocauda. Freshwat. Fish. 2024, 54, 67–74. [Google Scholar] [CrossRef]
- Štrus, J.; Žnidaršič, N.; Mrak, P.; Bogataj, U.; Vogt, G. Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles. Cell Tissue Res. 2019, 377, 415–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Q.; Jiang, G.L.; Li, L.D. Study of the digestive system and physiology of decapoda: A review. Mar. Sci. 2004, 28, 50–54. [Google Scholar] [CrossRef]
- Cho, J.-H.; Park, J.W.; Ryu, Y.-W.; Kim, K.-W.; Hur, S.-W. Morphology, histology, and histochemistry of the digestive tract of the Marbled Flounder Pseudopleuronectes yokohamae. Animals 2023, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Maria, J.; Kendall, D.; Carlo, M.; Fabrizia, R. Diet and habitat as determinants of intestine length in fishes. Rev Fish Biol Fish. 2024, 34, 1017–1034. [Google Scholar] [CrossRef]
- Rei, S.; Toshikazu, S.; Akio, T. Mouthpart morphology and wild diet of zoeae of the Ghost Shrimp, Nihonotrypaea harmandi (Decapoda: Axiidea: Callianassidae). J. Crustac. Biol. 2014, 34, 300–308. [Google Scholar] [CrossRef]
- Kari, L.; Jan, R. Functional Morphology of the mouthparts of Juvenile Lobsters, Homarus Americanus (Decapoda: Nephropidae), and Comparison with the Larval Stages. J. Crustac. Biol. 1992, 12, 467–510. [Google Scholar] [CrossRef]
- Raouf, K.; Nesreen, K.I. Preliminary investigation of direct age determination using band counts in the gastric mill of the Blue Swimmer Crab (Portunus pelagicus) in two salt-water lakes in the eastern mediterranean. J. Crustac. Biol. 2016, 36, 119–128. [Google Scholar] [CrossRef]
- Hideki, C.; Yukishige, K. Fine Structure and mineralization of the gastric mill in the Crayfish Procambarus Clarkii during intermolt stage. J. Crust. Biol. 2003, 23, 371–379. [Google Scholar] [CrossRef]
- Michael, S.; Ian, O. Evidence of complete gastric mill ossicle loss at ecdysis in the European green crab Carcinus maenas (Linnaeus, 1758) (Decapoda: Brachyura: Carcinidae). J. Crust. Biol. 2018, 38, 435–442. [Google Scholar] [CrossRef]
- Kalacheva, N.V.; Ginanova, T.T.; Kamenev, Y.O.; Maslennikov, S.I.; Dolmatov, I.Y. Morphology and ultrastructure of digestive system in pre-zoea and zoea I larvae of red king crab, Paralithodes camtschaticus (Tilesius, 1815). Cell Tissue Res. 2024, 395, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Factor, J.R. Development and metamorphosis of the digestive system of larval lobsters, Homarus americanus (Decapoda: Nephropidae). J Morphol. 1981, 169, 225–242. [Google Scholar] [CrossRef]
- BO, Q.K.; Lu, Y.Z.; Ma, C.; Mi, H.J.; Jia, L.; Meng, Y.G.; Yu, Y.G.; Geng, X.Y. Reproductive biology and biochemical changes in female mantis shrimp Oratosquilla oratoria (Stomatopoda) with ovary development from the Tianjin coastal zone of Bohai Bay. Aquaculture 2020, 534, 736239. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Bae, H.; Kim, H.-G.; Oh, C.-W. Growth and reproduction of the Japanese mantis shrimp, Oratosquilla oratoria (De Haan 1844) in the coastal area of Tongyeong. Korea. Ocean Sci. J. 2017, 52, 257–265. [Google Scholar] [CrossRef]
- Lou, F.R.; Ren, Z.J.; Tang, Y.Z.; Han, Z.Q. Full-length transcriptome reveals the circularly polarized light response-related molecular genetic characteristics of Oratosquilla oratoria. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101183. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.Y.; Huang, Q.; Li, H.J.; Lou, F.R. Comparative transcriptomics revealed the ecological trap effect of linearly polarized light on Oratosquilla oratoria. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 50, 101234. [Google Scholar] [CrossRef]
- Reddy, A.R. The structure, mechanism and development of the gastric armature in stomatopoda with a discussion as to its evolution in Decapoda. Proc. Indian Acad. Sci. 1935, 1, 650–675. [Google Scholar] [CrossRef]
- An, J.Z.; Xu, H.L.; Wang, Y.H.; Tian, Y.G.; Zhang, S.L. Histological and morphological study on the pyloric stomach, intestine and midgut gland of Mantis shrimp (Oratosquilla oratoria). Hebei Fish. 2018, 8, 14–16. [Google Scholar] [CrossRef]
- Wang, C.L.; Xu, S.L.; Mei, W.X. A biological basic character of oratosquilla oratoria. J. Zhejiang Fish. Univ. 1996, 01, 60–62. [Google Scholar]
- Boudriot, F.; Reutter, K. Ultrastructure of the taste buds in the blind cave fish Astyanax jordani (“Anoptichthys”) and the sighted river fish Astyanax mexicanus (Teleostei, Characidae). J. Comp. Neurol. 2001, 434, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.P.C.; Pereira, R.T.; Rosa, P.V. Morphology of the digestive system in carnivorous freshwater dourado Salminus brasiliensis. J. Fish Biol. 2021, 99, 1222–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Zheng, J.S.; Wang, H.L. Advances in the digestive system research of Decapod, Crustacea. Per. Ocean Univ. China. 2000, 30, 115–121. Available online: https://qikan.cqvip.com/Qikan/Article/Detail?id=1001110083 (accessed on 15 October 2024).
- Rigdon, R.H.; Mensik, D.J. Gastrointestinal tract of Penaeus aztecus Ives, 1891 (Decapoda, Natantia), a histologic study. Crustaceana 1976, 30, 164–168. Available online: http://www.jstor.org/stable/20102307 (accessed on 20 October 2024).
- Schembri, P.J. Functional morphology of the mouthparts and associated structures of Pagurus rubricatus (Crustacea: Decapoda: Anomura) with special reference to feeding and grooming. Zoomorphology 1982, 101, 17–38. [Google Scholar] [CrossRef]
- Garm, A. Mechanical functions of setae from the mouth apparatus of seven species of decapod crustaceans. J. Morphol. 2004, 260, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Tang, D.; Guo, H.Y.; Shen, C.C.; Wu, L.; Luo, Y.Q. Evolution of digestive enzyme genes associated with dietary diversity of crabs. Genetica. 2020, 148, 87–99. [Google Scholar] [CrossRef]
- Vogt, G.; Štrus, J. Diseases of the shrimp Palaemon elegans (Crustacea: Decapoda) in the Bay of Piran, Adriatic Sea. Nat. Hist. 1998, 32, 1795–1806. [Google Scholar] [CrossRef]
- Günter, V. Synthesis of digestive enzymes, food processing, and nutrient absorption in decapod crustaceans: A comparison to the mammalian model of digestion. Zool 2021, 147, 125945. [Google Scholar] [CrossRef]
- Veróonica, A.; Gimenez, F. Digestive physiology of three species of decapod crustaceans of Argentina. J. Shellfish Res. 2013, 32, 767–777. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Yan, S.F. Histological and histochemical studies on the digestive tract of panulirus stimpsoni. J. Jimei Univ. (Nat. Sci.). 2009, 14, 217–223. [Google Scholar] [CrossRef]
- Patwardhan, S.S. On the structure and mechanism of the gastric mill in Decapoda. Proc. Indian Acad. Sci. 1935, 2, 155–174. [Google Scholar] [CrossRef]
- Huxley, T.H. The Crayfish: An Introduction to the Study of Zoology; Kegan Paul & Co.: London, UK, 1880; pp. 29–60. Available online: https://www.gutenberg.org/cache/epub/58924/pg58924-images.html (accessed on 3 November 2024).
- Krings, W.; Brütt, J.O.; Gorb, S.N. Mechanical properties, degree of sclerotisation and elemental composition of the gastric mill in the red swamp crayfish Procambarus clarkii (Decapoda, Crustacea). Sci. Rep. 2022, 12, 17799. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.A.; Collins, P.A.; Renata, L.-G.; Magalhães, C.; Torres, M.V.; Williner, V. A comparative study of the gastric ossicles of Trichodactylidae crabs (Brachyura: Decapoda) with comments on the role of diet and phylogeny in shaping morphological traits. Peer J. 2018, 6, e5028. [Google Scholar] [CrossRef] [PubMed]
- Castejón, D.; Rotllant, G.; Ribes, E.; Durfort, M.; Guerao, G. Foregut morphology and ontogeny of the spider crab Maja brachydactyla (Brachyura, Majoidea, Majidae). J. Morphol. 2015, 276, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- McGaw, I.J.; Curtis, D.L. A review of gastric processing in decapod crustaceans. J. Comp. Physiol. B 2013, 183, 443–465. [Google Scholar] [CrossRef]
- Li, C.L.; Cao, F.J.; Huang, X.H.; Liu, C.W. Histological observation on digestive system of Panulirus homaru with light and scanning electron microscopy. J. Trop. Oceanogr. 2008, 27, 72–78. [Google Scholar] [CrossRef]
- Hutchinson, E.; Matthews, R.T.; Ross, E.; Hagedorn, S.; Butler, M.J. Gastric mill ossicles record chronological age in the Caribbean spiny lobster (Panulirus argus). Fish. Res. 2024, 277, 107083. [Google Scholar] [CrossRef]
- Herrera-Álvarez, L.; Fernandez, I.; Benito, J.; Pardos, F. Ultrastructure of the midgut and hindgut of Derocheilocaris remanei (Crustacea, Mystacocarida). J. Morphol. 2000, 244, 177–189. [Google Scholar] [CrossRef]
- Chen, K.Z.; Bao, Y.; He, W.H. Studies on the anatomy and histology of the digestive system of the Penaeus orientalis (crustacea, decapoda). Per. Ocean Univ. China 1988, 18, 43–53. [Google Scholar] [CrossRef]
- Dall, W. The functional anatomy of the digestive tract of a shrimp Metapenaeus bennettae Racek & Dall (Crustacea: Decapoda: Penaeidae). Aust. J. Zool. 1967, 15, 699. [Google Scholar] [CrossRef]
- Ou, Y.S.; Wu, X.P.; Yan, X.H.; Luo, H.F.; Zhou, C.H. Histological studies of the digestive system of Cambrus clarkii (Crustacea). J. Nanchang Univ. Nat. Sci. 2002, 26, 92–95. [Google Scholar] [CrossRef]
- Liang, H.F.; Weng, S.P.; He, J.G. Study on Anatomy and histology of digestive system of Panulirus ornatus. J. Guangdong Ocean Univ. 2011, 31, 1–5. [Google Scholar] [CrossRef]
- Castejón, D.; Rotllant, G.; Ribes, E.; Guerao, G. Morphological description of the midgut tract and midgut-hindgut junction in the larvae of the spider crab Maja brachydactyla Balss, 1922 (Malacostraca: Decapoda). Arthropod Struct. Dev. 2022, 70, 101168. [Google Scholar] [CrossRef]
- Lobo-da-Cunha, A.; Alves, Â.; Oliveira, E.; Calado, G. Functional histology and ultrastructure of the digestive tract in two Species of Chitons (Mollusca, Polyplacophora). J. Mar. Sci. Eng. 2022, 10, 160. [Google Scholar] [CrossRef]
- Deyashi, M.; Chakraborty, S.B. Histomorphology and ultrastructure of the hepatopancreas of a brachyuran crab, Varuna litterata (Fabricius, 1798). Zoomorphology 2024, 143, 615–629. [Google Scholar] [CrossRef]
- Stumpp, M.; Saborowski, R.; Jungblut, S.; Liu, H.C.; Wilhelm, H. Dietary preferences of brachyuran crabs from Taiwan for marine or terrestrial food sources: Evidence based on fatty acid trophic markers. Front. Zool. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.C.; Liu, W.B.; Liu, J.D.; Zhang, C.Y.; Zhang, L.; Gao, F.; Zhang, D.D.; Chi, C. Dietary supplementation with icariin affects estrogen synthesis, vitellogenesis, and oocyte development in the Chinese Mitten Crab, Eriocheir sinensis. Front. Mar. Sci. 2020, 7, 161. [Google Scholar] [CrossRef]
- Hopkin, S.P.; Nott, J.A. Some observations on concentrically structured, intracellular granules in the hepatopancreas of the shore crab Carcinus maenas (L.). J. Mar. Biol. Ass. 1979, 59, 867–877. [Google Scholar] [CrossRef]
- Vogt, G.; Stöcker, W.; Storch, V.; Zwilling, R. Biosynthesis of Astacus protease, a digestive enzyme from crayfish. Histochemistry. 1989, 91, 373–381. [Google Scholar] [CrossRef]
- Vannier, J.; Liu, J.; Lerosey-Aubril, R.; Vinther, J.; Daley, A.C. Sophisticated digestive systems in early arthropods. Nat. Commun. 2014, 5, 3641. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.M.; Xuan, R.J.; Li, Y.J.; Zou, E.M.; Wang, L. Morphological observation and histological studies on the digestive system of Sinopotamon henanense. J. Fish China 2014, 38, 956–964. [Google Scholar] [CrossRef]
- Qiu, G.F. Ultrastructural studies on the cells of midgut gland in the Macrobrachium nipponense. J. Fish. China 1997, 21, 233–239. [Google Scholar]



| Index | Parameters |
|---|---|
| Midgut length (mm) | 66.28 ± 7.73 |
| Hindgut length (mm) | 12.88 ± 2.23 |
| Hepatopancreas weight (g) | 0.74 ± 0.33 |
| MGP (%) | 59.39 ± 3.36 |
| ILI (%) | 70.82 ± 3.21 |
| HSI (%) | 3.83 ± 0.56 |
| Structures | Labrum | Esophagus | Cardiac Stomach | Cardio-Pyloric Valve | Pyloric Stomach | Midgut | Hindgut |
|---|---|---|---|---|---|---|---|
| Chitin thick | 155.70 ± 20.42 b | 70.4 ± 2.71 d | 15.53 ± 1.13 e | 84.06 ± 6.18 c | 359.43 ± 15.75 a | - | - |
| Mucosal epithelial layer or Villus height | 43.41 ± 5.92 d | 60.5 ± 8.94 cd | 60.26 ± 5.27 cd | 84.83 ± 9.25 c | 200.39 ± 29.08 b | 217.41 ± 42.64 b | 695.96 ± 116.71 a |
| Submucosal layer thick | 920.72 ± 10.37 b | 616.78 ± 40.72 c | 1091.13 ± 58.33 a | 189.62 ± 8.97 d | 117.01 ± 8.21 e | 87.55 ± 7.75 f | 19.24 ± 3.63 g |
| Longitudinal muscle thick | - | - | 250.81 ± 8.65 b | 416.1 ± 21.4 a | - | - | 74.58 ± 16.04 c |
| Circular muscle thick | 58.46 ± 12.68 c | 166.48 ± 41.08 a | 107.45 ± 19.91 b | - | 115.48 ± 10.08 b | 54.70 ± 4.44 c | - |
| Outer membrane thick | 170.07 ± 3.69 a | 39.57 ± 1.21 d | 56.19 ± 3.27 c | 21.41 ± 3.18 e | 70.48 ± 9.15 b | 19.74 ± 0.75 e | 9.35 ± 2.83 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Lou, F.; Yang, P.; Qiu, S.; Wang, L. Morphology and Histology of the Digestive System of Japanese Mantis Shrimp (Oratosquilla oratoria). Fishes 2025, 10, 71. https://doi.org/10.3390/fishes10020071
Wang R, Lou F, Yang P, Qiu S, Wang L. Morphology and Histology of the Digestive System of Japanese Mantis Shrimp (Oratosquilla oratoria). Fishes. 2025; 10(2):71. https://doi.org/10.3390/fishes10020071
Chicago/Turabian StyleWang, Ran, Fangrui Lou, Pei Yang, Shengyao Qiu, and Lei Wang. 2025. "Morphology and Histology of the Digestive System of Japanese Mantis Shrimp (Oratosquilla oratoria)" Fishes 10, no. 2: 71. https://doi.org/10.3390/fishes10020071
APA StyleWang, R., Lou, F., Yang, P., Qiu, S., & Wang, L. (2025). Morphology and Histology of the Digestive System of Japanese Mantis Shrimp (Oratosquilla oratoria). Fishes, 10(2), 71. https://doi.org/10.3390/fishes10020071






