Abstract
The clustered regularly interspaced short palindromic repeat-associated protein (CRISPR-Cas) system is considered a potential game-changer in the aquaculture sector. The CRISPR-Cas9 system is derived from an adaptive mechanism of the immune system of some prokaryotes. The CRISPR/Cas9 system potentially accelerates the rate of sustained genetic gain for the aquaculture and seafood production sectors. Unlike conventional genome-editing techniques, CRISPR/Cas9 is more cost-effective, user-friendly, and extremely precise. It enables overcoming large-scale challenges in aquaculture. Traits such as high fertility, external fertilization, shorter generation time, well-established breeding methods, and the ability to raise larvae offer potential benefits for applying CRISPR/Cas9 genome editing in most aquacultural species. The use of genome editing accelerates precise breeding where desired modifications are made to the target gene. There is a high likelihood that the intended alterations will be achieved, resulting in the transmission of the desired trait to the next generation. In this paper, we review how the CRISPR system evolved, its basic categories and different Cas systems, as well as the molecular mechanism of CRISPR/Cas. We also highlight and discuss the potential applications of CRISPR/Cas in the aquaculture industry. Moreover, the challenges of using CRISPR/Cas technology are briefly discussed. This pathway charts a course to a future in which genome editing has the potential to enable aquaculture to fulfill world food requirements with public and ecological safety.
Key Contribution:
This paper provides an overview of CRISPR/Cas9 applications in aquaculture, highlighting their potential for transformative genetic enhancements while addressing challenges and concerns regarding public acceptance.
1. Introduction
Aquaculture is one of the leading industries in the global food supply, addressing world food security concerns resulting from global climate change, population growth, and resource scarcity [1]. By 2050, the world population is expected to reach 9.7 billion, necessitating an increase of 25% to 70% in food production. To overcome these obstacles, it is essential to provide rich and healthy diets, which is where aquaculture plays a role [2]. In 2018, global aquaculture contributed 46 percent of the overall world production of fish as well as 52 percent of human fishery product consumption [3]. The sustainable development and continued growth of the aquaculture sector rely on high-protein feeds in order to cover the requirements of an increasing world population [4,5]. Aquaculture is an important source of animal protein for human consumption [6]. The high-quality, easy-to-digest protein of fishery products containing high levels of essential amino acids (EAAs) is regarded as superior to other sources of protein such as bovine, dairy, and egg products. It is the principal source of essential fatty acids (EFAs) for a healthy human diet, including n-3 polyunsaturated fatty acids (PUFAs), namely docosahexaenoic acid (DHA; 22:6n-3) and eicosapentaenoic acid (EPA; 20:5n-3). In addition, aquacultural products also contain a wide range of vitamins, notably vitamin A, and minerals not readily available from many land-based sources [7].
Genome editing provides new opportunities, and its applications in the aquaculture industry may be a clever tool to achieve maximum efficiency and allow more cost-effective availability of nutrient-rich food worldwide [8]. Initially, zinc finger nucleases (ZFNs) and transcription activator-like endonuclease (TALEN) technologies predominated in genome engineering. Currently, the most advanced genome-engineering technique, the clustered regularly interspaced short palindromic repeats (CRISPRs) system, has become the most widely used worldwide [9,10] and is significantly more cost-effective, user-friendly, and efficient for site-specific genome editing than ZFNs and TALENs. The CRISPR/Cas approach has been used in various livestock and fish species, with no sign of a slowdown in progress [11].
The CRISPR-Cas system is an acquired immune system found in many archaea and bacteria, ensuring sequence-specific defense mechanisms to protect against detectable foreign nucleic acids. The CRISPR loci consist of the CRISPR assembly, composed of short repeats directly interspersed with short variable DNA segments (known as spacers), flanked by various Cas genes [12]. There is more than one type of CRISPR/Cas system, including CRISPR/Cas9, CRISPR/Cas12, and CRISPR/Cas13 [12]. Nevertheless, due to its notably high efficacy and accuracy, CRISPR/Cas9 is the most widely used in several scientific fields [13].
CRISPR/Cas9 enables precise genome editing to improve crucial traits, including sex determination and growth rates (including length, weight gain, and the development of muscle fibers) [14,15,16]. Furthermore, it is a powerful approach to improving fish disease resistance by addressing pathogen recognition traits or immune-associated genes, thus avoiding over-dependence on chemical therapeutics and antibiotics [17,18,19]. This technique may radically transform modern aquaculture by improving essential traits in many fish species genetically. For instance, it successfully removed the germ cells involved in sexual differentiation from reproductive cells in Atlantic salmon (Salmo salar) [20,21], enhanced the feed conversion ratio to boost growth rates in yellow catfish (Pelteobagrus fulvidraco) [22], and achieved effective genetic mutations in Nile tilapia (Oreochromis niloticus), reducing the occurrence of undesirable effects [23].
The protection of the environment is crucial to the sustainable development of the aquaculture sector. CRISPR/Cas9 technology is not limited to improving the desired characteristics of fish but also considers environmental sustainability and the interests of consumers. Some researchers have altered fish to minimize the amount of nitrogen-rich waste they produce, thereby reducing the environmental effects of aquaculture. Moreover, they have enhanced the fish’s nutritional composition, improving n-3 PUFA levels and minimizing undesirable components. The fads2 gene for fatty acid desaturation in rainbow trout (Oncorhynchus mykiss) was modified to optimize the n-3 PUFA synthesis [4]. Nevertheless, public and consumer acceptance of this technology is hampered by various factors, including negative public perception and possible ecological effects [24], which have generated ethical concerns regarding genome editing in animals, especially using gene knock-in and knockout techniques. Commercializing CRISPR/Cas9-engineered products requires risk assessment and consumer approval. Regulatory approval and consumer trust in genetically modified fish are affected by environmental considerations, safety, and health credibility [25]. To earn regulatory approval and consumer confidence, it is essential to provide comprehensive clarification on how fish traits are modified, meet regulatory guidelines, and provide supporting data on genetically modified fish to address these concerns [4]. The CRISPR/Cas9 system still lacks comprehensive studies on its various implementations and the limitations it presents in editing major desired traits in different fish species. Previous reviews have mainly focused on describing the principles of this technique, highlighting its benefits and applications to improve economically important traits [26]. Hence, the purpose of this review is to discuss and review research advancements aimed at enhancing various target traits in aquaculture, including growth, disease resistance, reproductive confinement, omega-3 fatty acid metabolism, and pigmentation by using CRISPR/Cas9 technology.
2. History
In 1987, Ishino et al. [27] made a remarkable discovery in the genome of Escherichia coli, revealing a series of repetitive elements that they called “tandemly interspaced repeats” by using traditional Sanger sequencing. Thereafter, in 1993 and 1995 Mojica et al. [28,29] discovered them within two species of Archaea. The importance of these new sequences was obscure until then. In 1993, Mojica [29] was working on Haloferax mediterranei, a highly saline water-resistant archaeal species. He uncovered 30 base-pair (bp) clustered repeats in the DNA sequence of this microorganism, which intrigued him, as they had never been detected in any other microbes. These sequences consisted of inverted short palindromic repetitive repeats. He performed a literature search and came across the Ishino et al. [27] report of identical clustered repetitions found in another microbe, a gram-negative bacterium. His findings in various organisms confirmed that these clustered DNA repeats were retained and must have a significant role to play in microbes, which do not have a large genome. He pursued studies of diverse microorganisms to unravel the meaning of these repetitive clusters. Regularly spaced clustered palindromic repeats were originally named tandem repeats and short regularly spaced repeats. The bacterial sequence was first discovered as regularly interspaced CRISPR/Cas9 in 2002. Whole-genome sequencing technology has been applied to investigate CRISPR, showing that this structure is widely found in plasmid and viral systems and may encode a bacterial immune system. Then, the biochemical and genetic studies that followed supported the hypothesis, revealing that the CRISPR/Cas9 system is implicated in the identification and shielding of mobile genetic elements. Thereafter, in 2005, three different groups of researchers detected the presence of similarities between various types of DNA, including viral DNA, and CRISPR spacers [30,31]. Subsequently, Makarova et al. [32] hypothesized that the CRISPR/Cas system presented a possible adaptive defense mechanism for the immune system in bacteria. In 2007, Barrangou et al. [33], in their study of Streptococcus thermophilus, demonstrated experimentally that CRISPR has a role in the bacterial immune system. Following that, in 2008, Broun’s laboratory proved the antiviral role of CRISPR spacer sequences in host cells in association with Cas proteins. The RNA-guided Cas proteins mainly target specific DNA sequences and block horizontal transfer, sometimes also affecting RNA [34,35]. Prokaryotic cells can thereby react swiftly and efficiently to the foreign DNA threat and guard themselves against infection, thanks to the CRISPR/Cas system’s recognition and memory mechanisms.
This innate immune system presented a source of inspiration for scientists interested in applying CRISPR as a genome-editing tool [36]. In 2012, Jinek and Gasiunas, members of two teams of researchers [3,10] in independent laboratories succeeded in recreating the CRISPR/Cas system in vitro, using only Cas9 and a small artificially designed guide RNA (gRNA) to cut DNA in bacteria, thereby creating groundbreaking genome-editing technology commonly identified as CRISPR/Cas9. This versatile technology has been adopted by scientists from different scientific domains as a targeting tool since 2013. Cong et al. [36] reported the first use of the CRISPR/Cas9 technology in mammalian cell editing through the successful application of CRISPR elements from S. thermophilus and S. pyogenes. In addition, it was shown that CRISPR could be used in the manipulation of mammalian genetic material in another study [37].
3. Classification of CRISPR/Cas Systems
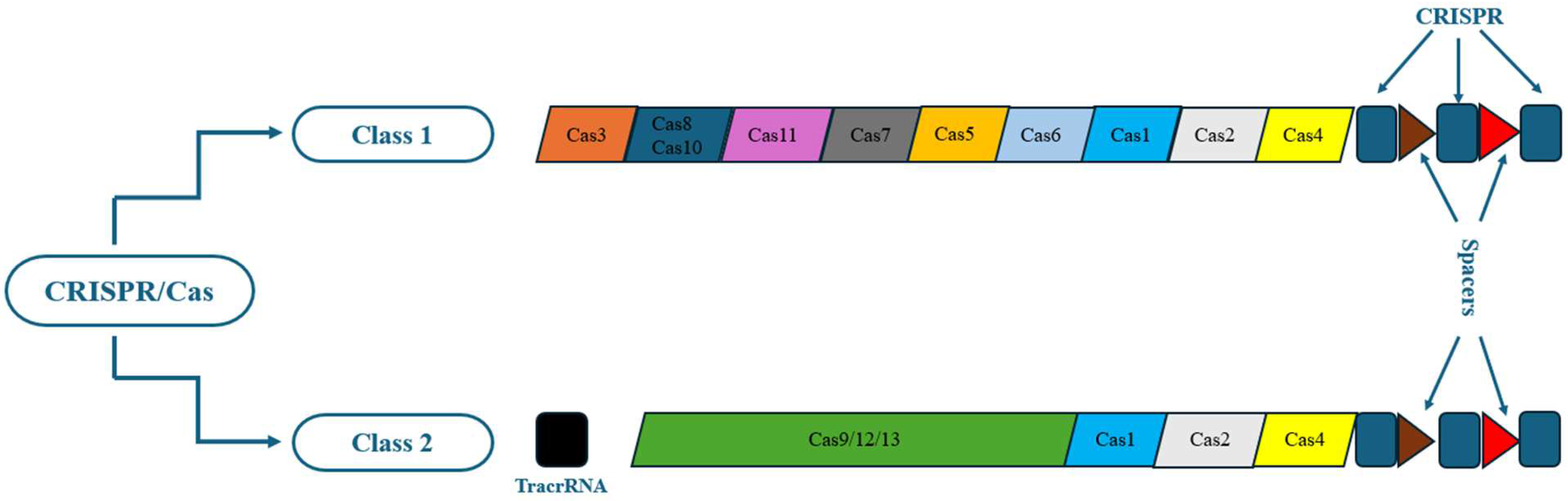
According to the function and structure of the Cas proteins, there are two main categories of CRISPR/Cas systems (class I and class II), which are in turn further subdivided into six types (types I–VI). Class I includes three types: I, III, and IV, while class II consists of types II, V, and VI [38]. The class I members use multiple subunit Cas protein complexes, unlike the class II members, which employ single-subunit Cas protein complexes. Class I emerges in bacteria and archaea, such as hyperthermophiles, whose elements comprise 90% of all currently recognized CRISPR-Cas loci [38].
In comparison, class II consists of 10% of all detected CRISPR-Cas loci and exists almost predominantly in bacteria [38]. Types I and III have not been applied to gene editing due to their complexity. Types IV–VI have been recognized recently. The most widely utilized CRISPR/Cas9 genetic engineering technology is the CRISPR type II system. The structure of CRISPR/Cas9 type II is comparatively user-friendly and has been extensively examined [39,40] (Figure 1).
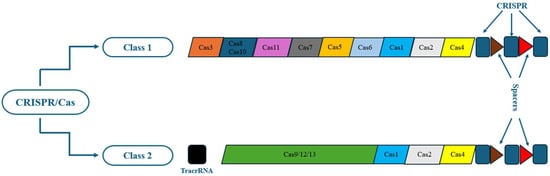
Figure 1.
Classes and types of CRISPR/Cas systems.
4. Mechanism of Action
The CRISPR/Cas9 system includes the gRNA and the Cas9 endonuclease, the two fundamental elements of CRISPR/Cas9 technology [41]. The Cas9 protein, which was the first Cas protein utilized in genetic modification, was isolated from S. pyogenes [42]. This is a large multi-subunit endonuclease with 1368 amino acids that break up the targeted DNA to produce a double-strand cleavage and is commonly described as the “genetic scissor” [43]. Cas9 is made up of two parts, known as the recognition (REC) lobe and the nuclease (NUC) lobe [44]. The REC lobe comprises the REC1 and REC2 regions, which are involved in binding to the guide RNA, while the NUC lobe consists of the RuvC, HNH, and Protospacer Adjacent Motif (PAM) regions. The RuvC and HNH regions are responsible for cleaving single strands of DNA, whereas the PAM targeting site contributes to the specificity of the PAM and triggers binding to target DNA [45]. The gRNA consists of two RNA molecules: the CRISPR RNA (crRNA) and the trans-activating CRISPR RNA (tracrRNA) [46]. The crRNA consists of a 20-nucleotide (nt) sequence that is found to be complementary to the target DNA sequence, whereas tracrRNA is a long-looped sequence that acts as a linking scaffold around the Cas9 endonuclease [47]. The gRNA in prokaryotes is typically utilized for targeting viral DNA, but in genetic modification technology, it may be synthesized using a combined crRNA and tracrRNA to generate a single gRNA to be used for targeting practically any DNA sequence that is intended to be genetically edited [46].
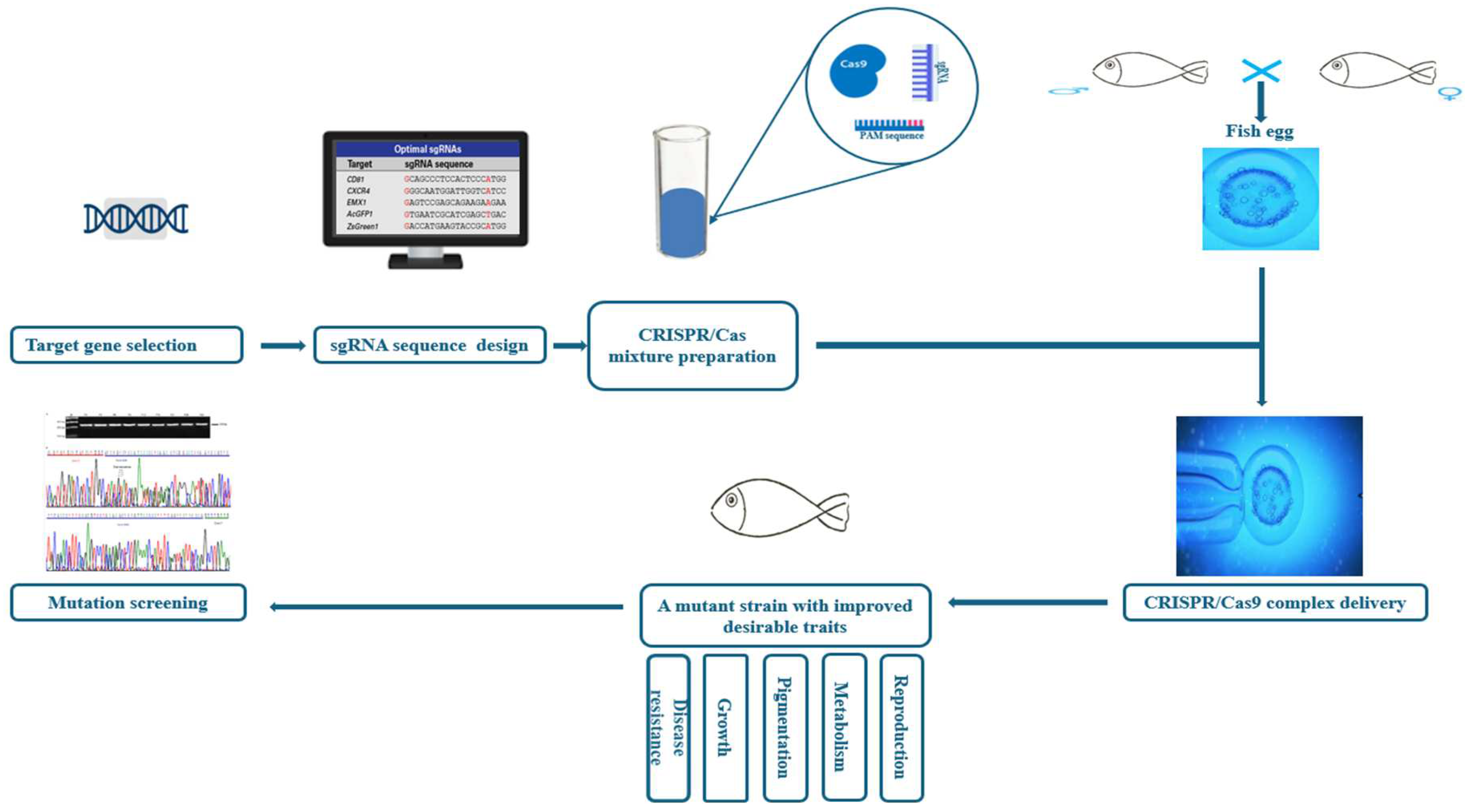
The CRISPR/Cas9 system operates through three phases: adaptation, expression, and interference [48]. Generally, in the adaptation stage, a complex of Cas proteins encounters an invasive foreign DNA (approximately 30 bp), binds to it, and triggers two DNA double-strand breaks. This sequence is called the short PAM. Intertwined between two repeats of the CRISPR system, the short DNA segment liberated from invading phages or plasmids is called a protospacer and serves as a spacer [49]. The CRISPR array is expressed and transcribed during the expression phase to form a long CRISPR precursor RNA (pre-cRNA). This pre-cRNA is then processed with Cas proteins and helper factors into a mature short gRNA [50]. During the interference stage, host cells are defended against infection through the combined mechanism of crRNA and Cas proteins, which recognize the foreign nucleic acid and ensure its cleavage [51]. The endonuclease most commonly applied in genome-editing technology, the Cas9 protein, recognizes the PAM sequences (NGG) [52]. Once it has identified a desired target region that has the appropriate PAM, Cas9 triggers DNA cleavage locally, thus creating an RNA–DNA hybrid [50]. Subsequently, the Cas9 protein cleaves the DNA, resulting in a double-strand break. Eventually, the double-strand break is repaired through either homology-directed repair or non-homologous end-joining pathways by the host repair machinery (Figure 2) [53].
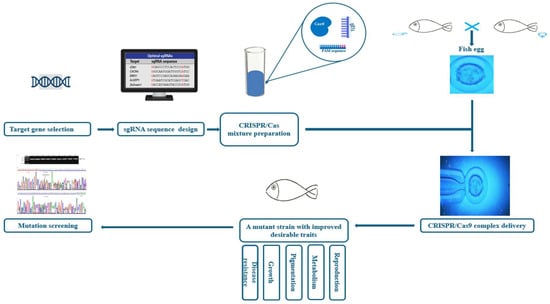
Figure 2.
The target traits and steps of CRISPR/Cas9 applications in aquaculture.
5. Applications of CRISPR/Cas in the Aquaculture Industry
The emergence of these more productive and cost-effective genome-editing (GE) technologies, particularly CRISPR/Cas, has prompted scientists to suggest GE as a potentially promising solution to various recent concerns in the aquaculture industry (Table 1, Figure 2). According to the literature, the first application of CRISPR/Cas9 on aquaculture species was performed by Li and colleagues on Nile tilapia (Oreochromis niloticus), making it the first aquaculture species genetically edited by CRISPR/Cas9 [54].

Table 1.
CRISPR/Cas9 system applications in aquaculture.
5.1. Disease Resistance
Disease resistance is an increasingly crucial issue for the aquaculture industry, and genetic enhancement of disease resistance represents a primary breeding goal. Gene editing experiments to enhance immunity and disease resistance have been carried out [11]. To reduce threats to aquatic animals and address viral infections and invasive pathogens, CRISPR/Cas9 gene-editing technology offers promising opportunities. In this context, many studies have been conducted to generate stocks more resistant to the threat of different infections [19]. For instance, it has been reported that CRISPR/Cas9-assisted genome editing and integrated transgenesis of vector-modified antimicrobial peptide (AMP) genes effectively modify the innate immune system of wild fish [78]. In a further trial, CRISPR/Cas9-assisted microinjection technology has been employed to incorporate the AMP genes cecropin (cec) and cathelicidin (cath) into the channel catfish (Ictalurus punctatus) genome, generating double AMP-integrated embryos (*_cec+/*_cath+) displaying a high degree of effectiveness. After bacterial infection, both single-AMP and double-AMP-integrated stocks exhibited considerably enhanced survival performance compared to wild-type stocks. The results of statistical regression showed that weight and sex had no impact on survival, whereas the cec and cath transgenes significantly improved survival after bacterial infection. The results proved that high-efficiency dual-gene integrated genetic strains can be produced using CRISPR/Cas9 technology and demonstrated the potential role of these transgenes in improving disease resistance in wild fish [84]. GAB3-GRB2-associated binding protein 3 (gab3), one of the members of the GRB2-associated binding protein gene group, functions as a scaffold protein for growth factor and cytokine signaling pathways [85]. Previous genome-wide association studies have demonstrated that the gab3 gene is a key factor in resistance to viral nerve necrosis (NNV) in Asian sea bass (Lates calcarifer). Knock-down of the gab3 gene in L. calcarifer cells with NNV exposure significantly reduced viral RNA [80]. Similarly, the knockout of gab3 in zebrafish (Danio rerio) improved survival and resistance to NNV. Hence, the knockout of gab3 appears to be an effective method of blocking NNV replication [81]. Wang et al. [86] achieved the integration of the exogenous alligator (Alligator sinensis) cathelicidin gene (As-cath) into the targeted luteinizing hormone (lh) site in Ictalurus punctatus by using CRISPR/Cas9-assisted technology. The edited fish showed higher disease resistance and decreased fertility compared to the wild-type fish [86]. In another study, the role of the carotenoid isomeroxygenase gene (EcNinaB-X1) in the immune system of ridgetail white prawn (Exopalaemon carinicauda) was investigated using CRISPR/Cas9-mediated gene editing. The EcNinaB-X1 knockout E. carinicauda showed significantly reduced mortality compared to the wild type after bacterial attack [87]. Dehler et al. [82] performed the knockout of the signal transducer and activator of transcription (stat2) in the Chinook salmon (Oncorhynchus tshawytscha) cell line to investigate its role by using CRISPR/Cas9. The results revealed the generation of epizootic hematopoietic necrosis virus (EHNV) DNA and viral hemorrhagic septicemia virus (VHSV) viral RNA particles, demonstrating the function of the stat2 gene in resistance against viral infection [82].
Generally, modified genes targeting disease resistance in aquaculture species offer significant potential to enhance survival rates and aquaculture productivity while decreasing reliance on antibiotics and other therapies. However, only a small handful of genes involved in disease resistance have been successfully targeted [81].
5.2. Reproduction
CRISPR/Cas9 technology is being harnessed to delve deeper into the genetic mechanisms underlying fish reproduction. By targeting and modifying specific genes expressed within fish gonads, scientists are gaining valuable insights into the complex processes of sex differentiation and germ cell development. This research has the potential to shed light on the evolutionary history of fishes and to develop novel strategies for aquaculture and conservation.
It is believed that the genes dnd (dead end), nanos2 (nanos C2HC-type zinc finger 2), and nanos3 (nanos C2HC-type zinc finger 3) play roles in the formation and maintenance of primordial germ cells (PGCs). Therefore, the disruption of these genes by knockout techniques may result in sterility in fish [54,88]. For example, when the dnd gene in Atlantic salmon was silenced using CRISPR/Cas9, the resulting F0 mutants lacked germ cells, confirming the gene’s crucial role in germline development [69]. Remarkably, despite the absence of germ cells, dnd mutant fish still developed testes and ovaries, suggesting that gonadal differentiation in Atlantic salmon can occur independently of germ cells [20,88]. In addition to dnd, CRISPR/Cas9-mediated editing of nanos2 and nanos3 genes in Nile tilapia resulted in germ cell-deficient gonads [54]. Several sex differentiation genes, including steroidogenic factor 1 (sf-1), double sex and mab-3-related transcription factor 1 (dmrt1), and gonadal somatic cell-derived factor (gsdf), have been studied using CRISPR/Cas9 technology. These genes play roles in regulating sex development [54,60,61]. The knockout of the sf-1 gene in Nile tilapia led to gonadal dysfunction and feminization in XY fish. Additionally, the disruption of sf-1 in XX fish resulted in the decreased expression of cytochrome P450, family 19, subfamily A, polypeptide 1a (cyp19a1a), fork-head box L2 (foxl2), and serum estradiol-17 beta. Conversely, the disruption of sf-1 in XY fish resulted in the increased expression of these genes (cyp19a1a, foxl2, serum estradiol-17 beta). Interestingly, treating sf-1-disrupted XY fish with 17α-methyltestosterone could partially rescue gonadal differentiation, while estradiol-17 beta had limited success in rescuing gonadal differentiation in sf-1-disrupted XX fish. These findings suggest that sf-1 plays a crucial role in regulating steroidogenesis and reproduction in tilapia [61]. In addition, several tilapia genes such as foxh1, cyp11c1, and cyp19a1b have been targeted with CRISPR/Cas technology with different effects and results. In addition to Nile tilapia, studies on other edible fish species, including starlet (Acipenser ruthenus), olive flounder (Paralichthys olivaceus), gibel carp (Carassius gibelio), yellow catfish (Pelteobagrus fulvidraco), and southern catfish (Silurus meridionalis), have been conducted to investigate genes related to reproduction and development [56,84,89,90,91]. Recently, CRISPR/Cas9 technology was used to create all-female common carp (Cyprinus carpio) by silencing the cyp17a1 gene [92].
5.3. Growth and Muscle Development
Genetic improvement programs may have multiple objectives, with growth enhancement being a universally desired trait. Over the years, researchers have focused on different genes associated with growth, including the growth hormone (gh), insulin-like growth factors (igf-I and igf-II), growth hormone-inhibiting hormone (ghih), and myostatin (mstn) genes [93]. MSTN is a protein that inhibits the growth of skeletal muscle and is part of the transforming growth factor-beta (TGF-β) family. It influences myogenesis by interacting with Smad proteins and holds the muscle cells in the G0/G1 and G2 phases of the cell cycle [94]. CRISPR/Cas9 gene editing has been used to enhance growth by disabling the mstn gene in various species such as the common carp [95], blunt snout bream (Megalobrama amblycephala) [73], channel catfish [74], olive flounder (Paralichthys olivaceus) [75], red sea bream (Pagrus major) [76], and Pacific oyster (Crassostrea gigas) [23]. The CRISPR/Cas9-mediated knockout of mstn in channel catfish resulted in a significant increase in body weight of 29% [74]. Moreover, the knockout of this gene in red sea bream using CRISPR/Cas9 resulted in an increase in muscle mass of 16%, reduced body length, and improved feed efficiency, leading to faster growth compared to other fishes [76,96]. CRISPR/Cas9 gene editing was also used in the disruption of the mstn gene in mud loach (Misgurnus anguillicaudatus), resulting in the first genetically modified individuals with enhanced growth [96]. In addition to mstn, CRISPR/Cas9 has been used to target the leptin receptor gene in tiger puffer (Takifugu rubripes). Disrupting this gene, which regulates appetite, can lead to increased feeding and potentially enhanced growth [76]. This technique was also applied to Pacific oysters by targeting the myosin essential light chain (MELC) gene and disrupting it. The results revealed a significant impact on muscle formation in the mutant strains. Hence, CRISPR/Cas9 offers a promising approach to enhancing aquaculture sustainability in the future by improving traits such as growth and reproduction.
5.4. Pigmentation
Pigmentation is another desirable trait in aquaculture; it can influence consumer preference [97]. Genes associated with pigmentation have been studied in various fishes using three different genome-editing technologies (TALEN, ZFN, and CRISPR/Cas9). These efforts aimed to establish standardized methods for studying pigmentation phenotypes [88]. Several genes, such as slc24a5, play a crucial role in pigmentation, and they are highly conserved among vertebrate species [70]. CRISPR/Cas9-mediated gene editing of slc45a2 in Atlantic salmon led to a loss of gene function, resulting in different degrees of pigment losses among the F0 generation [69]. The CRISPR/Cas9 technique has been used to inactivate the oculocutaneous albinism II (oca2) gene in cavefish (Astyanax mexicanus) and a cichlid (Astatotilapia calliptera) [70,71], as well as the asip gene in Oujiang color common carp (Cyprinus carpio var. color) [72,98]. The research findings suggest that the genes involved in melanogenesis in fish play a significant role in determining their color variation, which can have implications for aquaculture and ornamental fish breeding [70,71,72,98,99]. In addition, Nile tilapia has also been used to apply this technique to study the functions and effects of certain genes such as hps4, csf1ra, and mitf on pigmentation and body color patterns. Moreover, understanding pigmentation traits in fish can contribute to biosafety measures, as unique pigmentation patterns can help identify escaped genetically modified fish [20,55,69,100].
5.5. Omega-3-Fatty Acid Metabolism and Other Traits
Fish oil is a rich source of omega-3 long-chain polyunsaturated fatty acids (n − 3 LC-PUFAs), particularly EPA and DHA. These polyunsaturated fatty acids have been extensively studied and shown to have numerous health benefits. EPA and DHA play crucial roles in different physiological processes such as heart health, brain function, and inflammation. They have been linked to a reduced risk of heart disease, stroke, and type 2 diabetes. Additionally, omega-3 fatty acids have been shown to improve cognitive function, reduce inflammation, and support healthy skin and hair. Dietary supplementation is the primary way to fulfill the human body’s need for LC-PUFAs, as humans cannot produce them efficiently on their own. Fish raised in aquaculture typically have lower levels of n-3 LC-PUFAs than wild fish, especially when they are fed diets that replace fish oil with vegetable oils. The primary sources of omega-3 LC-PUFAs for humans are fish, particularly Atlantic salmon, which can produce these fatty acids through the actions of enzymes called fatty acyl elongases (Elovls) and fatty acyl desaturases (Fads). It would be valuable to study the molecular mechanisms involved in the production and regulation of PUFAs [83,101]. CRISPR/Cas9 was used to knock out the elovl2 gene in Atlantic salmon. Datsomor et al. [83,101] found that elovl2 is essential for producing 22:6n-3 in multiple tissues and that the body’s own PUFAs are necessary for regulating the activity of genes involved in fat production. Fish with the knocked-out elovl2 gene had lower levels of 22:6n-3 but higher levels of 20:5n-3 and docosapentaenoic acid (22:5n-3) in their liver, brain, and white muscle, indicating that the elongation process was inhibited. Additionally, salmon with the knocked-out elovl2 gene had higher levels of arachidonic acid (20:4n-6) in their brain and white muscle. The decrease in 22:6n-3 production led to an increase in the expression of certain genes involved in fatty acids production, such as sterol regulatory element-binding protein-1 (srebp-1), fatty acid synthase-b, Δ6fad-a, Δ5fad, and elovl5, in the liver. Thus, the study indicates that elovl2 is crucial for the final steps of PUFA synthesis in fish and that srebp-1 plays a key role in regulating the body’s production of PUFAs in Atlantic salmon. This discovery can reduce the reliance on live feed and/or fish oil in aquaculture diets, leading to economic and environmental benefits [83,101].
6. Discussion
Although genome-editing technology has proven highly valuable for studying the functions of genes as well as enhancing key traits, many of these approaches face ethical concerns and technological obstacles in the aquaculture industry [85]. The first major challenge concerning the application of CRISPR technology in aquaculture sector production lies in the manner of efficiently delivering CRISPR into fertilized eggs at the one-cell stage. The duration between the fertilization of the egg and the first cell division in fish differs from one species to another, and incubation temperature also impacts the time available for effective CRISPR-Cas9 application [102]. Therefore, it is crucial to know the duration between fertilization and the first cell division in order to avoid missing the critical phase for genome editing. While the microinjection of the Cas9/gRNA mixture directly into single-cell-stage fertilized eggs is efficient for producing approximately 100 individual eggs to investigate gene functions, for breeding applications, 100 microinjected eggs can prove inadequate [81]. In addition, in certain special situations, such as in salmonids or olive flounder, the chorion of the egg may be too tough to be microinjected using a standard needle. Nevertheless, insertion can be performed with electroporation or by using a heavy-duty needle for egg microinjection [84,102]. Furthermore, despite the sequencing and assembly of the genomes of various species, many of the sequenced genomes are still not properly assembled and annotated. As a result, most gene functions are not fully revealed. The number of genes identified as being involved in critical features of aquaculture species, such as disease resistance and growth, is extremely limited, hampering the potential uses of CRISPR/Cas9 [103]. Moreover, it is crucial to minimize the potential for off-target impacts and there is the issue of mosaicism within the founder generation in aquaculture species. Mosaic individuals may not transmit the edit to subsequent generations. Off-target modification can lead to undesirable results for the individual concerned. To overcome this issue, in vitro transcription (IVT) of gRNA and Cas9 can be used in contrast to CRISPR/Cas9 plasmids. In addition, to reduce the occurrence of mosaicism, the Cas9 protein can be used instead of Cas9 RNA. These attempts have reduced, though not eradicated, this challenge in zebrafish [104,105].
Public acceptance and the need for government approval are the main obstacles facing the application of genome editing in the aquaculture industry. Among various aquaculture species, myostatin-knockout red sea bream and leptin receptor knockout tiger puffer and olive flounder are genome-edited species that are currently commercially produced [106]. Comparing genome editing and genetically modified organisms (GMOs), the former does not insert any exotic genetic material into aquaculture species; instead, it enhances a specific desired trait through the modification of a specific DNA sequence of the species [107]. Thus, the organisms that are modified using GE differ from GMOs. Nevertheless, the GE tools are still largely at the research and development stage, with limited applicability in aquaculture systems, as most lines are still in development. The main reasons behind this are that the majority of the general public remains unconvinced, and the worldwide regulatory framework for GE is unclear [81].
7. Conclusions and Future Directions
CRISPR/Cas technology has emerged as one of the most widespread of existing GE technologies; thus, most of the commercialized GE aquaculture products planned will be produced using CRISPR/Cas technology. This technology offers great opportunities to develop wide-ranging solutions to many obstacles facing the aquaculture industry. The use of CRISPR/Cas9 technology in the aquaculture sector has shown significant potential for gaining deeper insights into improving various economically important traits. Furthermore, new techniques and technologies associated with CRISPR/Cas9 technology, such as high-throughput sequencing and genome-wide research, can contribute to uncovering more key genes involved in various traits of aquatic species, opening the possibility of further research into functional traits to improve genetic selection programs as well as enhance aquacultural productivity.
Author Contributions
Conceptualization, A.B. and D.W.; methodology, S.S. and A.A.; software, B.N.U.; validation, S.T.; writing—original draft preparation, A.B., S.S. and A.A.; writing—review and editing, D.W. and M.B.; visualization, H.A.; supervision, D.W.; project administration, G.A.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific and Technological Research Council of Türkiye grant number 222O095).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kidane, L.; Kejela, A. Food Security and Environment Conservation through Sustainable Use of Wild and Semi-Wild Edible Plants: A Case Study in Berek Natural Forest, Oromia Special Zone, Ethiopia. Agric. Food Secur. 2021, 10, 29. [Google Scholar] [CrossRef]
- Dewali, S.; Sharma, N.; Melkani, D.; Arya, M.; Kathayat, N.; Panda, A.K.; Bisht, S.S. Aquaculture: Contributions to Global Food Security. In Emerging Solutions in Sustainable Food and Nutrition Security; Springer International Publishing: Cham, Germany, 2023; pp. 123–139. [Google Scholar]
- Guenard, R. Poisson from a Petri Dish. Inform 2021, 32, 6–10. [Google Scholar]
- Zhu, M.; Sumana, S.L.; Abdullateef, M.M.; Falayi, O.C.; Shui, Y.; Zhang, C.; Zhu, J.; Su, S. CRISPR/Cas9 Technology for Enhancing Desirable Traits of Fish Species in Aquaculture. Int. J. Mol. Sci. 2024, 25, 9299. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.N.; Singh, P.; Giri, S.S. Potentiality of New Feed Ingredients for Aquaculture: A Review. Agric. Rev. 2018, 39, 282–291. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Hussain, S.M.; Bano, A.A.; Ali, S.; Rizwan, M.; Adrees, M.; Zahoor, A.F.; Sarker, P.K.; Hussain, M.; Arsalan, M.Z.-H.; Yong, J.W.H.; et al. Substitution of Fishmeal: Highlights of Potential Plant Protein Sources for Aquaculture Sustainability. Heliyon 2024, 10, e26573. [Google Scholar] [CrossRef]
- Hallerman, E. Genome Editing in Cultured Fishes. CABI Agric. Biosci. 2021, 2, 46. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, V.; Behera, B.K.; Parhi, J.; Mohapatra, S.; Chakraborty, T.; Das, B.K. CRISPR/Cas Genome Editing—Can It Become a Game Changer in Future Fisheries Sector? Front. Mar. Sci. 2022, 9, 924475. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Haft, D.H.; et al. An Updated Evolutionary Classification of CRISPR–Cas Systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Krzowski, L.; Saluk-Bijak, J.; Bijak, M. Various Aspects of a Gene Editing System—CRISPR–Cas9. Int. J. Mol. Sci. 2020, 21, 9604. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, T.; Yang, L.; Su, Y.; Zhao, C.; Li, L.; Cai, J.; Dai, X.; Wang, D.; Zhou, L. Generation of Fast Growth Nile Tilapia (Oreochromis niloticus) by Myostatin Gene Mutation. Aquaculture 2023, 562, 738762. [Google Scholar] [CrossRef]
- Kamachi, Y.; Kawahara, A. CRISPR-Cas9-Mediated Genome Modifications in Zebrafish. In Genome Editing in Animals: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 313–324. [Google Scholar]
- Miao, M.; Li, S.; Yuan, J.; Liu, P.; Fang, X.; Zhang, C.; Zhang, X.; Li, F. CRISPR/Cas9-Mediated Gene Mutation of EcIAG Leads to Sex Reversal in the Male Ridgetail White Prawn Exopalaemon carinicauda. Front. Endocrinol. 2023, 14, 1266641. [Google Scholar] [CrossRef]
- Ma, J.; Fan, Y.; Zhou, Y.; Liu, W.; Jiang, N.; Zhang, J.; Zeng, L. Efficient Resistance to Grass Carp Reovirus Infection in JAM-A Knockout Cells Using CRISPR/Cas9. Fish Shellfish Immunol. 2018, 76, 206–215. [Google Scholar] [CrossRef]
- Jao, L.-E.; Wente, S.R.; Chen, W. Efficient Multiplex Biallelic Zebrafish Genome Editing Using a CRISPR Nuclease System. Proc. Natl. Acad. Sci. USA 2013, 110, 13904–13909. [Google Scholar] [CrossRef]
- Mokrani, A.; Li, J.; Li, Q.; Liu, S. Toward Understanding Mechanistic Regulation of Body Size and Growth Control in Bivalve Mollusks. Rev. Aquac. 2024, 17, e12962. [Google Scholar] [CrossRef]
- Wargelius, A.; Leininger, S.; Skaftnesmo, K.O.; Kleppe, L.; Andersson, E.; Taranger, G.L.; Schulz, R.W.; Edvardsen, R.B. Dnd Knockout Ablates Germ Cells and Demonstrates Germ Cell Independent Sex Differentiation in Atlantic Salmon. Sci. Rep. 2016, 6, 21284. [Google Scholar] [CrossRef]
- Gutási, A.; Hammer, S.E.; El-Matbouli, M.; Saleh, M. Review: Recent Applications of Gene Editing in Fish Species and Aquatic Medicine. Animals 2023, 13, 1250. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Dong, Z.; Dong, X.; Chi, J.; Chen, H.; Zhao, Q.; Li, K. A New Strain of Yellow Catfish Carrying Genome Edited Myostatin Alleles Exhibits Double Muscling Phenotype with Hyperplasia. Aquaculture 2020, 523, 735187. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Li, Q.; Xu, R.; Yue, C.; Du, S. Targeted Gene Disruption in Pacific Oyster Based on CRISPR/Cas9 Ribonucleoprotein Complexes. Mar. Biotechnol. 2019, 21, 301–309. [Google Scholar] [CrossRef]
- Menchaca, A.; Anegon, I.; Whitelaw, C.B.A.; Baldassarre, H.; Crispo, M. New Insights and Current Tools for Genetically Engineered (GE) Sheep and Goats. Theriogenology 2016, 86, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sumana, S.L.; Chen, H.; Shui, Y.; Zhang, C.; Yu, F.; Zhu, J.; Su, S. Effect of Dietary Selenium on the Growth and Immune Systems of Fish. Animals 2023, 13, 2978. [Google Scholar] [CrossRef] [PubMed]
- Gratacap, R.L.; Regan, T.; Dehler, C.E.; Martin, S.A.M.; Boudinot, P.; Collet, B.; Houston, R.D. Efficient CRISPR/Cas9 Genome Editing in a Salmonid Fish Cell Line Using a Lentivirus Delivery System. BMC Biotechnol 2020, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide Sequence of the Iap Gene, Responsible for Alkaline Phosphatase Isozyme Conversion in Escherichia Coli, and Identification of the Gene Product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Ferrer, C.; Juez, G.; Rodríguez-Valera, F. Long Stretches of Short Tandem Repeats Are Present in the Largest Replicons of the Archaea Haloferax mediterranei and Haloferax volcanii and Could Be Involved in Replicon Partitioning. Mol. Microbiol. 1995, 17, 85–93. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Juez, G.; Rodriguez-Valera, F. Transcription at Different Salinities of Haloferax mediterranei Sequences Adjacent to Partially Modified Pst I Sites. Mol. Microbiol. 1993, 9, 613–621. [Google Scholar] [CrossRef]
- Kansal, R. The CRISPR-Cas System and Clinical Applications of CRISPR-Based Gene Editing in Hematology with a Focus on Inherited Germline Predisposition to Hematologic Malignancies. Genes 2024, 15, 863. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Diez-Villasenor, C.; Garcia-Martinez, J.; Soria, E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Makarova, K.S.; Grishin, N.V.; Shabalina, S.A.; Wolf, Y.I.; Koonin, E.V. A Putative RNA-Interference-Based Immune System in Prokaryotes: Computational Analysis of the Predicted Enzymatic Machinery, Functional Analogies with Eukaryotic RNAi, and Hypothetical Mechanisms of Action. Biol. Direct. 2006, 1, 7. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Brouns, S.J.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.H.; Snijders, A.P.L.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; van der Oost, J. Small CRISPR RNAs Guide Antiviral Defense in Prokaryotes. Science 2008, 321, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9–CrRNA Ribonucleoprotein Complex Mediates Specific DNA Cleavage for Adaptive Immunity in Bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Lin, C.-Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double Nicking by RNA-Guided CRISPR Cas9 for Enhanced Genome Editing Specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Severi, A.A.; Akbari, B. CRISPR-Cas9 Delivery Strategies and Applications: Review and Update. Genesis 2024, 62, e23598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, H.; Cui, Y.; Cong, L.; Zhang, D. Application of Different Types of CRISPR/Cas-Based Systems in Bacteria. Microb. Cell Fact. 2020, 19, 172. [Google Scholar] [CrossRef]
- Khoshandam, M.; Soltaninejad, H.; Mousazadeh, M.; Hamidieh, A.A.; Hosseinkhani, S. Clinical Applications of the CRISPR/Cas9 Genome-Editing System: Delivery Options and Challenges in Precision Medicine. Genes Dis. 2024, 11, 268–282. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; El-Tanani, M.; Tambuwala, M.M. Principles of CRISPR-Cas9 Technology: Advancements in Genome Editing and Emerging Trends in Drug Delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105338. [Google Scholar] [CrossRef]
- H El-Ashry, A. The CRISPR/Cas System: Gene Editing by Bacterial Defense. Nov. Res. Microbiol. J. 2023, 7, 2101–2115. [Google Scholar] [CrossRef]
- Parvathy, S.T. Genome Editing: A Safe Alternative to Genetic Engineering of Crops. In Genetic Engineering of Crop Plants for Food and Health Security; Springer Nature Singapore: Singapore, 2024; pp. 327–372. [Google Scholar]
- Hosen, A.; Nishat, M.N.H.; Soaib, M.M.H.; Sharkar, O.S.; Sahabuddin, M.; Sharif, I.H.; Bhajan, S.K. A Review: CRISPR Cas System and the Mechanism With an Inhibition of Binding of CRISPR Cas-9. Nano Select. 2024, 6, e202400009. [Google Scholar] [CrossRef]
- Saha, A.; Ahsan, M.; Arantes, P.R.; Schmitz, M.; Chanez, C.; Jinek, M.; Palermo, G. An Alpha-Helical Lid Guides the Target DNA toward Catalysis in CRISPR-Cas12a. Nat. Commun. 2024, 15, 1473. [Google Scholar] [CrossRef] [PubMed]
- Krysler, A.R.; Cromwell, C.R.; Tu, T.; Jovel, J.; Hubbard, B.P. Guide RNAs Containing Universal Bases Enable Cas9/Cas12a Recognition of Polymorphic Sequences. Nat. Commun. 2022, 13, 1617. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Wang, Y.; Chen, H.; Sun, Z.S.; Ju, X.-D. Recent Progress in CRISPR/Cas9 Technology. J. Genet. Genom. 2016, 43, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Mochi, J.; Jani, J.; Joshi, S.; Pappachan, A. CRISPR-Cas9: Chronology and Evolution. In CRISPR-Cas System in Translational Biotechnology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–21. [Google Scholar]
- Bhatia, S.; Pooja; Yadav, S.K. CRISPR-Cas for Genome Editing: Classification, Mechanism, Designing and Applications. Int. J. Biol. Macromol. 2023, 238, 124054. [Google Scholar] [CrossRef]
- Schmidheini, L.; Mathis, N.; Marquart, K.F.; Rothgangl, T.; Kissling, L.; Böck, D.; Chanez, C.; Wang, J.P.; Jinek, M.; Schwank, G. Continuous Directed Evolution of a Compact CjCas9 Variant with Broad PAM Compatibility. Nat. Chem. Biol. 2024, 20, 333–343. [Google Scholar] [CrossRef]
- Chavez, M.; Chen, X.; Finn, P.B.; Qi, L.S. Advances in CRISPR Therapeutics. Nat. Rev. Nephrol. 2023, 19, 9–22. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Wang, X.; Shi, L. The Immune System of Prokaryotes: Potential Applications and Implications for Gene Editing. Biotechnol. J. 2024, 19, 2300352. [Google Scholar] [CrossRef]
- Ahmad, A.; Munir, A.; Zafar, H.; Zahoor, M.K.; Hassan, S.; Khan, S.H. Tracking Footprints of CRISPR-Based Genome Editing. In Global Regulatory Outlook for CRISPRized Plants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 113–145. [Google Scholar]
- Li, M.; Yang, H.; Zhao, J.; Fang, L.; Shi, H.; Li, M.; Sun, Y.; Zhang, X.; Jiang, D.; Zhou, L.; et al. Efficient and Heritable Gene Targeting in Tilapia by CRISPR/Cas9. Genetics 2014, 197, 591–599. [Google Scholar] [CrossRef]
- Güralp, H.; Skaftnesmo, K.O.; Kjærner-Semb, E.; Straume, A.H.; Kleppe, L.; Schulz, R.W.; Edvardsen, R.B.; Wargelius, A. Rescue of Germ Cells in Dnd Crispant Embryos Opens the Possibility to Produce Inherited Sterility in Atlantic Salmon. Sci. Rep. 2020, 10, 18042. [Google Scholar] [CrossRef]
- Baloch, A.R.; Franěk, R.; Tichopád, T.; Fučíková, M.; Rodina, M.; Pšenička, M. Dnd1 Knockout in Sturgeons By CRISPR/Cas9 Generates Germ Cell Free Host for Surrogate Production. Animals 2019, 9, 174. [Google Scholar] [CrossRef]
- Xu, Z.; Zhuo, M.; Xiao, H.; Tao, W.; Wang, D. Maternal Dnd1 Is Essential for Migration and Maintenance of PGCs in Nile Tilapia at Larval Stage. Aquac. Rep. 2024, 38, 102319. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, H.; Jie, M.; Dai, S.; Wu, X.; Li, M.; Wang, D. Amh Regulate Female Folliculogenesis and Fertility in a Dose-Dependent Manner through Amhr2 in Nile Tilapia. Mol. Cell Endocrinol. 2020, 499, 110593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Yang, H.; Li, M.; Shi, H.; Zhang, X.; Wang, D. Gsdf Is a Downstream Gene of Dmrt1 That Functions in the Male Sex Determination Pathway of the Nile Tilapia. Mol. Reprod. Dev. 2016, 83, 497–508. [Google Scholar] [CrossRef]
- Xie, Q.-P.; He, X.; Sui, Y.-N.; Chen, L.-L.; Sun, L.-N.; Wang, D.-S. Haploinsufficiency of SF-1 Causes Female to Male Sex Reversal in Nile Tilapia, Oreochromis niloticus. Endocrinology 2016, 157, 2500–2514. [Google Scholar] [CrossRef]
- Tao, B.; Tan, J.; Chen, L.; Xu, Y.; Liao, X.; Li, Y.; Chen, J.; Song, Y.; Hu, W. CRISPR/Cas9 System-Based Myostatin-Targeted Disruption Promotes Somatic Growth and Adipogenesis in Loach, Misgurnus anguillicaudatus. Aquaculture 2021, 544, 737097. [Google Scholar] [CrossRef]
- Zheng, Q.; Xiao, H.; Shi, H.; Wang, T.; Sun, L.; Tao, W.; Kocher, T.D.; Li, M.; Wang, D. Loss of Cyp11c1 Causes Delayed Spermatogenesis Due to the Absence of 11-Ketotestosterone. J. Endocrinol. 2020, 244, 487–499. [Google Scholar] [CrossRef]
- Zhang, X.; Min, Q.; Li, M.; Liu, X.; Li, M.; Wang, D. Mutation of Cyp19a1b Results in Sterile Males Due to Efferent Duct Obstruction in Nile Tilapia. Mol. Reprod. Dev. 2019, 86, 1224–1235. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Liu, S.; Zhao, C.; Miao, Y.; Jin, L.; Wang, D.; Zhou, L. Cyp17a1 Is Required for Female Sex Determination and Male Fertility by Regulating Sex Steroid Biosynthesis in Fish. Endocrinology 2021, 162, bqab205. [Google Scholar] [CrossRef]
- Lu, B.; Wang, C.; Liang, G.; Xu, M.; Kocher, T.D.; Sun, L.; Wang, D. Generation of Ornamental Nile Tilapia with Distinct Gray and Black Body Color Pattern by Csf1ra Mutation. Aquac. Rep. 2022, 23, 101077. [Google Scholar] [CrossRef]
- Wang, C.; Kocher, T.D.; Wu, J.; Li, L.P.; Liang, G.; Lu, B.; Xu, J.; Chen, X.; Wang, D. Knockout of Microphthalmia-Associated Transcription Factor (mitf) Confers a Red and Yellow Tilapia with Few Pigmented Melanophores. Aquaculture 2023, 565, 739151. [Google Scholar] [CrossRef]
- Wang, C.; Kocher, T.D.; Lu, B.; Xu, J.; Wang, D. Knockout of Hermansky-Pudlak Syndrome 4 (Hps4) Leads to Silver-White Tilapia Lacking Melanosomes. Aquaculture 2022, 559, 738420. [Google Scholar] [CrossRef]
- Edvardsen, R.B.; Leininger, S.; Kleppe, L.; Skaftnesmo, K.O.; Wargelius, A. Targeted Mutagenesis in Atlantic Salmon (Salmo salar L.) Using the CRISPR/Cas9 System Induces Complete Knockout Individuals in the F0 Generation. PLoS ONE 2014, 9, e108622. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, H.; Wang, Y.; Adamski, K.; Rohner, N.; Kowalko, J.E. CRISPR Mutagenesis Confirms the Role of Oca2 in Melanin Pigmentation in Astyanax Mexicanus. Dev. Biol. 2018, 441, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.; Elkin, J.; Marconi, A.; Turner, G.F.; Smith, A.M.; Joyce, D.; Miska, E.A.; Juntti, S.A.; Santos, M.E. Oca2 Targeting Using CRISPR/Cas9 in the Malawi Cichlid Astatotilapia calliptera. R. Soc. Open Sci. 2022, 9, 220077. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Du, J.; Si, Z.; Yang, H.; Xu, X.; Wang, C. ASIP Disruption via CRISPR/Cas9 System Induces Black Patches Dispersion in Oujiang Color Common Carp. Aquaculture 2019, 498, 230–235. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.-D.; Nissa, M.; Chen, J.; Zou, S.-M. Disruption of Mstna and Mstnb Gene through CRISPR/Cas9 Leads to Elevated Muscle Mass in Blunt Snout Bream (Megalobrama amblycephala). Aquaculture 2020, 528, 735597. [Google Scholar] [CrossRef]
- Khalil, K.; Elayat, M.; Khalifa, E.; Daghash, S.; Elaswad, A.; Miller, M.; Abdelrahman, H.; Ye, Z.; Odin, R.; Drescher, D.; et al. Generation of Myostatin Gene-Edited Channel Catfish (Ictalurus punctatus) via Zygote Injection of CRISPR/Cas9 System. Sci. Rep. 2017, 7, 7301. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.Y.; Kim, J.-W.; Kim, H.-C.; Noh, J.K.; Kim, Y.-O.; Hwang, H.-K.; Kim, W.-J.; Yeo, S.-Y.; An, C.M.; et al. CRISPR/Cas9-Mediated Myostatin Disruption Enhances Muscle Mass in the Olive Flounder Paralichthys olivaceus. Aquaculture 2019, 512, 734336. [Google Scholar] [CrossRef]
- Kishimoto, K.; Washio, Y.; Yoshiura, Y.; Toyoda, A.; Ueno, T.; Fukuyama, H.; Kato, K.; Kinoshita, M. Production of a Breed of Red Sea Bream Pagrus Major with an Increase of Skeletal Muscle Mass and Reduced Body Length by Genome Editing with CRISPR/Cas9. Aquaculture 2018, 495, 415–427. [Google Scholar] [CrossRef]
- Li, M.; Dai, S.; Liu, X.; Xiao, H.; Wang, D. A Detailed Procedure for CRISPR/Cas9-Mediated Gene Editing in Tilapia. Hydrobiologia 2021, 848, 3865–3881. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y. Enhancing Aquaculture Disease Resistance: Antimicrobial Peptides and Gene Editing. Rev. Aquac. 2024, 16, 433–451. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Bruce, T.J.; Wise, A.L.; Zeng, P.; Cao, G.; Simora, R.M.C.; Bern, L.; Shang, M.; Li, S.; et al. CRISPR/Cas9 Microinjection of Transgenic Embryos Enhances the Dual-Gene Integration Efficiency of Antimicrobial Peptide Genes for Bacterial Resistance in Channel Catfish, Ictalurus punctatus. Aquaculture 2023, 575, 739725. [Google Scholar] [CrossRef]
- Yang, Z.; Wong, S.M.; Yue, G.H. Characterization of GAB3 and Its Association with NNV Resistance in the Asian Seabass. Fish Shellfish Immunol. 2020, 104, 18–24. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, Y.; Tay, Y.X.; Yue, G.H. Genome Editing and Its Applications in Genetic Improvement in Aquaculture. Rev. Aquac. 2022, 14, 178–191. [Google Scholar] [CrossRef]
- Dehler, C.E.; Lester, K.; Della Pelle, G.; Jouneau, L.; Houel, A.; Collins, C.; Dovgan, T.; Machat, R.; Zou, J.; Boudinot, P.; et al. Viral Resistance and IFN Signaling in STAT2 Knockout Fish Cells. J. Immunol. 2019, 203, 465–475. [Google Scholar] [CrossRef]
- Datsomor, A.K.; Zic, N.; Li, K.; Olsen, R.E.; Jin, Y.; Vik, J.O.; Edvardsen, R.B.; Grammes, F.; Wargelius, A.; Winge, P. CRISPR/Cas9-Mediated Ablation of Elovl2 in Atlantic Salmon (Salmo salar L.) Inhibits Elongation of Polyunsaturated Fatty Acids and Induces Srebp-1 and Target Genes. Sci. Rep. 2019, 9, 7533. [Google Scholar] [CrossRef]
- Wang, L.; Tan, X.; Wu, Z.; Wang, L.; Jiao, S.; Zou, Y.; Ji, G.; You, F. Targeted Mutagenesis in the Olive Flounder (Paralichthys olivaceus) Using the CRISPR/Cas9 System with Electroporation. Biologia 2021, 76, 1297–1304. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, G.; Lee, M.; Yeo, S.; Yue, G.H. Genes for Editing to Improve Economic Traits in Aquaculture Fish Species. Aquac. Fish. 2024, 10, 1–18. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Xing, D.; Bruce, T.J.; Li, S.; Bern, L.; Shang, M.; Johnson, A.; Simora, R.M.C.; Coogan, M.; et al. Generation of Eco-Friendly and Disease-Resistant Channel Catfish (Ictalurus punctatus) Harboring the Alligator Cathelicidin Gene via CRISPR/Cas9 Engineering. Engineering 2024, 39, 273–286. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, C.; Liu, M.; Liu, Y.; Wang, W.; Cheng, W.; Yang, F.; Zhang, J. CRISPR/Cas9-Mediated Deletion of One Carotenoid Isomerooxygenase Gene (EcNinaB-X1) from Exopalaemon carinicauda. Fish Shellfish Immunol. 2020, 97, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ge, W. Genome Editing in Fishes and Their Applications. Gen. Comp. Endocrinol. 2018, 257, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, R.; Ma, H.; Dong, R.; Liu, Z.; Jiang, W.; Tao, W.; Wang, D. Retinoic Acid Triggers Meiosis Initiation via Stra8-Dependent Pathway in Southern Catfish, Silurus meridionalis. Gen. Comp. Endocrinol. 2016, 232, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Dan, C.; Lin, Q.; Gong, G.; Yang, T.; Xiong, S.; Xiong, Y.; Huang, P.; Gui, J.-F.; Mei, J. A Novel PDZ Domain-Containing Gene Is Essential for Male Sex Differentiation and Maintenance in Yellow Catfish (Pelteobagrus fulvidraco). Sci. Bull. 2018, 63, 1420–1430. [Google Scholar] [CrossRef]
- Gan, R.-H.; Wang, Y.; Li, Z.; Yu, Z.-X.; Li, X.-Y.; Tong, J.-F.; Wang, Z.-W.; Zhang, X.-J.; Zhou, L.; Gui, J.-F. Functional Divergence of Multiple Duplicated Foxl2 Homeologs and Alleles in a Recurrent Polyploid Fish. Mol. Biol. Evol. 2021, 38, 1995–2013. [Google Scholar] [CrossRef]
- Zhai, G.; Shu, T.; Chen, K.; Lou, Q.; Jia, J.; Huang, J.; Shi, C.; Jin, X.; He, J.; Jiang, D.; et al. Successful Production of an All-Female Common Carp (Cyprinus carpio L.) Population Using Cyp17a1-Deficient Neomale Carp. Engineering 2022, 8, 181–189. [Google Scholar] [CrossRef]
- Gui, J.; Zhu, Z. Molecular Basis and Genetic Improvement of Economically Important Traits in Aquaculture Animals. Chin. Sci. Bull. 2012, 57, 1751–1760. [Google Scholar] [CrossRef]
- Hill, J.J.; Davies, M.V.; Pearson, A.A.; Wang, J.H.; Hewick, R.M.; Wolfman, N.M.; Qiu, Y. The Myostatin Propeptide and the Follistatin-Related Gene Are Inhibitory Binding Proteins of Myostatin in Normal Serum. J. Biol. Chem. 2002, 277, 40735–40741. [Google Scholar] [CrossRef]
- Zhong, Z.; Niu, P.; Wang, M.; Huang, G.; Xu, S.; Sun, Y.; Xu, X.; Hou, Y.; Sun, X.; Yan, Y.; et al. Targeted Disruption of Sp7 and Myostatin with CRISPR-Cas9 Results in Severe Bone Defects and More Muscular Cells in Common Carp. Sci. Rep. 2016, 6, 22953. [Google Scholar] [CrossRef]
- Ohama, M.; Washio, Y.; Kishimoto, K.; Kinoshita, M.; Kato, K. Growth Performance of Myostatin Knockout Red Sea Bream Pagrus major Juveniles Produced by Genome Editing with CRISPR/Cas9. Aquaculture 2020, 529, 735672. [Google Scholar] [CrossRef]
- Colihueque, N.; Araneda, C. Appearance Traits in Fish Farming: Progress from Classical Genetics to Genomics, Providing Insight into Current and Potential Genetic Improvement. Front. Genet. 2014, 5, 251. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Chen, H.; Si, Z.; Hou, X.; Yang, H.; Xu, X.; Wang, J.; Wang, C. Shrunk and Scattered Black Spots Turn out Due to MC1R Knockout in a White-Black Oujiang Color Common Carp (Cyprinus carpio Var. Color). Aquaculture 2020, 518, 734822. [Google Scholar] [CrossRef]
- Xu, X.; Chen, H.; Mandal, B.K.; Si, Z.; Wang, J.; Wang, C. Duplicated Tyr Disruption Using CRISPR/Cas9 Reveals Melanophore Formation in Oujiang Color Common Carp (Cyprinus carpio Var. Color). Reprod. Breed. 2022, 2, 37–45. [Google Scholar] [CrossRef]
- Hege Straume, A.; Kjaerner-Semb, E.; Skaftnesmo, K.O.; Güralp, H.; Lillico, S.; Wargelius, A.; Brudvik Edvardsen, R. A Refinement to Gene Editing in Atlantic Salmon Using Asymmetrical Oligonucleotide. bioRxiv 2021. [Google Scholar] [CrossRef]
- Datsomor, A.K.; Olsen, R.E.; Zic, N.; Madaro, A.; Bones, A.M.; Edvardsen, R.B.; Wargelius, A.; Winge, P. CRISPR/Cas9-Mediated Editing of Δ5 and Δ6 Desaturases Impairs Δ8-Desaturation and Docosahexaenoic Acid Synthesis in Atlantic Salmon (Salmo salar L.). Sci. Rep. 2019, 9, 16888. [Google Scholar] [CrossRef]
- Xiang, G.; Zhang, X.; An, C.; Cheng, C.; Wang, H. Temperature Effect on CRISPR-Cas9 Mediated Genome Editing. J. Genet. Genom. 2017, 44, 199–205. [Google Scholar] [CrossRef]
- Yue, G.H.; Wang, L. Current Status of Genome Sequencing and Its Applications in Aquaculture. Aquaculture 2017, 468, 337–347. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable Editing of a Target Base in Genomic DNA without Double-Stranded DNA Cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef]
- Mehravar, M.; Shirazi, A.; Nazari, M.; Banan, M. Mosaicism in CRISPR/Cas9-Mediated Genome Editing. Dev. Biol. 2019, 445, 156–162. [Google Scholar] [CrossRef]
- Wray-Cahen, D.; Hallerman, E.; Tizard, M. Global Regulatory Policies for Animal Biotechnology: Overview, Opportunities and Challenges. Front. Genome Ed. 2024, 6, 1467080. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M.; et al. Harnessing Genomics to Fast-Track Genetic Improvement in Aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).