Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet Formulation
2.2. Feeding Trial
2.3. Fish Sampling
2.4. Growth, Feed Utilization, and Biological Indices
2.5. Proximate Composition and Apparent Digestibility of Nutrients Analysis
2.6. Physicochemical Quality and Textural Properties of Muscle
2.7. Determination of Flesh Amino Acid, Free Amino Acid, and Fatty Acid
2.8. Muscle Biochemical Parameters Analysis
2.9. Statistical Analysis
3. Results
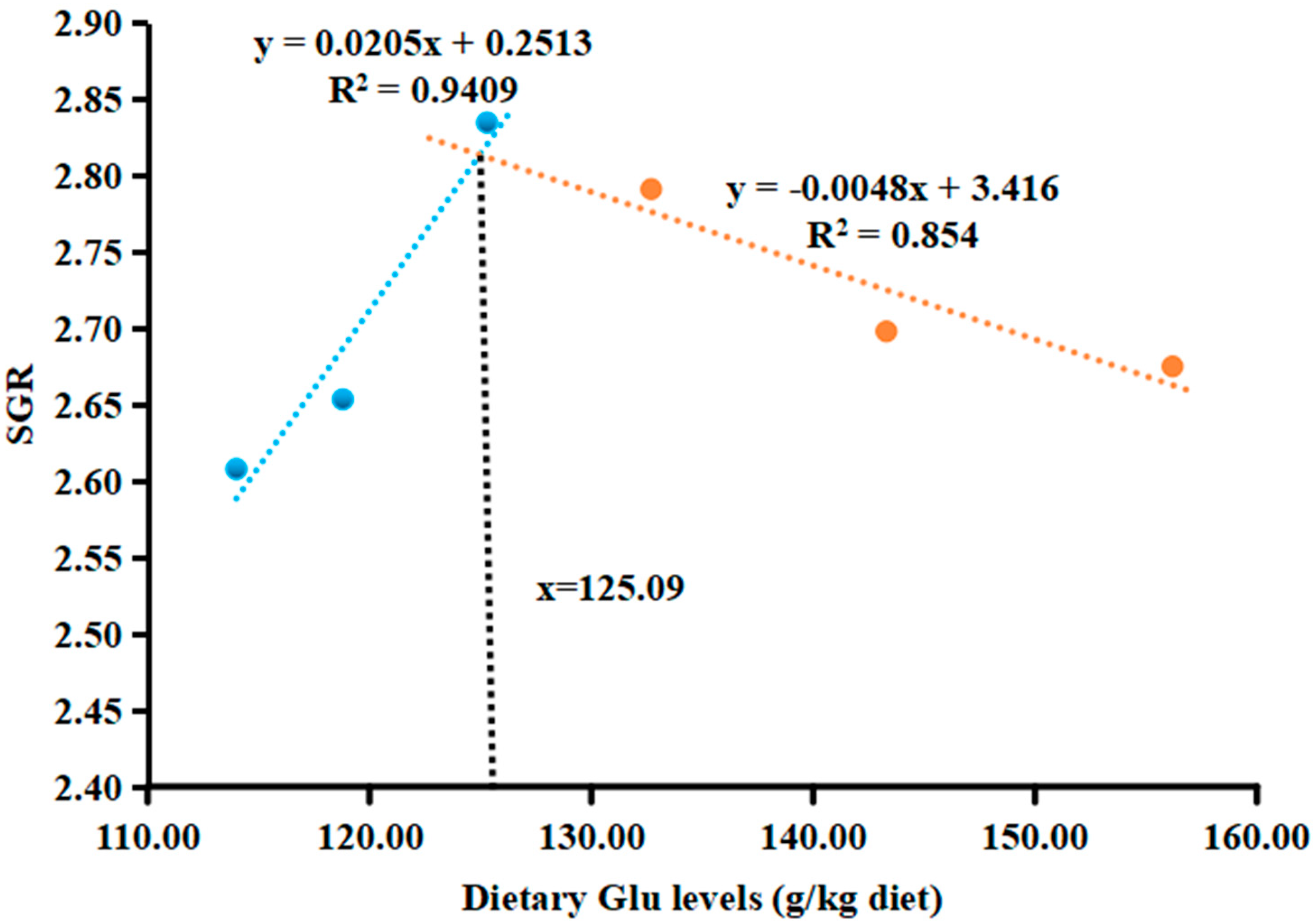
3.1. Growth Performance
3.2. Body Proximate Compositions, Biometric Parameters and Nutrients Apparent Digestibility
3.3. Muscle Nutritional Components, Amino Acid and Fatty Acid Profiles
3.4. Physicochemical Quality and Textural Properties of Muscle
3.5. Flesh Biochemical Indices
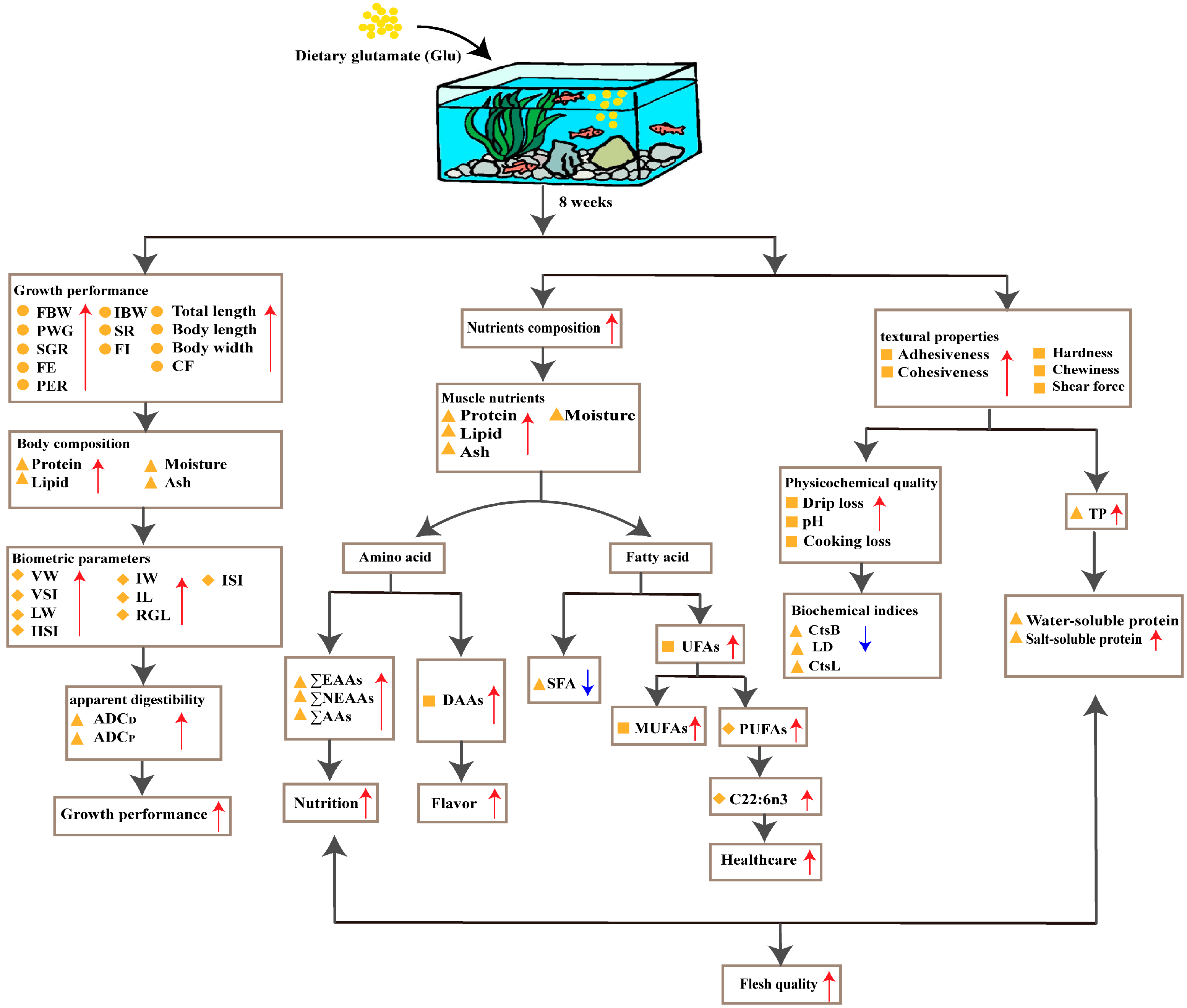
4. Discussion
4.1. Dietary Glu Effects on the Growth Performance, Body Compositions, and Apparent Digestibility
4.2. Effects of Dietary Glu Levels on the Proximate Composition, Amino Acid, and Fatty Acid Profiles in Muscle
4.3. Effects of Dietary Glu Levels on the Muscle Textural Properties, Physicochemical Quality, and Biochemical Indices
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, S.; Wu, G. Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids 2020, 52, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Solares, A.; Viegas, I.; Salgado, M.C.; Siles, A.M.; Sáez, A.; Metón, I.; Baanante, I.V.; Fernández, F. Diets supplemented with glutamate or glutamine improve protein retention and modulate gene expression of key enzymes of hepatic metabolism in gilthead seabream (Sparus aurata) juveniles. Aquaculture 2015, 444, 79–87. [Google Scholar] [CrossRef]
- Yoshida, C.; Maekawa, M.; Bannai, M.; Yamamoto, T. Glutamate promotes nucleotide synthesis in the gut and improves availability of soybean meal feed in rainbow trout (Oncorhynchus mykiss). Springerplus 2016, 5, 1021. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Zhou, X.Q.; Zeng, X.Y.; Feng, L.; Liu, Y.; Jiang, W.D.; Li, S.H.; Li, D.B.; Wu, X.Q.; et al. Effects of dietary glutamate supplementation on growth performance, digestive enzyme activities and antioxidant capacity in intestine of grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2015, 21, 935–941. [Google Scholar] [CrossRef]
- Belghit, I.; Philip, A.J.P.; Maas, R.M.; Lock, E.; Eding, E.H.; Espe, M.; Schrama, J.W. Impact of dietary glutamate and glycine on growth and nutrient utilization in rainbow trout (Oncorhynchus mykiss). Aquaculture 2023, 568, 739311. [Google Scholar] [CrossRef]
- Subandiyono, S.; Hastuti, S. Growth performances, feed utilization and hematological parameters of the carp (Cyprinus carpio), according to the dietary glutamate. Aquac. Aquar. Conserv. Legis. 2022, 15, 830–839. [Google Scholar]
- Larsson, T.; Koppang, E.O.; Espe, M.; Terjesen, B.F.; Krasnov, A.; Moreno, H.M.; Rørvik, K.; Thomassen, M.; Mørkøre, T. Fillet quality and health of Atlantic salmon (Salmo salar L.) fed a diet supplemented with glutamate. Aquaculture 2014, 426–427, 288–295. [Google Scholar] [CrossRef]
- Nursten, H.E. Umami: A basic taste-physiology, biochemistry, nutrition, food science. Biochem. Soc. Trans. 1987, 16, 1095–1096. [Google Scholar] [CrossRef]
- Rogers, P.J.; Blundell, J.E. Umami and appetite: Effects of monosodium glutamate on hunger and food intake in human subjects. Physiol. Behav. 1990, 48, 801–804. [Google Scholar] [CrossRef]
- Ladeira, A.L.F.; Rusth, R.C.T.; Carneiro, C.L.D.S.; Campelo, D.A.V.; Morante, V.H.P.; Luz, R.K.; Carneiro, A.P.S.; Salaro, A.L. Dietary monosodium glutamate supplementation during the feed training of pacamã (Lophiosilurus alexandri): Growth performance and intestinal histomorphometry. Aquac. Res. 2021, 52, 356–363. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, J.; Shi, M.; Liu, Y.; Qu, K.; Tan, B.; Yang, H.; Xie, S. Effects of replacing fishmeal with different proportions of mixed protein source in the diet of largemouth bass (Micropterus salmoides). Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 49, 101181. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, W.; Zhou, M.; Gao, H.; Lu, X.; Yu, Z.; Sun, M.; Huang, Y. Effects of dietary L-glutamic acid on the growth performance, gene expression associated with muscle growth-related gene expression, and intestinal health of juvenile largemouth bass (Micropterus salmoides). Fishes 2024, 9, 312. [Google Scholar] [CrossRef]
- Sarma, D.; Akhtar, M.S.; Das, P.; Das, P.; Shahi, N.; Ciji, A.; Mahanta, P.C.; Yengkokpam, S.; Debnath, D. Nutritional quality in terms of amino acid and fatty acid of five coldwater fish species: Implications to human health. Natl. Acad. Sci. Lett. 2013, 36, 385–391. [Google Scholar] [CrossRef]
- Cai, L.; Tong, F.; Tang, T.; Ao, Z.; Wei, Z.; Yang, F.; Shu, Y.; Liu, S.; Mai, K. Comparative evaluation of nutritional value and flavor quality of muscle in triploid and diploid common carp: Application of genetic improvement in fish quality. Aquaculture 2021, 541, 736780. [Google Scholar] [CrossRef]
- Yang, H.; Li, X.; Rahman, M.M.; Leng, X. Dietary supplementation of leucine improved the flesh quality of largemouth bass, Micropterus salmoides through TOR, FoxO3a and MRFs regulation. Aquaculture 2023, 566, 739237. [Google Scholar] [CrossRef]
- Liu, X.; Feng, L.; Jiang, W.; Wu, P.; Jiang, J.; Yang, D.; Tang, L.; Kuang, S.; Shi, H.; Zhou, X.; et al. (2-Carboxyethyl)dimethylsulfonium Bromide (Br-DMPT) improves muscle flesh quality and antioxidant status of on-growing grass carp (Ctenopharyngodon idella) fed non-fish meal diets. Aquaculture 2020, 521, 735065. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Free amino acids in muscle of Norway lobster (Nephrops novergicus (L.)) in controlled and modified atmospheres during chilled storage. Food Chem. 2004, 86, 85–91. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. F 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Yamanaka, H.; Shimada, R. Post-mortem biochemical changes in the muscle of japanese spiny lobster during storage. Fish. Sci. 1996, 62, 821–824. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Zhao, J.; Chen, D.; Zhou, X.; et al. Effects of dietary glutamate supplementation on flesh quality, antioxidant defense and gene expression related to lipid metabolism and myogenic regulation in Jian carp (Cyprinus carpio var. Jian). Aquaculture 2019, 502, 212–222. [Google Scholar] [CrossRef]
- Dong, B.; Wu, L.; Wang, Y.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; Xie, S.; Liu, Z.; Jin, J. Glutamate improves flesh quality and muscle growth of triploid crucian carp. Aquac. Rep. 2023, 33, 101832. [Google Scholar] [CrossRef]
- Wen, M.; Wu, P.; Jiang, W.; Liu, Y.; Wu, C.; Zhong, C.; Li, S.; Tang, L.; Feng, L.; Zhou, X. Dietary threonine improves muscle nutritional value and muscle hardness associated with collagen synthesis in grass carp (Ctenopharyngodon idella). Food Chem. 2023, 422, 136223. [Google Scholar] [CrossRef] [PubMed]
- Tie, H.; Wu, P.; Jiang, W.; Liu, Y.; Kuang, S.; Zeng, Y.; Jiang, J.; Tang, L.; Zhou, X.; Feng, L. Dietary nucleotides supplementation affect the physicochemical properties, amino acid and fatty acid constituents, apoptosis and antioxidant mechanisms in grass carp (Ctenopharyngodon idellus) muscle. Aquaculture 2019, 502, 312–325. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.; Kuang, S.; Jiang, J.; Li, S.; Tang, L.; Zhou, X. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, H.; Jia, M.; Zhou, H.; Zhang, Y.; Xu, W.; Zhang, W.; Mai, K. Effects of dietary Antarctic krill Euphausia superba meal on growth performance and muscle quality of triploid rainbow trout Oncorhynchus mykiss farmed in sea water. Aquaculture 2019, 509, 72–84. [Google Scholar] [CrossRef]
- Jiang, Q.; Yan, M.; Zhao, Y.; Zhou, X.; Yin, L.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Wang, Y.; et al. Dietary isoleucine improved flesh quality, muscle antioxidant capacity, and muscle growth associated with AKT/TOR/S6K1 and AKT/FOXO3a signaling in hybrid bagrid catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). J. Anim. Sci. Biotechnol. 2021, 12, 53. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Zhu, X.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Huang, X.; Chen, D.; Yang, S.; et al. Dietary methionine hydroxy analogue supplementation benefits on growth, intestinal antioxidant status and microbiota in juvenile largemouth bass Micropterus salmoides. Aquaculture 2022, 556, 738279. [Google Scholar] [CrossRef]
- Hu, Z.; Li, H.; Liu, S.; Xue, R.; Sun, J.; Ji, H. Assessment of black soldier fly (Hermetia illucens) larvae meal as a potential substitute for soybean meal on growth performance and flesh quality of grass carp Ctenopharyngodon idellus. Anim. Nutr. 2023, 14, 425–449. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, Z.; Zhao, J.; Feng, L.; Jiang, W.; Wu, P.; Zhao, J.; Liu, H.; Jiang, J. Evaluation of glycyrrhetinic acid in attenuating adverse effects of a high-fat diet in largemouth bass (Micropterus salmoides). Anim. Nutr. 2024, 19, 248–260. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Zhang, C.; Bian, Y.; Yao, W.; Xu, Z.; Wang, Y.; Li, X.; Leng, X. Effects of replacing fishmeal with cottonseed protein concentrate on growth performance, flesh quality and gossypol deposition of largemouth bass (Micropterus salmoides). Aquaculture 2022, 548, 737551. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, X.; Guo, C.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Luo, W.; Huang, X.; Chen, D.; et al. Dietary isoleucine affects muscle fatty acid and amino acid profiles through regulating lipid metabolism and autophagy in hybrid catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). Anim. Nutr. 2022, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Jiang, W.; Wu, P.; Liu, Y.; Ren, H.; Tang, L.; Li, S.; Zhong, C.; Zhang, R.; Feng, L.; et al. Improvement of flesh quality, muscle growth and protein deposition in adult grass carp (Ctenopharyngodon idella): The role of tryptophan. Aquaculture 2023, 577, 740005. [Google Scholar] [CrossRef]
- Sigholt, T.; Erikson, U.; Rustad, T.; Johansen, S.; Seland, A. Handling stress and storage temperature affect meat quality of farmed-raised atlantic salmon (Salmo Salar). J. Food Sci. 2006, 62, 898–905. [Google Scholar]
- Wei, Z.; Ma, J.; Pan, X.; Mu, H.; Li, J.; Shentu, J.; Zhang, W.; Mai, K. Dietary hydroxyproline improves the growth and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 2016, 464, 497–504. [Google Scholar] [CrossRef]
- Palomino Ramos, A.R.; Campelo, D.A.V.; Carneiro, C.L.D.S.; Zuanon, J.A.S.; Da Matta, S.L.P.; Furuya, W.M.; Salaro, A.L. Optimal dietary L-glutamine level improves growth performance and intestinal histomorphometry of juvenile giant trahira (Hoplias lacerdae), a Neotropical carnivorous fish species. Aquaculture 2022, 547, 737469. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, T.; Li, Q.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Zhao, J.; Zhou, X.; Jiang, J. Effect of dietary L-glutamate levels on growth, digestive and absorptive capability, and intestinal physical barrier function in Jian carp (Cyprinus carpio var. Jian). Anim. Nutr. 2020, 6, 198–209. [Google Scholar] [CrossRef]
- Zhelyazkov, G.; Stratev, D. Effect of monosodium glutamate on growth performance and blood biochemical parameters of rainbow trout (Oncorhynchus mykiss W.). Vet. World 2019, 12, 1008–1012. [Google Scholar] [CrossRef]
- Zhelyazkov, G. Effect of monosodium glutamate dietary supplementation on some productive traits of common carp (Cyprinus carpio L.), cultivated in net cages. Agric. Sci. Technol. 2018, 10, 204–207. [Google Scholar]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar]
- Hamza, N.L.; Mhetli, M.; Khemis, I.B.; Cahu, C.; Kestemont, P. Effect of dietary phospholipid levels on performance, enzyme activities and fatty acid composition of pikeperch (Sander lucioperca) larvae. Aquaculture 2008, 275, 274–282. [Google Scholar] [CrossRef]
- Saleh, N.E.; Wassef, E.A.; Kamel, M.A.; El-Haroun, E.R.; El-Tahan, R.A. Beneficial effects of soybean lecithin and vitamin C combination in fingerlings gilthead seabream (Sparus aurata) diets on; fish performance, oxidation status and genes expression responses. Aquaculture 2022, 546, 737345. [Google Scholar] [CrossRef]
- Buentello, J.A.; Gatlin, D.M. Preliminary observations on the effects of water hardness on free taurine and other amino acids in plasma and muscle of channel catfish (Ictalurus punctatus). N. Am. J. Aquac. 2002, 64, 95–102. [Google Scholar] [CrossRef]
- Pedersen, H.E.; Sissons, J.W. Effect of antigenic soybean protein on the physiology and morphology of the gut in the preruminant calf. Can. J. Anim. Sci. 1984, 64, 183–184. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Fan, S.; Zhou, Z.; Zhou, R.; Wu, C.; Gong, D.; Wen, M.; Wang, Y.; Tao, M.; et al. Comparative analysis of muscle nutrient in two types of hybrid bream and native bream. Reprod. Breed. 2022, 2, 71–77. [Google Scholar] [CrossRef]
- Su, Q.; Yang, J.; Yang, Z.; Kong, Q.; Xiao, G.; Liu, D.; Tang, H. Toxicology of aspartame to largemouth bass (Micropterus salmoides) on the basis of antioxidant capacity, liver histology and the intestinal microbiota. Anim. Feed Sci. Technol. 2025, 320, 116225. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar]
- Jiang, W.; Wu, P.; Tang, R.; Liu, Y.; Kuang, S.; Jiang, J.; Tang, L.; Tang, W.; Zhang, Y.; Zhou, X.; et al. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef]
- Michelle, B.; Kristina, P.; Penny, K.E. Saturated fatty acids and cardiovascular disease: Replacements for saturated fat to reduce cardiovascular risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; Legrand, P. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: Where does the evidence stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar]
- Bhaskar, N.; Miyashita, K. Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)-A review. Food Rev. Int. 2007, 22, 291–307. [Google Scholar]
- de Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner–Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, Y.; Liu, L.; Li, Y.; Huang, X.; Zhong, Y.; Tang, T.; Zhang, J.; Chu, W.; Shen, Y. Effects of high-fat diet on muscle textural properties, antioxidant status and autophagy of Chinese soft-shelled turtle (Pelodiscus sinensis). Aquaculture 2019, 511, 734228. [Google Scholar] [CrossRef]
- Song, D.; Yun, Y.; Mi, J.; Luo, J.; Zhou, Q. Effects of faba bean on growth performance and fillet texture of Yellow River carp, Cyprinus carpio haematopterus. Aquac. Rep. 2020, 17, 100379. [Google Scholar]
- Xu, X.; Li, X.; Xu, Z.; Yang, H.; Lin, X.; Leng, X. Replacing fishmeal with cottonseed protein concentrate in practical diet of largemouth bass (Micropterus salmoides): Growth, flesh quality and metabolomics. Aquaculture 2024, 579, 740164. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Lerfall, J.; Roth, B.; Skare, E.F.; Henriksen, A.; Betten, T.; Dziatkowiak-Stefaniak, M.A.; Rotabakk, B.T. Pre-mortem stress and the subsequent effect on flesh quality of pre-rigor filleted Atlantic salmon (Salmo salar L.) during ice storage. Food Chem. 2015, 175, 157–165. [Google Scholar] [CrossRef]
- Li, T.; Hu, W.; Li, J.; Zhang, X.; Zhu, J.; Li, X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea). Food Control 2012, 25, 101–106. [Google Scholar]
- Einen, O.; Mørkøre, T.; Rørå, A.M.B.; Thomassen, M.S. Feed ration prior to slaughter-a potential tool for managing product quality of Atlantic salmon (Salmo salar). Aquaculture 1999, 178, 149–169. [Google Scholar] [CrossRef]
- Morkore, T.; Rodbotten, M.; Vogt, G.; Fjaera, S.O.; Kristiansen, I.O.; Manseth, E. Relevance of season and nucleotide catabolism on changes in fillet quality during chilled storage of raw Atlantic salmon (Salmo salar L.). Food Chem. 2010, 119, 1417–1425. [Google Scholar] [CrossRef]
- Haard, N.F. Control of chemical composition and food quality attributes of cultured fish. Food Res. Int. 1992, 25, 289–307. [Google Scholar] [CrossRef]
| Ingredients | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Fish meal | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 | 21.00 |
| Chicken powder | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Extruded soybean meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Clostridium autoethanogenum protein | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Monosodium glutamate 1 | 0.00 | 0.60 | 1.20 | 1.80 | 2.40 | 3.00 |
| Soy protein concentrate | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 | 7.50 |
| High gluten wheat flour | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Cassava starch | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soya bean oil | 4.72 | 4.72 | 4.72 | 4.72 | 4.72 | 4.72 |
| Soy lecithin oil powder | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Lysine | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 |
| DL-methionine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Calcium phosphate | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Microcrystalline cellulose | 3.00 | 2.40 | 1.80 | 1.20 | 0.60 | 0.00 |
| Choline chloride | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Premix 2 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient content (%) (analyzed, % dry matter) 3 | ||||||
| Crude protein | 50.34 | 51.21 | 51.92 | 51.60 | 52.24 | 52.26 |
| Crude lipid | 10.54 | 11.02 | 10.34 | 11.23 | 10.51 | 11.08 |
| Ash | 11.09 | 11.09 | 10.87 | 10.15 | 10.98 | 10.64 |
| Measured Glu value | 11.40 | 11.88 | 12.53 | 13.27 | 14.33 | 15.62 |
| Parameters | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Essential amino acids (EAAs) | ||||||
| Methionine | 1.13 | 1.12 | 1.24 | 1.18 | 1.20 | 1.14 |
| Lysine | 3.45 | 3.43 | 3.38 | 3.81 | 3.66 | 3.59 |
| Threonine | 1.47 | 1.44 | 1.57 | 1.47 | 1.55 | 1.50 |
| Isoleucine | 1.90 | 1.93 | 2.11 | 2.16 | 2.08 | 2.14 |
| Histidine | 0.99 | 0.88 | 0.95 | 0.88 | 0.92 | 1.00 |
| Valine | 2.13 | 2.06 | 2.22 | 2.07 | 2.18 | 2.28 |
| Leucine | 3.17 | 3.03 | 3.28 | 3.05 | 3.23 | 3.28 |
| Arginine | 2.54 | 2.33 | 2.54 | 2.69 | 2.48 | 2.52 |
| Phenylalanine | 1.78 | 1.66 | 1.81 | 1.68 | 1.77 | 1.65 |
| Non-essential amino acids (NEAAs) | ||||||
| Aspartic acid | 4.20 | 4.08 | 3.78 | 3.79 | 4.03 | 4.37 |
| Serine | 1.85 | 1.78 | 1.94 | 1.79 | 1.89 | 1.70 |
| Glutamic acid | 11.40 | 11.88 | 12.53 | 13.27 | 14.33 | 15.62 |
| Glycine | 2.50 | 2.40 | 2.54 | 2.35 | 2.49 | 2.43 |
| Alanine | 2.48 | 2.39 | 2.56 | 2.37 | 2.50 | 2.28 |
| Cysteine | 0.31 | 0.29 | 0.31 | 0.30 | 0.30 | 0.33 |
| Proline | 1.98 | 1.88 | 2.03 | 1.60 | 1.69 | 1.80 |
| Tyrosine | 1.47 | 1.44 | 1.57 | 1.47 | 1.55 | 1.57 |
| ∑AAs | 44.72 | 43.98 | 46.33 | 45.89 | 47.81 | 49.16 |
| Items 1 | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| FBW | 103.30 ± 2.95 c | 106.45 ± 2.93 bc | 118.54 ± 4.84 a | 115.41 ± 4.91 ab | 108.19 ± 4.99 abc | 107.70 ± 7.02 abc |
| PWG 3 | 331.07 ± 16.75 c | 342.02 ± 10.83 bc | 389.27 ± 15.55 a | 377.76 ± 21.69 ab | 353.38 ± 17.58 abc | 348.02 ± 26.67 abc |
| SGR 4 | 2.61 ± 0.07 c | 2.65 ± 0.04 bc | 2.83 ± 0.06 a | 2.79 ± 0.08 ab | 2.70 ± 0.07 abc | 2.67 ± 0.10 abc |
| FI 5 | 73.19 ± 4.15 | 72.85 ± 1.64 | 70.68 ± 2.82 | 74.00 ± 2.13 | 71.07 ± 1.25 | 69.48 ± 0.61 |
| FE 6 | 108.54 ± 3.95 b | 113.06 ± 2.25 b | 133.61 ± 7.63 a | 123.43 ± 8.21 ab | 118.58 ± 4.79 ab | 120.36 ± 8.99 ab |
| PER 7 | 2.16 ± 0.08 b | 2.21 ± 0.04 b | 2.57 ± 0.15 a | 2.39 ± 0.16 ab | 2.27 ± 0.09 b | 2.30 ± 0.17 ab |
| CF 8 | 1.90 ± 0.15 bc | 1.94 ± 0.16 abc | 2.05 ± 0.13 ab | 2.06 ± 0.15 a | 1.89 ± 0.16 c | 1.92 ± 0.09 abc |
| Items | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Moisture | 67.85 ± 0.41 | 69.25 ± 2.26 | 68.75 ± 0.47 | 67.25 ± 0.75 | 67.70 ± 0.52 | 69.28 ± 0.39 |
| Protein | 17.17 ± 0.35 b | 17.32 ± 0.95 ab | 18.43 ± 0.33 a | 18.31 ± 0.24 ab | 17.92 ± 0.35 ab | 17.44 ± 0.40 ab |
| Lipid | 7.53 ± 0.25 ab | 7.39 ± 0.60 ab | 7.37 ± 0.15 ab | 8.01 ± 0.20 a | 7.83 ± 0.12 ab | 7.26 ± 0.21 b |
| Ash | 3.92 ± 0.17 | 3.82 ± 0.24 | 3.94 ± 0.03 | 3.78 ± 0.13 | 3.98 ± 0.08 | 3.97 ± 0.08 |
| ADCD 2 | 66.78 ± 0.12 b | 66.28 ± 0.29 b | 69.30 ± 1.01 a | 70.66 ± 0.28 a | 66.77 ± 0.91 b | 66.09 ± 2.38 b |
| ADCP 3 | 93.74 ± 0.09 b | 93.84 ± 0.19 ab | 94.32 ± 0.36 a | 94.39 ± 0.17 a | 93.88 ± 0.14 ab | 93.72 ± 0.36 b |
| Items 1 | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| VW | 6.94 ± 0.58 c | 8.43 ± 0.43 b | 8.65 ± 1.07 b | 9.63 ± 0.80 a | 8.07 ± 0.57 b | 6.85 ± 0.43 c |
| VSI 2 | 6.50 ± 0.50 | 6.52 ± 0.38 | 6.60 ± 0.48 | 6.92 ± 0.47 | 6.92 ± 0.55 | 6.93 ± 0.46 |
| LW | 1.75 ± 0.11 c | 2.17 ± 0.17 ab | 2.40 ± 0.38 a | 2.26 ± 0.29 ab | 2.07 ± 0.24 b | 1.77 ± 0.12 c |
| HIS 3 | 1.65 ± 0.09 bc | 1.68 ± 0.14 abc | 1.84 ± 0.23 a | 1.61 ± 0.10 c | 1.77 ± 0.18 abc | 1.78 ± 0.12 ab |
| IW | 0.69 ± 0.05 b | 0.80 ± 0.04 a | 0.85 ± 0.06 a | 0.86 ± 0.15 a | 0.68 ± 0.04 b | 0.62 ± 0.06 b |
| ISI 4 | 0.65 ± 0.05 | 0.63 ± 0.03 | 0.65 ± 0.05 | 0.61 ± 0.10 | 0.60 ± 0.04 | 0.63 ± 0.07 |
| IL | 13.24 ± 1.02 cd | 14.24 ± 0.77 abc | 14.72 ± 1.04 ab | 15.05 ± 1.84 a | 13.70 ± 0.57 bc | 12.25 ± 0.61 d |
| RGL 5 | 74.67 ± 5.85 ab | 75.46 ± 4.20 ab | 79.42 ± 5.58 a | 79.72 ± 10.90 a | 74.57 ± 4.10 ab | 70.79 ± 3.01 b |
| Items | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Moisture | 77.28 ± 0.01 | 76.70 ± 0.00 | 76.55 ± 0.01 | 77.04 ± 0.00 | 76.45 ± 0.00 | 77.38 ± 0.00 |
| Protein | 18.71 ± 0.02 d | 19.19 ± 0.03 c | 19.74 ± 0.01 a | 19.21 ± 0.01 c | 19.57 ± 0.04 b | 18.62 ± 0.02 e |
| Lipid | 3.49 ± 0.03 bc | 3.62 ± 0.04 a | 3.53 ± 0.00 b | 3.46 ± 0.02 bcd | 3.39 ± 0.02 d | 3.41 ± 0.00 cd |
| Ash | 1.23 ± 0.03 | 1.28 ± 0.00 | 1.26 ± 0.02 | 1.33 ± 0.05 | 1.27 ± 0.02 | 1.27 ± 0.02 |
| Parameters | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| EAA | ||||||
| Met | 2.27 ± 0.25 a | 2.30 ± 0.03 a | 2.34 ± 0.11 a | 1.98 ± 0.01 b | 1.71 ± 0.13 bc | 1.56 ± 0.07 c |
| Lys | 6.53 ± 0.32 b | 6.67 ± 0.34 b | 7.70 ± 0.21 a | 6.14 ± 0.08 b | 5.89 ± 0.13 b | 5.80 ± 0.87 b |
| Thr | 3.13 ± 0.08 b | 3.52 ± 0.03 ab | 3.84 ± 0.02 a | 3.35 ± 0.41 ab | 3.30 ± 0.48 ab | 3.44 ± 0.14 ab |
| Ile | 3.27 ± 0.18 | 3.19 ± 0.20 | 3.38 ± 0.38 | 3.16 ± 0.12 | 3.48 ± 0.36 | 3.43 ± 0.26 |
| His | 1.44 ± 0.28 | 1.72 ± 0.11 | 1.92 ± 0.03 | 1.69 ± 0.17 | 1.66 ± 0.28 | 1.46 ± 0.22 |
| Val | 3.52 ± 0.22 | 3.42 ± 0.20 | 3.78 ± 0.16 | 3.40 ± 0.15 | 3.31 ± 0.39 | 3.58 ± 0.19 |
| Leu | 5.68 ± 0.19 | 5.52 ± 0.30 | 5.89 ± 0.48 | 5.82 ± 0.57 | 5.91 ± 0.79 | 5.24 ± 0.70 |
| Arg | 4.11 ± 0.08 | 4.36 ± 0.24 | 4.57 ± 0.22 | 4.31 ± 0.44 | 4.47 ± 0.16 | 4.58 ± 0.30 |
| Phe | 3.12 ± 0.17 | 3.06 ± 0.23 | 2.93 ± 0.16 | 3.04 ± 0.15 | 3.05 ± 0.29 | 2.69 ± 0.25 |
| NEAA | ||||||
| Asp | 7.17 ± 0.15 | 7.27 ± 0.36 | 7.85 ± 0.54 | 7.93 ± 0.70 | 7.27 ± 0.14 | 7.64 ± 0.01 |
| Ser | 2.73 ± 0.08 | 3.13 ± 0.01 | 3.20 ± 0.47 | 2.97 ± 0.41 | 2.99 ± 0.49 | 3.36 ± 0.23 |
| Glu | 10.61 ± 0.25 b | 11.03 ± 0.42 b | 12.26 ± 0.25 a | 11.26 ± 0.52 b | 11.12 ± 0.32 b | 10.46 ± 0.03 b |
| Gly | 3.31 ± 0.12 b | 3.68 ± 0.25 ab | 4.04 ± 0.04 ab | 3.65 ± 0.44 b | 3.44 ± 0.53 b | 4.46 ± 0.39 a |
| Ala | 4.17 ± 0.09 | 4.44 ± 0.26 | 4.42 ± 0.41 | 4.42 ± 0.43 | 4.11 ± 0.21 | 3.98 ± 0.55 |
| Cys | 0.34 ± 0.13 | 0.37 ± 0.14 | 0.25 ± 0.05 | 0.33 ± 0.15 | 0.25 ± 0.11 | 0.22 ± 0.09 |
| Pro | 2.50 ± 0.35 ab | 2.70 ± 0.05 ab | 2.76 ± 0.12 a | 2.44 ± 0.32 ab | 2.74 ± 0.15 a | 2.01 ± 0.54 b |
| Tyr | 2.58 ± 0.19 | 2.53 ± 0.14 | 2.48 ± 0.16 | 2.50 ± 0.16 | 2.62 ± 0.30 | 2.67 ± 0.21 |
| ∑EAAs | 33.08 ± 1.12 ab | 33.77 ± 1.12 ab | 36.36 ± 0.41 a | 32.90 ± 2.03 b | 32.78 ± 1.65 b | 31.76 ± 1.83 b |
| ∑NEAAs | 33.40 ± 0.87 b | 35.14 ± 1.15 ab | 37.26 ± 1.61 a | 35.51 ± 2.72 ab | 34.54 ± 1.42 ab | 34.79 ± 0.48 ab |
| ∑EAAs:∑NEAAs | 0.99 ± 0.02 a | 0.96 ± 0.02 ab | 0.98 ± 0.03 ab | 0.93 ± 0.03 ab | 0.95 ± 0.01 ab | 0.91 ± 0.04 b |
| ∑EAAs:∑AAs | 0.50 ± 0.01 a | 0.49 ± 0.01 ab | 0.49 ± 0.01 ab | 0.48 ± 0.01 ab | 0.49 ± 0.00 ab | 0.48 ± 0.01 b |
| ∑AAs | 66.48 ± 1.85 b | 68.90 ± 2.17 ab | 73.62 ± 1.96 a | 68.41 ± 4.67 ab | 67.32 ± 3.06 ab | 66.55 ± 2.31 b |
| Parameters | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Asp | 0.10 ± 0.02 b | 0.15 ± 0.02 ab | 0.16 ± 0.02 a | 0.13 ± 0.01 ab | 0.15 ± 0.02 ab | 0.16 ± 0.03 a |
| Thr | 0.92 ± 0.06 | 1.34 ± 0.39 | 1.39 ± 0.34 | 1.43 ± 0.18 | 1.33 ± 0.21 | 0.98 ± 0.10 |
| Ser | 0.31 ± 0.02 | 0.57 ± 0.27 | 0.44 ± 0.15 | 0.50 ± 0.12 | 0.60 ± 0.19 | 0.51 ± 0.10 |
| Glu | 0.45 ± 0.16 | 0.48 ± 0.37 | 0.59 ± 0.33 | 0.72 ± 0.43 | 0.51 ± 0.23 | 0.37 ± 0.12 |
| Gly | 7.12 ± 1.27 b | 9.17 ± 2.41 ab | 11.67 ± 0.93 a | 11.00 ± 1.73 ab | 8.72 ± 0.69 ab | 9.34 ± 2.89 ab |
| Ala | 2.03 ± 0.50 b | 2.77 ± 0.24 ab | 2.67 ± 0.45 ab | 2.69 ± 0.42 ab | 3.18 ± 0.19 a | 2.49 ± 0.53 ab |
| Val | 0.19 ± 0.02 | 0.27 ± 0.06 | 0.25 ± 0.03 | 0.28 ± 0.04 | 0.29 ± 0.03 | 0.18 ± 0.11 |
| Cys | 0.15 ± 0.05 b | 0.23 ± 0.01 a | 0.25 ± 0.04 a | 0.21 ± 0.00 a | 0.24 ± 0.01 a | 0.22 ± 0.03 a |
| Met | 0.12 ± 0.02 c | 0.16 ± 0.01 ab | 0.17 ± 0.01 a | 0.16 ± 0.00 ab | 0.15 ± 0.01 abc | 0.14 ± 0.01 bc |
| Ile | 0.11 ± 0.04 b | 0.15 ± 0.01 ab | 0.16 ± 0.03 ab | 0.18 ± 0.02 a | 0.16 ± 0.01 ab | 0.13 ± 0.03 ab |
| Leu | 0.20 ± 0.05 b | 0.27 ± 0.05 ab | 0.28 ± 0.02 ab | 0.33 ± 0.04 a | 0.30 ± 0.02 ab | 0.25 ± 0.05 ab |
| Tyr | 0.07 ± 0.01 c | 0.10 ± 0.02 bc | 0.11 ± 0.01 ab | 0.13 ± 0.01 a | 0.09 ± 0.01 bc | 0.10 ± 0.00 bc |
| Phe | 0.09 ± 0.01 | 0.11 ± 0.03 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 |
| Lys | 0.81 ± 0.39 b | 1.13 ± 0.28 ab | 1.48 ± 0.37 a | 1.30 ± 0.18 ab | 1.07 ± 0.11 ab | 0.96 ± 0.12 ab |
| His | 4.09 ± 0.81 | 4.44 ± 0.78 | 4.54 ± 0.96 | 4.46 ± 0.54 | 4.01 ± 1.03 | 3.66 ± 1.20 |
| Arg | 0.04 ± 0.00 b | 0.06 ± 0.01 ab | 0.09 ± 0.04 a | 0.05 ± 0.01 ab | 0.07 ± 0.02 ab | 0.06 ± 0.00 ab |
| Pro | 0.47 ± 0.20 b | 0.71 ± 0.28 ab | 1.20 ± 0.20 a | 0.62 ± 0.34 ab | 0.24 ± 0.02 b | 0.25 ± 0.01 b |
| ∑AAs | 17.27 ± 0.75 d | 22.08 ± 2.39 bc | 25.56 ± 0.45 a | 24.32 ± 1.32 ab | 21.12 ± 0.35 bc | 19.82 ± 2.12 cd |
| EAAs | 6.57 ± 1.34 | 7.91 ± 0.56 | 8.47 ± 0.97 | 8.31 ± 0.27 | 7.48 ± 0.89 | 6.44 ± 1.43 |
| DAAs | 9.71 ± 0.87 b | 12.57 ± 2.63 ab | 15.09 ± 1.33 a | 14.55 ± 1.50 a | 12.55 ± 1.02 ab | 12.37 ± 2.67 ab |
| Items 1 | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| C6:0 | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.25 ± 0.04 | 0.24 ± 0.01 | 0.19 ± 0.06 |
| C14:0 | 0.98 ± 0.08 bc | 0.96 ± 0.03 c | 1.07 ± 0.10 bc | 1.28 ± 0.06 a | 1.14 ± 0.06 ab | 1.10 ± 0.10 abc |
| C15:0 | 0.21 ± 0.13 | 0.11 ± 0.01 | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.11 ± 0.01 | 0.12 ± 0.02 |
| C16:0 | 20.72 ± 1.01 | 20.93 ± 1.10 | 19.56 ± 0.39 | 20.47 ± 1.60 | 21.44 ± 0.89 | 21.99 ± 1.79 |
| C17:0 | 0.27 ± 0.10 | 0.18 ± 0.04 | 0.23 ± 0.14 | 0.26 ± 0.13 | 0.28 ± 0.16 | 0.27 ± 0.14 |
| C18:0 | 4.99 ± 0.44 | 5.05 ± 0.26 | 4.22 ± 0.39 | 4.28 ± 0.18 | 4.99 ± 0.24 | 5.02 ± 0.51 |
| C20:0 | 0.12 ± 0.05 | 0.11 ± 0.03 | 0.10 ± 0.04 | 0.13 ± 0.04 | 0.10 ± 0.06 | 0.08 ± 0.04 |
| C23:0 | 0.56 ± 0.05 a | 0.51 ± 0.05 ab | 0.45 ± 0.09 ab | 0.40 ± 0.02 b | 0.47 ± 0.04 ab | 0.51 ± 0.08 ab |
| SFAs | 28.08 ± 1.08 ab | 28.09 ± 1.20 ab | 25.95 ± 1.01 b | 27.19 ± 1.44 ab | 28.79 ± 0.47 ab | 29.29 ± 2.14 a |
| C16:1 | 3.03 ± 0.60 | 3.44 ± 0.62 | 3.92 ± 0.83 | 4.49 ± 1.18 | 3.86 ± 1.21 | 3.90 ± 0.80 |
| C18:1n9t | 0.28 ± 0.04 | 0.26 ± 0.05 | 0.26 ± 0.04 | 0.28 ± 0.03 | 0.25 ± 0.03 | 0.26 ± 0.03 |
| C18:1n9c | 23.87 ± 1.79 | 23.93 ± 1.62 | 24.11 ± 0.43 | 26.48 ± 1.18 | 25.78 ± 3.23 | 25.52 ± 2.38 |
| C20:1n9 | 0.08 ± 0.00 | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 |
| C22:1n9 | 0.09 ± 0.02 | 0.09 ± 0.03 | 0.05 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.07 ± 0.01 |
| C24:1n9 | 0.16 ± 0.03 a | 0.09 ± 0.02 b | 0.11 ± 0.02 ab | 0.11 ± 0.01 ab | 0.13 ± 0.02 ab | 0.11 ± 0.02 ab |
| MUFAs | 27.51 ± 2.34 | 27.90 ± 1.43 | 28.52 ± 1.22 | 31.51 ± 2.32 | 30.18 ± 4.39 | 29.94 ± 3.06 |
| C18:2n6c | 20.59 ± 0.76 | 20.61 ± 0.88 | 21.94 ± 0.09 | 19.22 ± 3.24 | 20.62 ± 3.90 | 19.23 ± 3.44 |
| C18:3n6 | 0.40 ± 0.04 | 0.36 ± 0.06 | 0.31 ± 0.08 | 0.34 ± 0.08 | 0.39 ± 0.06 | 0.35 ± 0.04 |
| C18:3n3 | 1.44 ± 0.14 | 1.44 ± 0.27 | 1.24 ± 0.33 | 1.20 ± 0.14 | 1.50 ± 0.29 | 1.37 ± 0.31 |
| C20:2 | 0.79 ± 0.03 | 0.80 ± 0.08 | 0.67 ± 0.13 | 0.73 ± 0.12 | 0.84 ± 0.14 | 0.79 ± 0.05 |
| C20:3n6 | 0.51 ± 0.04 | 0.52 ± 0.04 | 0.41 ± 0.02 | 0.40 ± 0.05 | 0.51 ± 0.07 | 0.50 ± 0.01 |
| C22:2 | 0.04 ± 0.00 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| C20:5n3 | 1.20 ± 0.23 | 1.12 ± 0.06 | 1.18 ± 0.09 | 1.16 ± 0.15 | 1.46 ± 0.15 | 1.39 ± 0.22 |
| C22:6n3 | 18.14 ± 1.78 a | 18.84 ± 1.45 a | 19.27 ± 0.78 a | 17.70 ± 0.48 ab | 15.07 ± 0.36 b | 16.55 ± 1.44 ab |
| PUFAs | 43.12 ± 2.79 | 43.72 ± 1.04 | 45.06 ± 0.37 | 40.79 ± 3.43 | 40.43 ± 4.45 | 40.24 ± 4.09 |
| UFAs | 70.63 ± 0.48 b | 71.61 ± 1.23 ab | 73.58 ± 1.20 a | 72.29 ± 1.24 ab | 70.61 ± 0.25 b | 70.18 ± 1.91 b |
| EFAs | 22.04 ± 0.89 | 22.06 ± 0.85 | 23.18 ± 0.28 | 20.41 ± 3.30 | 22.12 ± 4.19 | 20.60 ± 3.75 |
| n-3PUFA | 20.78 ± 2.07 ab | 21.40 ± 1.63 a | 21.69 ± 0.39 a | 20.05 ± 0.63 ab | 18.03 ± 0.36 b | 19.31 ± 1.59 ab |
| n-6PUFA | 21.51 ± 0.71 | 21.49 ± 0.80 | 22.66 ± 0.14 | 19.96 ± 3.34 | 21.51 ± 4.02 | 20.09 ± 3.44 |
| n-3/n-6 | 0.96 ± 0.07 | 1.00 ± 0.11 | 0.96 ± 0.02 | 1.03 ± 0.16 | 0.86 ± 0.13 | 0.99 ± 0.18 |
| Items | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| Cooking loss (%) | 22.99 ± 3.87 | 22.49 ± 2.92 | 21.95 ± 3.15 | 21.65 ± 3.47 | 21.91 ± 3.41 | 21.37 ± 4.05 |
| Drip loss (%) | 9.94 ± 2.50 a | 9.40 ± 2.67 ab | 8.05 ± 1.47 abc | 7.43 ± 1.15 bc | 6.44 ± 1.78 c | 6.94 ± 2.36 c |
| pH 45min | 6.65 ± 0.13 ab | 6.61 ± 0.15 abc | 6.60 ± 0.15 abc | 6.71 ± 0.12 a | 6.51 ± 0.19 bc | 6.47 ± 0.17 c |
| pH 24h | 6.29 ± 0.09 b | 6.28 ± 0.14 b | 6.39 ± 0.12 b | 6.53 ± 0.20 a | 6.32 ± 0.12 b | 6.29 ± 0.09 b |
| Hardness (N) | 3.90 ± 0.40 | 4.05 ± 0.57 | 3.81 ± 0.75 | 3.70 ± 0.82 | 3.88 ± 0.64 | 3.84 ± 0.73 |
| Adhesiveness (N.mm) | 0.45 ± 0.12 b | 0.53 ± 0.13 ab | 0.58 ± 0.09 ab | 0.56 ± 0.12 ab | 0.64 ± 0.13 a | 0.53 ± 0.22 ab |
| Cohesiveness (Ratio) | 0.57 ± 0.07 ab | 0.52 ± 0.10 b | 0.63 ± 0.08 a | 0.56 ± 0.11 ab | 0.57 ± 0.12 ab | 0.51 ± 0.07 b |
| Springiness (mm) | 9.61 ± 0.97 | 9.97 ± 0.03 | 9.24 ± 1.09 | 9.83 ± 0.26 | 9.96 ± 0.26 | 9.22 ± 1.42 |
| Gumminess (N) | 1.49 ± 0.58 b | 1.89 ± 0.59 ab | 2.35 ± 0.39 a | 2.3 ± 0.42 a | 1.91 ± 0.34 ab | 1.92 ± 0.52 ab |
| Chewiness (mj) | 9.97 ± 1.89 | 10.19 ± 2.01 | 12.13 ± 0.76 | 12.29 ± 2.05 | 12.44 ± 2.56 | 12.48 ± 3.24 |
| Shear force (N) | 3.76 ± 0.42 | 3.86 ± 0.47 | 3.89 ± 0.54 | 3.80 ± 0.45 | 3.55 ± 0.23 | 3.60 ± 0.32 |
| Parameters | G1 | G2 | G3 | G4 | G5 | G6 |
|---|---|---|---|---|---|---|
| CtsB (U/g protein) | 0.75 ± 0.01 a | 0.74 ± 0.01 b | 0.73 ± 0.00 c | 0.73 ± 0.01 c | 0.74 ± 0.01 b | 0.76 ± 0.01 a |
| CtsL (U/g protein) | 0.63 ± 0.00 | 0.63 ± 0.00 | 0.64 ± 0.00 | 0.64 ± 0.01 | 0.64 ± 0.01 | 0.63 ± 0.00 |
| Water-soluble protein | 2.45 ± 0.46 | 2.39 ± 0.11 | 2.40 ± 0.05 | 2.42 ± 0.10 | 2.37 ± 0.30 | 2.44 ± 0.14 |
| Salt-soluble protein | 2.18 ± 0.14 c | 2.26 ± 0.11 c | 2.49 ± 0.04 b | 2.61 ± 0.22 a | 2.50 ± 0.12 ab | 2.58 ± 0.09 ab |
| LD (mmol/g prot) | 2.34 ± 0.47 ab | 1.86 ± 0.25 ab | 1.45 ± 0.34 b | 1.51 ± 0.17 b | 1.95 ± 0.41 ab | 2.45 ± 0.51 a |
| TP (g/L) | 6.87 ± 1.17 b | 7.12 ± 1.07 b | 8.81 ± 1.34 a | 8.04 ± 0.92 ab | 7.20 ± 1.61 b | 7.07 ± 1.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Zhao, J.; Liu, H.; Zhang, H.; Shan, H.; Zong, J.; Cao, Q.; Jiang, J. Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass. Fishes 2025, 10, 151. https://doi.org/10.3390/fishes10040151
Zhao B, Zhao J, Liu H, Zhang H, Shan H, Zong J, Cao Q, Jiang J. Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass. Fishes. 2025; 10(4):151. https://doi.org/10.3390/fishes10040151
Chicago/Turabian StyleZhao, Bo, Ju Zhao, Haifeng Liu, Hailong Zhang, Hongying Shan, Jiali Zong, Quanquan Cao, and Jun Jiang. 2025. "Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass" Fishes 10, no. 4: 151. https://doi.org/10.3390/fishes10040151
APA StyleZhao, B., Zhao, J., Liu, H., Zhang, H., Shan, H., Zong, J., Cao, Q., & Jiang, J. (2025). Impact of Dietary Glutamate on Growth Performance and Flesh Quality of Largemouth Bass. Fishes, 10(4), 151. https://doi.org/10.3390/fishes10040151








