Impact of Faba Bean (Vicia faba L.) Diet and Subsequent Withdrawal on GIFT Tilapia (Oreochromis niloticus) Muscle Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Husbandry
2.3. Sampling Procedures
2.4. Chemical Analysis
2.5. Muscle Texture Analysis
2.6. Histological Analysis
2.7. Non-Specific Immunity and Antioxidant Activity Analysis
2.8. Biochemical Analysis
2.9. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
2.10. Statistical Analysis
3. Results
3.1. Growth Performances
3.2. Texture Analysis
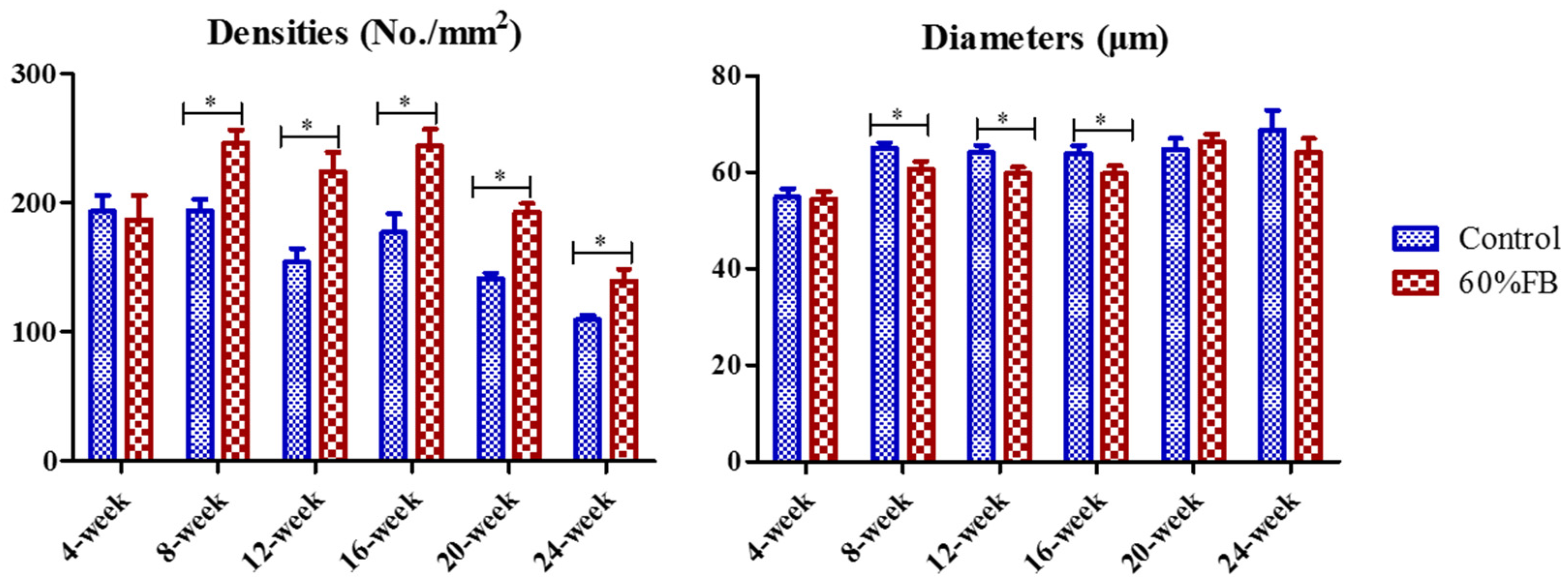
3.3. Histology Analysis
3.4. Non-Specific Immunity and Antioxidant Activity
3.5. Serum Biochemical Parameters
3.6. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Chen, J.; Liang, H.; Li, M.; Zhou, W.H.; Yang, Y.L.; Zhang, Z.; Ding, Q.W.; Ran, C.; Zhou, Z.G. Effect of crowding stress on liver health, gut permeability and gut microbiota of genetically improved farmed tilapia (GIFT, Oreochromis niloticus). Aquaculture 2025, 594, 741448. [Google Scholar] [CrossRef]
- Du, Z.Y.; Nie, P.; Liu, J. Genetic improvement for aquaculture species: A promising approach for aquaculture challenges and development. Rev. Aquac. 2021, 13, 1756–1757. [Google Scholar] [CrossRef]
- Wang, D.; Gao, H.; Song, D.; Wu, F. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Wu, Q.Q.; Huang, H.; Hao, S.X.; Cen, J.W.; Wei, Y.; Xiang, H.; Hu, X.; Zhao, Y.Q. Effect of hanging pulp on quality improvement and protein stability of prepared tilapia fillets. South China Fish. Sci. 2024, 4, 11–23. [Google Scholar]
- Qi, W.Y.; Wen, Y.; Wang, H.H.; Yue, L.; Chen, Z.J.; Yan, W.Q.; Zheng, Q.; Bao, Y.Z.; Zhang, Y.Y.; Kong, Q.L. Effects of electronic beams irradiation sterilization on cold storage life and sensory quality of tilapia fillets. J. Northwest A F Univ. (Nat. Sci. Ed.) 2020, 5, 138–145. [Google Scholar]
- Yilmaz, S. Effects of dietary caffeic acid supplement on antioxidant, immunological and liver gene expression responses, and resistance of Nile tilapia, Oreochromis niloticus to Aeromonas veronii. Fish Shellfish Immunol. 2019, 86, 384–392. [Google Scholar] [CrossRef]
- Li, Q.Q.; Huang, Y.; Zhang, X.Q.; Zou, C.Y.; Lin, L. Improvement of muscle quality in tilapia (Oreochromis niloticus) with dietary faba bean (Vicia faba L.). Front. Nutr. 2023, 10, 1153323. [Google Scholar] [CrossRef]
- Chen, L.J.; Kaneko, G.; Li, Y.C.; Xie, J.; Wang, G.J.; Li, Z.F.; Tian, J.J.; Zhang, K.; Gong, W.B.; Xia, Y.; et al. Reactive oxygen species (ROS)-mediated regulation of muscle texture in grass carp fed with dietary oxidants. Aquaculture 2021, 544, 737150. [Google Scholar] [CrossRef]
- Etemadi, F.; Barker, A.V.; Hashemi, M.; Zandvakili, O.R.; Park, Y. Nutrient accumulation in faba bean varieties. Commun. Soil Sci. Plant Anal. 2018, 49, 2064–2073. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Barker, A.V.; Zandvakili, O.R.; Liu, X.B. Agronomy, nutritional value, and medicinal application of faba bean (Vicia faba L.). Hortic. Plant J. 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Gu, B.; Masli, M.D.P.; Ganjyal, G.M. Whole faba bean flour exhibits unique expansion characteristics relative to the whole flours of lima, pinto, and red kidney beans during extrusion. J. Food Sci. 2020, 85, 404–413. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Q.Q.; Zhang, Y.; Chen, J.H.; Sun, Z.P.; Ren, C.H.; Zhang, Z.J.; Cheng, X.; Huang, Y.F. Nutritive value of faba bean (Vicia faba L.) as a feedstuff resource in livestock nutrition: A review. Food Sci. Nutr. 2021, 9, 5244–5262. [Google Scholar] [CrossRef]
- Cutrignelli, M.I.; Calabrò, S.; Bovera, F.; Tudisco, R.; D’Urso, S.; Marchiello, M.; Piccolo, V.; Infascelli, F. Effects of two protein sources and energy level of diet on the performance of young Marchigiana bulls. 2. Meat quality. Ital. J. Anim. Sci. 2008, 7, 271–285. [Google Scholar] [CrossRef]
- Lanza, M.; Priolo, A.; Biondi, L.; Bella, M.; Ben Salem, H. Replacement of cereal grains by orange pulp and carob pulp in faba bean-based diets fed to lambs: Effects on growth performance and meat quality. Anim. Res. 2001, 50, 21–30. [Google Scholar] [CrossRef]
- Chen, L.J.; Liu, J.; Kaneko, G.; Xie, J.; Wang, G.J.; Yu, D.G.; Li, Z.F.; Ma, L.L.; Qi, D.; Tian, J.J.; et al. Quantitative phosphoproteomic analysis of soft and firm grass carp muscle. Food Chem. 2020, 303, 125367. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wang, C.N.; Huang, K.; Yang, X.H.; Luo, Y.X.; Huang, J.; Wang, D.D.; Li, J.M.; Jie, B.F.; Tang, Z.Y.; et al. Exploring the muscle-hardening mechanisms via the muscle-gut axis in tilapia (Oreochromis niloticus) fed with faba bean (Vicia faba L.) supplementary diets. Aquac. Rep. 2024, 37, 102268. [Google Scholar] [CrossRef]
- Hao, M.L.; Yi, L.L.; Cheng, W.J.; Zhu, J.H.; Zhao, S.M. Lipidomics analysis reveals new insights into crisp grass carp associated with meat texture. Heliyon 2024, 10, e32179. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.L.; Cheng, W.J.; Xie, Y.X.; Li, C.T.; Zhu, J.H.; Yi, L.L.; Zhao, S.M. Identification of hub genes in meat quality of grass carp (Ctenopharyngodon idellus) fed with faba bean by muscle tissue transcriptomic analysis. Ital. J. Anim. Sci. 2024, 23, 594–606. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, K.T.; Gan, L.; Sun, J.J.; Guo, C.J.; Liu, L.; Huang, X.D. Intestinal microbiota of grass carp fed faba beans: Acomparative study. Microorganisms 2019, 7, 465. [Google Scholar] [CrossRef]
- Lin, W.L.; Zeng, Q.X.; Zhu, Z.W. Different changes in mastication between crisp grass carp (Ctenopharyngodon idellus C. et V) and grass carp (Ctenopharyngodon idellus) after heating: The relationship between texture and ultrastructure in muscle tissue. Food Res. Int. 2009, 42, 271–278. [Google Scholar] [CrossRef]
- Yu, E.M.; Zhang, H.F.; Li, Z.F.; Wang, G.J.; Wu, H.K.; Xie, J.; Yu, D.G.; Xia, Y.; Zhang, K.; Gong, W.B. Proteomic signature of muscle fibre hyperplasia in response to faba bean intake in grass carp. Sci. Rep. 2017, 7, 45950. [Google Scholar] [CrossRef]
- Ma, L.L.; Kaneko, G.; Wang, X.J.; Xie, J.; Tian, J.J.; Zhang, K.; Wang, G.J.; Yu, D.G.; Li, Z.F.; Gong, W.B.; et al. Effects of four faba bean extracts on growth parameters, textural quality, oxidative responses, and gut characteristics in grass carp. Aquaculture 2020, 516, 734620. [Google Scholar] [CrossRef]
- Yang, J.Q.; Peng, K.; Liang, Q.C.; Cao, J.M.; Huang, W.; Hu, J.R.; Zhao, H.X.; Cai, J.; Zhang, S.F.; Chen, B. Effects of high level of faba bean supplementation on growth performance, muscle nutritional composition and fishy smell substances of genetic improvement of farmed tilapia (Oreochromis niloticus). Chin. J. Anim. Nutr. 2024, 36, 7236–7249. [Google Scholar]
- Chen, M.Y.; Li, Q.Q.; Yang, L.; Lin, W.Q.; Qin, Z.D.; Liang, S.W.; Lin, L.; Xie, X. Effects of diet containing germinated faba bean (Vicia faba L.) on the intestinal health and gut microbial communities of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2024, 36, 102053. [Google Scholar] [CrossRef]
- Li, Q.Q.; Huang, Y.; Zhang, X.Q.; Qin, Z.D.; Zou, C.Y.; Tan, X.H.; Xie, X.; Liang, S.W.; Lin, L. Effects of faba beans (Vicia faba L.) on growth performance, textural quality and physiological indices of tilapia (Oreochromis niloticus). Aquaculture 2023, 574, 739640. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, J.M.; Fu, B.; Xie, J.; Wang, G.J.; Tian, J.J.; Xia, Y.; Yu, E.M. Textural quality, growth parameters and oxidative responses in Nile tilapia (Oreochromis niloticus) fed faba bean water extract diet. Peer J. 2022, 10, e13048. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Peng, K.; Chen, B.; Huang, W.; Cao, J.M.; Liang, Q.C.; Che, N.Q.; Peng, Z.; Lao, C.Y. Effect of faba bean and its active substances on growth performance, blood indexes, muscle antioxidant capacity and muscle quality of tilapia (Oreochromis niloticus). Chin. J. Anim. Nutr. 2023, 35, 6574–6586. [Google Scholar]
- Guo, H.J.; Liu, G.Q.; Tian, M.D.; Liu, C.S.; Zhang, H.R.; Wang, S.Q.; Wen, X.B.; Lin, F. Effect of faba bean Vicia faba L. water/alcohol extract on growth performance, antioxidant capacity, textural properties, and collagen deposition in the swim bladder of juvenile Nibea coibor. Fish Physiol. Biochem. 2024, 50, 1157–1169. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhu, J.; Zhang, L.; Ge, X.P.; Ren, M.C.; Liang, H. Dietary supplementation with Eucommia ulmoides leaf extract improved the intestinal antioxidant capacity, immune response, and disease resistance against Streptococcus agalactiae in genetically improved farmed tilapia (GIFT; Oreochromis niloticus). Antioxidants 2022, 11, 1800. [Google Scholar] [CrossRef]
- Xu, G.L.; Xing, W.; Yu, H.H.; Jiang, N.; Ma, Z.H.; Luo, L.; Li, T.L. Evaluation of Chlorogenic acid supplementation in koi (Cyprinus carpio) diet: Growth performance, body color, antioxidant activity, serum biochemical parameters, and immune response. Aquac. Nutr. 2022, 2022, 2717003. [Google Scholar] [CrossRef]
- Xu, G.L.; Xing, W.; Li, T.L.; Yu, H.H.; Wei, S.B.; Jiang, N.; Ma, Z.H.; Luo, L. Dietary grape seed proanthocyanidins improved growth, immunity, antioxidant, digestive enzymes activities, and intestinal microbiota of juvenile hybrid sturgeon (Acipenser baeri Brandt ♀ × A. schrenckii Brandt ♂). Aquac. Nutr. 2021, 27, 1983–1995. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.T.; Luo, L.; Ren, Y.Y.; Xing, W.; Xu, G.L.; Li, T.L.; Xue, M.; Yu, H.H.; Wu, Z.L. Dietary lipid levels affect growth performance, lipid metabolism, antioxidant and immune status of Amur sturgeon, Acipenser schrenckii. Aquac. Rep. 2023, 33, 101796. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Yang, H.L.; Ma, R.L.; Lin, W.Y. Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol. 2010, 29, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Xing, W.; Li, T.L.; Ma, Z.H.; Liu, C.X.; Jiang, N.; Luo, L. Effects of dietary raffinose on growth, non-specific immunity, intestinal morphology and microbiome of juvenile hybrid sturgeon (Acipenser baeri Brandt ♀ × A. schrenckii Brandt ♂). Fish Shellfish Immunol. 2018, 72, 237–246. [Google Scholar] [CrossRef]
- Zhou, C.P.; Lin, H.Z.; Ge, X.P.; Niu, J.; Wang, J.; Yang, Y.; Chen, L.X.; Huang, Z.; Yu, W.; Tan, X.H. Effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2025, 43, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Q.; Ding, Z.L.; Zhang, Y.X.; Zhou, P.X.; Wu, C.B.; Zhu, M.H.; Ye, J.Y. Types of carbohydrate in feed affect the growth performance, antioxidant capacity, immunity, and activity of digestive and carbohydrate metabolism enzymes in juvenile Macrobrachium nipponense. Aquaculture 2019, 512, 734282. [Google Scholar] [CrossRef]
- Ren, Z.L.; Wang, S.F.; Cai, Y.; Wu, Y.; Tian, L.J.; Liao, J.Q.; Wang, S.Q.; Jiang, L.; Guo, W.L.; Zhou, Y.C. Antioxidant capacity, non-specific immunity, histopathological analysis and immune-related genes expression in Nile tilapia Oreochromis niloticus infected with Aeromonas schubertii. Aquaculture 2020, 529, 735642. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouee, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crops Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, A.; Saint-Eve, A.; Maillard, M.N. Fava bean (Vicia faba L.) for food applications: From seed to ingredient processing and its effect on functional properties, antinutritional factors, flavor, and color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428. [Google Scholar] [CrossRef]
- Li, Z.F.; Yu, E.M.; Wang, G.J.; Yu, D.G.; Zhang, K.; Gong, W.B.; Xie, J. Broad Bean (Vicia faba L.) induces intestinal inflammation in grass carp (Ctenopharyngodon idellus C. et V) by increasing relative abundances of intestinal gram-negative and flagellated bacteria. Front. Microbiol. 2018, 9, 1913. [Google Scholar] [CrossRef]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Jiang, W.B.; Li, X.Q.; Ji, D.; Leng, X.J. Comparative study on effects of germinated broad bean and germinated pea on growth and flesh quality of grass carp, Ctenopharyngodon idella. J. Shanghai Ocean Univ. 2021, 30, 83–93. [Google Scholar]
- Lun, F.; Leng, X.J.; Meng, X.L.; Liu, X.M. Effect of feeding broad bean on muscle quality of tilapia. J. Shanghai Ocean Univ. 2017, 16, 83–86. [Google Scholar]
- Gan, L.; Li, X.X.; Pan, Q.; Wu, S.L.; Feng, T.; Ye, H. Effects of replacing soybean meal with faba bean meal on growth, feed utilization and antioxidant status of juvenile grass carp, Ctenopharyngodon idella. Aquac. Nutr. 2017, 23, 192–200. [Google Scholar] [CrossRef]
- Ouraji, H.; Zaretabar, A.; Rahmani, H. Performance of rainbow trout (Oncorhynchus mykiss) fingerlings fed diets containing different levels of faba bean (Vicia faba) meal. Aquaculture 2013, 416–417, 161–165. [Google Scholar] [CrossRef]
- Singh, A.; Bharati, R.C.; Manibhushan, N.; Pedpati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar]
- Song, D.Y.; Yun, Y.H.; Mi, J.L.; Luo, J.X.; Jin, M.; Nie, G.X.; Zhou, Q.C. Effects of faba bean on growth performance and fillet texture of Yellow River carp, Cyprinus carpio haematopterus. Aquac. Rep. 2020, 17, 100379. [Google Scholar] [CrossRef]
- Fu, B.; Peng, K.; Zhao, H.X.; Chen, B.; Wang, G.X.; Cao, J.M.; Huang, W. Research progress on the mechanism of fish embrittlement. Feed Res. 2023, 3, 140–143. [Google Scholar]
- Luo, X.M.; Shi, B.B. Influence of raw material fermentation on broad beans nutrients and anti-nutritional factors. Cereals Oils 2012, 8, 29–31. [Google Scholar]
- Jose, I.; Castanon, R.; Ronald, R. Effect of enzyme addition, autoclave treatment and fermenting on the nutritive value of field beans (Vicia faba L.). Anim. Feed Sci. Technol. 1989, 26, 71–79. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011.
- Li, Y.C.; Chen, B.; Zhang, J.M.; Wang, G.J.; Gong, W.B.; Tian, J.J.; Li, H.Y.; Zhang, K.; Xia, Y.; Li, Z.F.; et al. Combined effects of dietary faba bean water extract and vitamin K3 on growth performance, textural quality, intestinal characteristics, oxidative and immune responses in grass carp. Peer J. 2023, 11, e15733. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.P.; Araœjo, M.; Batista, S.; Peixoto, M.J.; Sousa-Pinto, I.; Brotas, V.; Cunha, L.M.; Rema, P. Carotenoid deposition, flesh quality and immunological response of Nile tilapia fed increasing levels of IMTA-cultivated Ulva spp. J. Appl. Phycol. 2016, 28, 691–701. [Google Scholar] [CrossRef]
- López-Albors, O.; Abdel, I.; Periago, M.J.; Ayala, D.; García Alcázar, A.; Martínez Graciá, C.; Nathanailides, C.; Vázquez, J.M. Temperature influence on the white muscle growth dynamics of the sea bass Dicentrarchus labrax, L. Flesh quality implications at commercial size. Aquaculture 2008, 277, 39–51. [Google Scholar] [CrossRef]
- Johnston, I.A.; Li, X.J.; Vieira, V.L.A.; Nickell, D.; Dingwall, A.; Alderson, R.; Campbell, P.; Bickerdike, R. Muscle and flesh quality traits in wild and farmed Atlantic salmon. Aquaculture 2016, 256, 323–336. [Google Scholar] [CrossRef]
- Xu, J.H.; Wang, Y.; Zeng, X.X.; Yang, G.; Wu, X.; Zhu, G.X.; Wang, H.H.; Bai, D.Q. Effects of muscle quality improved feed on the growth performance, muscle quality and antioxidant ability of Ictalurus punctatus Rafinesque. China Feed 2020, 21, 79–86. [Google Scholar]
- Joyce, W. Muscle growth and plasticity in teleost fish: The significance of evolutionarily diverse sarcomeric proteins. Rev. Fish Biol. Fisheries 2023, 33, 1311–1327. [Google Scholar] [CrossRef]
- Koganti, P.; Yao, J.B.; Cleveland, B.M. Molecular mechanisms regulating muscle plasticity in fish. Animals 2021, 7, 61. [Google Scholar] [CrossRef]
- Rowlerson, A.; Radaelli, G.; Mascarello, F.; Veggetti, A. Regeneration of skeletal muscle in two teleost fish: Sparus aurata and Brachydanio rerio. Cell Tissue Res. 1997, 289, 311–322. [Google Scholar] [CrossRef]
- Hurling, R.; Rodell, J.B.; Hunt, H.D. Fiber diameter and fish texture. J. Texture Stud. 1996, 27, 679–685. [Google Scholar] [CrossRef]
- Johnston, I.A.; Manthri, S.; Bickerdike, R.; Dingwall, A.; Luijkx, R.; Campbell, P.; Nickell, D.; Alderson, R. Growth performance, muscle structure and flesh quality in out-of-season atlantic salmon (Salmo salar) smolts reared under two different photoperiod regimes. Aquaculture 2004, 237, 281–300. [Google Scholar] [CrossRef]
- Li, Y.C.; Fu, B.; Zhang, J.M.; Wang, G.J.; Tian, J.J.; Li, H.Y.; Xia, Y.; Xie, J.; Yu, E.M. Comparative genome-wide methylation analysis reveals epigenetic regulation of muscle development in grass carp (Ctenopharyngodon idellus) fed with whole faba bean. Peer J. 2022, 10, e14403. [Google Scholar] [CrossRef]
- Randhir, R.; Vattem, D.; Shetty, K. Antioxidant enzyme response studies in H2O2-stressed porcine muscle tissue following treatment with oregano phenolic extracts. Process Biochem. 2005, 40, 2123–2134. [Google Scholar] [CrossRef]
- Chen, X.M.; Wang, Q.J.; Guo, Z.X.; Zhao, Y.L.; Gao, Y.Q.; Yu, T.; Chen, Y.K.; Zhang, D.M.; Wang, G.Q. Effects of dietary oxidized fish oil on growth performance and antioxidant defense mechanism of juvenile Rhynchocypris lagowski Dybowski. Aquaculture 2019, 512, 734368. [Google Scholar] [CrossRef]
- Song, D.Y.; Yun, Y.H.; He, Z.J.; Mi, J.L.; Luo, J.X.; Jin, M.; Zhou, Q.C.; Nie, G.X. Effects of faba bean (Vicia faba L.) on fillet quality of Yellow River carp (Cyprinus carpio) via the oxidative stress response. Food Chem. 2022, 388, 132953. [Google Scholar] [CrossRef] [PubMed]
- Alami-Durante, H.; Bazin, D.; Cluzeaud, M.; Fontagne-Dicharry, S.; Kaushik, S.; Geurden, I. Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 483, 273–285. [Google Scholar] [CrossRef]
- Kenta, K.; Youhei, W.; Yasutoshi, Y.; Toyoda, A.; Ueno, T.; Fukuyama, H.; Kato, K.; Kinoshita, M. Production of a breed of red sea bream Pagrus major with an increase of skeletal muscle muss and reduced body length by genome editing with CRISPYR/Cas9. Aquaculture 2018, 495, 415–427. [Google Scholar]
- Michelato, M.; Furuya, W.M.; Gatlin, D.M. Metabolic responses of Nile tilapia Oreochromis niloticus to methionine and taurine supplementation. Aquaculture 2018, 485, 66–72. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Wang, L.; Xu, S.; Zhou, J.; Xiang, A.; Yang, C. Sex- and age-dependent expression of Pax7, myf 5, MyoG, and myostatin in yak skeletal muscles. Genet. Mol. Res. 2016, 15, 7–9. [Google Scholar] [CrossRef]
- Bower, N.I.; Johnston, I.A. Discovery and characterization of nutritionally regulated genes associated with muscle growth in atlantic salmon. Physiol. Genomics 2010, 42A, 114–130. [Google Scholar]
- Yu, E.M.; Xie, J.; Wang, G.J.; Yu, D.G.; Gong, W.B.; Li, Z.F.; Wang, H.Y.; Xia, Y.; Wei, N. Gene expression profiling of grass carp (Ctenopharyngodon idellus) and crispy grass carp. Int. J. Genom. 2014, 2014, 639687. [Google Scholar]
- Shioya, I.; Takemura, S.; Ishizuka, R.; Yamaguchi, T. Variations in the proximate composition of muscle in cultured yellowtail Seriola quinqueradiata at different anatomical portions. Fish. Sci. 2012, 7, 725–733. [Google Scholar] [CrossRef]
- Xu, W.H.; Guo, H.H.; Chen, S.J.; Wang, Y.Z.; Lin, Z.H.; Huang, X.D.; Tang, H.J.; He, Y.H.; Sun, J.J.; Gan, L. Transcriptome analysis revealed changes of multiple genes involved in muscle hardness in grass carp (Ctenopharyngodon idellus) fed with faba bean meal. Food Chem. 2020, 314, 126205. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.M.; Ma, L.L.; Ji, H.; Li, Z.F.; Wang, G.J.; Xie, J.; Yu, D.G.; Kaneko, G.; Tian, J.J.; Zhang, K.; et al. Smad4-dependent regulation of type I collagen expression in the muscle of grass carp fed with faba bean. Gene 2019, 685, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.M.; Fu, B.; Wang, G.J.; Li, Z.F.; Ye, D.W.; Jiang, Y.; Ji, H.; Wang, X.; Yu, D.G.; Ehsan, H.; et al. Proteomic and metabolomic basis for improved textural quality in crisp grass carp (Ctenopharyngodon idellus C.et V) fed with a natural dietary pro-oxidant. Food Chem. 2020, 325, 126906. [Google Scholar] [CrossRef]
- Li, Z.M.; Leng, X.J.; Li, X.Q.; Zhu, R.J.; Guang, L.; Li, N.S. Analysis on growth performance, muscle quality, serum biochemical indexes and digestive enzyme activities of crispy grass carp. Jiangsu Agric. Sci. 2012, 40, 186–189. [Google Scholar]
- Liu, B.H.; Wang, G.J.; Yu, E.M.; Xie, J.; Yu, D.W.; Wang, H.Y.; Gong, W.B. Comparison and evaluation of nutrition composition in muscle of grass carp Ctenopharyngodon idellus fed with broad bean and common compound feed. South China Fish. Sci. 2011, 7, 58–65. [Google Scholar]


| Ingredient (g/kg Diet) a | 0% FB (Control Diet) | 60% FB |
|---|---|---|
| Lecithin oil | 2 | 2 |
| Choline chloride | 0.2 | 0.2 |
| Monocalcium phosphate | 1.8 | 1.8 |
| Wheat flour | 29 | 10 |
| Wheat middling | 29 | 0 |
| GIFT premix | 1 | 1 |
| Fish meal | 15 | 15 |
| Wheat gluten | 22 | 10 |
| Faba bean | 0 | 60 |
| Total | 100 | 100 |
| Proximate composition | ||
| Crude protein | 35.77 | 36.01 |
| Crude lipid | 4.25 | 4.53 |
| Ash | 4.42 | 4.77 |
| Moisture | 10.26 | 11.31 |
| Gross energy (MJ/kg) | 18.16 | 17.99 |
| Amino acid (% crude protein) | ||
| Aspartic acid | 12.12 | 10.87 |
| Threonine | 4.44 | 4.12 |
| Serine | 5.85 | 5.62 |
| Glutamic acid | 22.39 | 26.94 |
| Glycine | 4.60 | 5.13 |
| Alanine | 4.65 | 4.97 |
| Cysteine | 0.74 | 0.87 |
| Valine | 3.93 | 3.69 |
| Methionine | 1.87 | 1.47 |
| Isoleucine | 3.84 | 3.33 |
| Leucine | 8.74 | 8.07 |
| Tyrosine | 3.51 | 2.78 |
| Phenylalanine | 5.61 | 4.58 |
| Histidine | 4.62 | 4.19 |
| Lysine | 6.42 | 6.37 |
| Arginine | 6.69 | 7.03 |
| Gene Name | GeneBank No. | Sequences of Primers (5′ to 3′) | Tm (°C) | Amplification Size/bp |
|---|---|---|---|---|
| col1a-1 | NM_001279444.1 | F: GGACGCCATCAAGGTCTACT | 60 | 175 |
| R: GCCCTCACTGCCATACTCG | ||||
| col1a-2 | NM_001282897.1 | F: AGCACCGAGAACAAGAAGCA | 60 | 185 |
| R: TCCATGTAGGCAACGCTGT | ||||
| igf-1 | AY979869.1 | F: GTGCGATGTGCTGTATCTCCT | 60 | 178 |
| R: CCATAGCCTGTTGGTTTATTGA | ||||
| mstn | XM_003458832.5 | F: GTAATGATGGCAACTGAACCTG | 60 | 219 |
| R: CAAGGAGCGGATTCGTATGT | ||||
| myog | NM_001279526.1 | F: CCAGAGTGTCGTCGTCAAGC | 60 | 158 |
| R: GGTCAAGGCCCGCATG | ||||
| gapdh | XM_005455438.3 | F: ATCACTGCCACCCAGAAGAC | 60 | 207 |
| R: CAGACGGACTGTCAGGTCAA |
| 0% FB (Control) | 60% FB | p Value | |
|---|---|---|---|
| IBW (g/fish) | 258.44 ± 0.15 | 258.00 ± 0.42 | 0.37 |
| FBW (g/fish) | 562.63 ± 15.88 | 615.87 ± 21.34 | 0.12 |
| WGR (%) | 117.70 ± 6.19 | 138.73 ± 8.56 | 0.12 |
| SGR (%/day) | 0.69 ± 0.026 | 0.77 ± 0.031 | 0.11 |
| FCR | 1.59 ± 0.062 | 1.43 ± 0.083 | 0.18 |
| SR (%) | 98.89 ± 1.11 | 96.67 ± 1.92 | 0.37 |
| HSI (%) | 1.74 ± 0.23 | 2.16 ± 0.21 | 0.20 |
| VSI (%) | 7.36 ± 0.19 | 7.71 ± 0.36 | 0.42 |
| CF (%) | 3.35 ± 0.033 | 3.50 ± 0.089 | 0.12 |
| Sampling Time | Textural Parameters | 0% FB (Control) | 60% FB | p Value | |
|---|---|---|---|---|---|
| Crispy texture development period 1 | 4-week | Hardness | 34.47 ± 6.04 | 36.64 ± 8.99 | 0.86 |
| Adhesiveness | 0.18 ± 0.042 | 0.24 ± 0.068 | 0.51 | ||
| Cohesiveness | 0.37 ± 0.016 | 0.38 ± 0.030 | 0.78 | ||
| Springiness | 1.56 ± 0.12 | 1.56 ± 0.096 | 0.99 | ||
| Gumminess | 12.83 ± 2.34 | 13.00 ± 2.26 | 0.81 | ||
| Chewiness | 21.16 ± 4.91 | 20.82 ± 4.23 | 0.54 | ||
| Shear force | 25.56 ± 6.13 | 26.08 ± 2.90 | 0.66 | ||
| 8-week | Hardness | 56.28 ± 1.27 | 67.36 ± 2.42 * | 0.010 | |
| Adhesiveness | 0.22 ± 0.053 | 0.20 ± 0.031 | 0.72 | ||
| Cohesiveness | 0.30 ± 0.012 | 0.34 ± 0.021 | 0.19 | ||
| Springiness | 1.53 ± 0.13 | 1.93 ± 0.074 * | 0.018 | ||
| Gumminess | 20.14 ± 0.86 | 22.96 ± 1.04 | 0.56 | ||
| Chewiness | 27.24 ± 0.32 | 36.08 ± 1.50 * | 0.029 | ||
| Shear force | 28.16 ± 3.21 | 47.84 ± 3.20 * | 0.008 | ||
| 12-week | Hardness | 63.17 ± 2.78 | 75.90 ± 1.30 * | 0.016 | |
| Adhesiveness | 0.18 ± 0.004 | 0.21 ± 0.017 | 0.19 | ||
| Cohesiveness | 0.32 ± 0.020 | 0.32 ± 0.026 | 0.93 | ||
| Springiness | 1.92 ± 0.029 | 2.18 ± 0.055 * | 0.002 | ||
| Gumminess | 23.81 ± 2.79 | 24.74 ± 3.08 | 0.83 | ||
| Chewiness | 32.60 ± 3.07 | 46.85 ± 2.02 * | 0.008 | ||
| Shear force | 32.66 ± 1.54 | 52.78 ± 2.98 * | 0.000 | ||
| 16-week | Hardness | 67.71 ± 1.61 | 80.69 ± 1.73 * | 0.002 | |
| Adhesiveness | 0.17 ± 0.0058 | 0.22 ± 0.035 | 0.89 | ||
| Cohesiveness | 0.27 ± 0.0098 | 0.30 ± 0.0088 | 0.054 | ||
| Springiness | 1.69 ± 0.028 | 1.95 ± 0.090 * | 0.000 | ||
| Gumminess | 20.37 ± 1.59 | 26.01 ± 1.69 | 0.13 | ||
| Chewiness | 34.34 ± 2.85 | 50.82 ± 4.22 * | 0.005 | ||
| Shear force | 35.52 ± 0.98 | 56.26 ± 2.84 * | 0.000 | ||
| Crispy texture retention period 2 | 20-week | Hardness | 72.74 ± 1.33 | 103.41 ± 1.56 * | 0.000 |
| Adhesiveness | 0.28 ± 0.025 | 0.21 ± 0.014 | 0.099 | ||
| Cohesiveness | 0.28 ± 0.016 | 0.30 ± 0.012 | 0.37 | ||
| Springiness | 1.76 ± 0.040 | 2.37 ± 0.038 * | 0.000 | ||
| Gumminess | 18.43 ± 0.58 | 23.14 ± 1.93 | 0.18 | ||
| Chewiness | 35.06 ± 1.99 | 59.80 ± 5.31 * | 0.038 | ||
| Shear force | 44.58 ± 0.83 | 54.31 ± 1.76 * | 0.000 | ||
| 24-week | Hardness | 89.71 ± 3.60 | 100.94 ± 1.04 * | 0.024 | |
| Adhesiveness | 0.28 ± 0.097 | 0.33 ± 0.063 | 0.66 | ||
| Cohesiveness | 0.22 ± 0.0086 | 0.23 ± 0.0081 | 0.54 | ||
| Springiness | 2.04 ± 0.036 | 2.34 ± 0.015 * | 0.000 | ||
| Gumminess | 19.87 ± 1.39 | 22.28 ± 1.08 | 0.22 | ||
| Chewiness | 40.41 ± 2.38 | 52.18 ± 2.37 * | 0.013 | ||
| Shear force | 56.67 ± 3.80 | 84.14 ± 5.98 * | 0.017 |
| Sampling Time | Parameters | 0% FB (Control) | 60% FB | p Value | |
|---|---|---|---|---|---|
| Crispy texture development period 1 | 4-week | SOD (U/mL) | 49.04 ± 1.31 | 54.36 ± 0.82 * | 0.014 |
| MDA (nmol/mL) | 2.28 ± 0.31 * | 1.46 ± 0.19 | 0.047 | ||
| CAT (U/mL) | 2.26 ± 0.35 | 1.80 ± 0.27 | 0.092 | ||
| MPO (U/L) | 29.66 ± 4.32 | 31.46 ± 4.51 | 0.78 | ||
| C3 (μg/mL) | 273.79 ± 6.58 | 248.12 ± 18.87 | 0.23 | ||
| LZM (U/L) | 1.81 ± 0.09 | 2.03 ± 0.24 | 0.40 | ||
| 8-week | SOD (U/mL) | 52.78 ± 1.09 | 53.64 ± 0.89 | 0.58 | |
| MDA (nmol/mL) | 2.42 ± 0.23 | 1.96 ± 0.14 | 0.12 | ||
| CAT (U/mL) | 2.82 ± 0.72 | 2.97 ± 0.55 | 0.87 | ||
| MPO (U/L) | 29.00 ± 3.98 | 24.91 ± 2.83 | 0.42 | ||
| C3 (μg/mL) | 294.19 ± 13.05 | 290.72 ± 20.54 | 0.89 | ||
| LZM (U/L) | 2.25 ± 0.18 | 2.49 ± 0.20 | 0.38 | ||
| 12-week | SOD (U/mL) | 51.90 ± 2.09 | 55.33 ± 1.77 | 0.28 | |
| MDA (nmol/mL) | 1.83 ± 0.19 | 1.74 ± 0.26 | 0.79 | ||
| CAT (U/mL) | 2.52 ± 0.37 | 2.01 ± 0.16 | 0.23 | ||
| MPO (U/L) | 21.63 ± 3.29 | 16.06 ± 1.52 | 0.15 | ||
| C3 (μg/mL) | 255.34 ± 11.52 | 219.52 ± 13.12 | 0.067 | ||
| LZM (U/L) | 1.81 ± 0.09 | 2.03 ± 0.24 | 0.15 | ||
| 16-week | SOD (U/mL) | 53.73 ± 0.26 | 55.72 ± 3.59 | 0.59 | |
| MDA (nmol/mL) | 2.17 ± 0.24 | 1.62 ± 0.23 | 0.13 | ||
| CAT (U/mL) | 2.38 ± 0.27 | 2.22 ± 0.23 | 0.67 | ||
| MPO (U/L) | 19.50 ± 1.94 | 21.30 ± 2.35 | 0.57 | ||
| C3 (μg/mL) | 280.77 ± 15.50 | 285.64 ± 17.96 | 0.84 | ||
| LZM (U/L) | 1.84 ± 0.068 | 2.02 ± 0.061 | 0.092 | ||
| Crispy texture retention period 2 | 20-week | SOD (U/mL) | 59.58 ± 7.92 | 53.22 ± 0.89 | 0.46 |
| MDA (nmol/mL) | 2.56 ± 0.21 | 2.63 ± 0.21 | 0.83 | ||
| CAT (U/mL) | 2.20 ± 0.14 | 2.63 ± 0.45 | 0.39 | ||
| MPO (U/L) | 18.93 ± 2.73 | 24.58 ± 4.70 | 0.34 | ||
| C3 (μg/mL) | 434.83 ± 32.33 | 401.49 ± 10.87 | 0.37 | ||
| LZM (U/L) | 2.26 ± 0.22 | 2.34 ± 0.18 | 0.79 | ||
| 24-week | SOD (U/mL) | 51.54 ± 0.39 | 53.15 ± 1.00 | 0.18 | |
| MDA (nmol/mL) | 2.52 ± 0.10 | 2.16 ± 0.23 | 0.21 | ||
| CAT (U/mL) | 1.42 ± 0.22 | 1.96 ± 0.15 | 0.087 | ||
| MPO (U/L) | 20.24 ± 1.34 | 23.43 ± 1.67 | 0.19 | ||
| C3 (μg/mL) | 430.67 ± 29.79 | 387.37 ± 42.04 | 0.21 | ||
| LZM (U/L) | 2.26 ± 0.22 | 2.21 ± 0.14 | 0.84 |
| Sampling Time | Parameters | 0% FB (Control) | 60% FB | p Value | |
|---|---|---|---|---|---|
| Crispy texture development period 1 | 4-week | AST (U/L) | 4.74 ± 0.44 | 4.34 ± 0.37 | 0.50 |
| ALT (U/L) | 1.71 ± 0.23 | 2.16 ± 0.39 | 0.33 | ||
| TG (mmol/L) | 1.23 ± 0.22 | 0.98 ± 0.11 | 0.33 | ||
| TC (mmol/L) | 2.69 ± 0.22 | 2.49 ± 0.19 | 0.51 | ||
| HDLC (mmol/L) | 0.37 ± 0.037 | 0.42 ± 0.020 | 0.21 | ||
| 8-week | AST (U/L) | 4.11 ± 0.38 | 3.53 ± 0.35 | 0.29 | |
| ALT (U/L) | 1.43 ± 0.15 | 2.37 ± 0.46 | 0.078 | ||
| TG (mmol/L) | 0.69 ± 0.076 | 0.72 ± 0.073 | 0.75 | ||
| TC (mmol/L) | 2.47 ± 0.094 | 2.95 ± 0.20 | 0.054 | ||
| HDLC (mmol/L) | 0.42 ± 0.057 | 0.31 ± 0.044 | 0.17 | ||
| 12-week | AST (U/L) | 3.43 ± 0.30 | 3.31 ± 0.10 | 0.27 | |
| ALT (U/L) | 2.11 ± 0.17 | 2.45 ± 0.23 | 0.71 | ||
| TG (mmol/L) | 0.83 ± 0.15 | 0.67 ± 0.083 | 0.38 | ||
| TC (mmol/L) | 2.66 ± 0.28 | 2.26 ± 0.14 | 0.23 | ||
| HDLC (mmol/L) | 0.42 ± 0.015 | 0.43 ± 0.013 | 0.59 | ||
| 16-week | AST (U/L) | 3.19 ± 0.24 | 3.65 ± 0.17 | 0.20 | |
| ALT (U/L) | 1.73 ± 0.28 | 2.67 ± 0.38 | 0.075 | ||
| TG (mmol/L) | 0.87 ± 0.090 | 0.75 ± 0.070 | 0.44 | ||
| TC (mmol/L) | 2.44 ± 0.10 | 2.76 ± 0.15 | 0.22 | ||
| HDLC (mmol/L) | 0.32 ± 0.026 | 0.39 ± 0.025 | 0.083 | ||
| Crispy texture retention period 2 | 20-week | AST (U/L) | 3.58 ± 0.088 | 3.60 ± 0.87 | 0.98 |
| ALT (U/L) | 1.95 ± 0.18 | 2.38 ± 0.27 | 0.23 | ||
| TG (mmol/L) | 1.30 ± 0.18 | 1.45 ± 0.089 | 0.47 | ||
| TC (mmol/L) | 4.35 ± 0.33 | 3.73 ± 0.087 | 0.12 | ||
| HDLC (mmol/L) | 0.44 ± 0.076 | 0.48 ± 0.086 | 0.74 | ||
| 24-week | AST (U/L) | 3.65 ± 0.052 | 3.52 ± 0.17 | 0.45 | |
| ALT (U/L) | 1.94 ± 0.29 | 1.87 ± 0.30 | 0.87 | ||
| TG (mmol/L) | 0.82 ± 0.084 | 1.11 ± 0.21 | 0.26 | ||
| TC (mmol/L) | 4.23 ± 0.28 | 3.73 ± 0.15 | 0.18 | ||
| HDLC (mmol/L) | 0.57 ± 0.050 | 0.51 ± 0.048 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Xing, W.; Li, T.; Wei, S.; Zhang, Y.; Song, T.; Yu, H.; Luo, L. Impact of Faba Bean (Vicia faba L.) Diet and Subsequent Withdrawal on GIFT Tilapia (Oreochromis niloticus) Muscle Quality. Fishes 2025, 10, 170. https://doi.org/10.3390/fishes10040170
Xu G, Xing W, Li T, Wei S, Zhang Y, Song T, Yu H, Luo L. Impact of Faba Bean (Vicia faba L.) Diet and Subsequent Withdrawal on GIFT Tilapia (Oreochromis niloticus) Muscle Quality. Fishes. 2025; 10(4):170. https://doi.org/10.3390/fishes10040170
Chicago/Turabian StyleXu, Guanling, Wei Xing, Tieliang Li, Shibo Wei, Ying Zhang, Tingting Song, Huanhuan Yu, and Lin Luo. 2025. "Impact of Faba Bean (Vicia faba L.) Diet and Subsequent Withdrawal on GIFT Tilapia (Oreochromis niloticus) Muscle Quality" Fishes 10, no. 4: 170. https://doi.org/10.3390/fishes10040170
APA StyleXu, G., Xing, W., Li, T., Wei, S., Zhang, Y., Song, T., Yu, H., & Luo, L. (2025). Impact of Faba Bean (Vicia faba L.) Diet and Subsequent Withdrawal on GIFT Tilapia (Oreochromis niloticus) Muscle Quality. Fishes, 10(4), 170. https://doi.org/10.3390/fishes10040170






