Abstract
Surf zones are important nursery grounds for fish larvae and juveniles. However, little is known about fish larvae and juveniles in the surf zone along the coast of Jiangsu Province. To describe the species composition of fish larvae and juveniles, monthly collections were conducted at eight stations during the spring tide from February 2024 to January 2025. The fish larvae and juveniles were sampled using a seine net (1m × 4m; 1 mm mesh aperture), with sampling repeated three times at each station per month. A total of 1435 fish larvae and juveniles were collected, belonging to 42 species and 37 genera in 21 families. Almost half of them were postflexion larvae. Gobiidae, with 14 species, was the most diverse family. Based on the index of relative importance (IRI) result, the dominant species was Amoya pflaumi, accounting for 35.33% of the total number of individuals collected. Common species were Mugil cephalus, Amblychaeturichthys hexanema, Tridentiger trigonocephalus, Acanthogobius ommaturus, Mugilogobius abei, Thryssa mystax, Periophthalmus modestus, Sillago sihama, and Mugilogobius myxodermus. All other species were classified as rare. No fish larvae or juveniles were collected in February 2024 and January 2025. The species number, catch per unit effort (CPUE), Margalef’s richness index, Pielou’s evenness index, and Shannon–Wiener diversity index showed similar trends, and significant differences could be found for each parameter among sampling months. The cluster analysis from the ten months (excluding February 2024 and January 2025) indicated a significant seasonal change in the community structure of fish larvae and juveniles. Fish larvae and juveniles were abundant and diverse at one station near an aquaculture tidal flat for seaweed. This study provides essential basic data to support the management and conservation of fishery resources in the surf zone along the coast of Jiangsu Province.
Keywords:
early life stages of fish; species composition; diversity patterns; littoral zone; Yangtze River Estuary Key Contribution:
This is the first study investigating the species composition of the fish larvae and juveniles in the surf zone along the coast of Jiangsu Province. A new larval distribution record of Elops machnata and Trachidermus fasciatus has been found in the present survey.
1. Introduction
Jiangsu Province is located in the east part of mainland China, and its coast is at the central section of the Yellow Sea area. It has the longest length of coastline and the biggest tidal flat in China [1]. Characterized by predominantly flat terrain and plains, the coast exhibits the following three primary coastline variants: sandy, muddy, and bedrock coasts. Among them, the muddy coast comprises approximately 93% of the total coastline extent [2]. The coast is facing major threats from human activities and natural factors. Over the past two decades, the Jiangsu coastline has significantly changed, the natural has coastline decreased while the artificial coastline has increased, and many coastal reclamation projects have been conducted around the tidal flats for aquaculture ponds, port engineering, and artificial levees. Humans’ lack of awareness of nature protection and over-exploitation lead to serious damage to wetland ecosystems [3,4,5]. Adjacent to Jiangsu lie four major fishing grounds (Lvsi, Haizhou Bay, Yangtze River Estuary, and Dasha), which constitute one of China’s primary marine fishery bases [6]. The main fish caught in this area include Collichthys lucidus and Larimichthys polyactis; both are economically important species [7]. However, several studies have shown that the yield of some economic fish species has decreased. Their biological characteristics show a trend of a shorter body length of the largest captured individuals, a shorter body length of spawning adults, and a younger age structure [8,9,10]. The population density of adult fish has decreased, making it difficult to form large-scale fishing seasons. Instead, there is an increase in the occurrence of small-sized fish, young fish, and low-value fish [11]. The fish larvae and juveniles are important stages during the early developmental stage, which directly affects the replenishment of fish resources [12]. Fish larvae and juveniles are also one of the important links in the food web [13]. Knowledge of fish larvae and juveniles’ assemblages provides fundamental information that is valuable for conservation and management. There has already been some research about the fish larvae and juveniles in Jiangsu, but most of it has focused on the river, lake, and offshore areas [14,15,16,17]. Only a small amount of information is available about the early stages of fish in the coastline area, mainly along the northern coast of the Yangtze River Estuary [18].
Surf zones are an integral part of the coast and are areas where waves break as they approach the interface between land and sea, and serve as nurseries, refuges, foraging areas, and dispersal corridors for organisms, especially marine fish, in their early life stages [19,20]. In this study, the species composition of fish larvae and juveniles in the surf zone along the coast of Jiangsu was examined and spatial and temporal variations were observed, which could provide the main basis for the management of fish resources in Jiangsu.
2. Materials and Methods
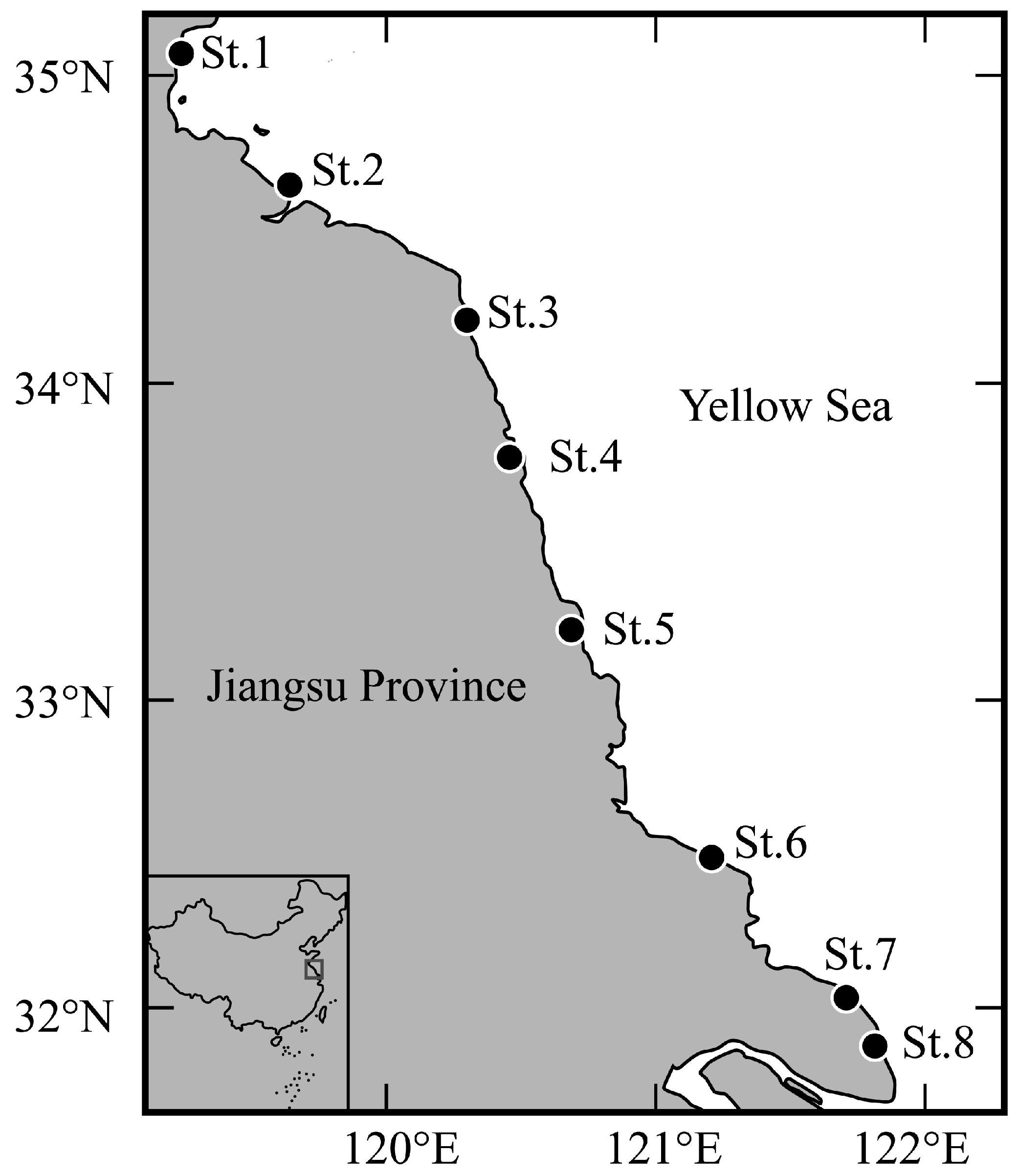
At eight stations (St.1–St.8; Figure 1 and Table 1) in the surf zone along the coast of Jiangsu Province, sampling was conducted once per month using a seine net (1 × 4 m; 1 mm mesh aperture) during the spring tide from February 2024 to January 2025. The net was pulled by two samplers parallel to the shoreline at a depth of 0.2–1.0 m over a distance of a 50 m transect. Three replicate tows were completed at each sampling station, with all samples immediately preserved in 5% seawater formalin for the subsequent laboratory analysis. Water temperature (°C) and salinity (ppt) were measured on site using a thermometer and a digital salinometer, respectively, during the sampling period.
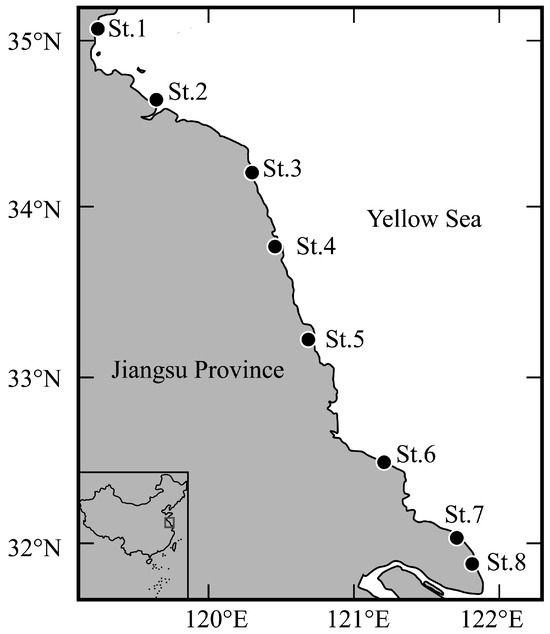
Figure 1.
Sampling stations in the surf zone along the coast of Jiangsu Province. Solid circles indicate the sampling stations.

Table 1.
Situation of sampling stations in the surf zone along the coast of Jiangsu Province.
Fish larvae and juveniles were counted and identified to the lowest possible taxon with the information currently available [6,21,22]. Unlabeled lengths indicated body length (BL) (notochord length for preflexion and flexion larvae and standard length for postflexion larvae, juveniles, and young fish).
The catch per unit effort (CPUE; individual/hauls) for each month or at each station was used in the following equation:
where n is the total number of collected individuals for each month or at each station and E is the total number of hauls for each month or at each station.
The index of relative importance (IRI) was calculated for each species by the following formula:
where N% is the percentage of the specimen number of each species among the total number of fish species and F% is the species frequency per species sampled as a percentage. Accordingly, each species was defined as dominant (IRI ≥ 100), common (100 > IRI ≥ 10), or rare (IRI < 10) [19].
Diversity studies were analyzed using Margalef’s richness index (D), Shannon–Wiener’s diversity index (H′), and Pielou’s evenness index (J) [18].
where S is the number of fish larvae and juveniles, N is the number of species, and Pi is the proportion of individuals in taxa i.
The CPUE data were square-root-transformed to reduce the effects of extremum and then subjected to a non-metric multi-dimensional scaling ordination (n-MDS) using Bray–Curtis’s similarity index [20]. For visualization, a two-dimensional n-MDS plot was generated with cluster points (CLUSTER) to illustrate the relationships among samples. An analysis of similarities (ANOSIM) was used to test differences between communities by month or station, and the percentage similarity analysis (similarity of percentage analysis, or SIMPER) was used to ascertain the similarity and the typical species within a similar community structure. Primer 6.0 [23] was used to perform this analysis.
Differences in the species number, CPUE, Margalef’s richness index, Shannon–Wiener’s diversity index, and Pielou’s evenness index among all sampling months or stations were tested using the Kruskal–Wallis test. Furthermore, Pearson correlation coefficients were used to analyze the relationships between environmental (water temperature and salinity) and biotic variables (species number, CPUE, Margalef’s richness index, Shannon–Wiener’s diversity index, and Pielou’s evenness index). These analyses were carried out using SPSS 19.0 [24].
3. Results
3.1. Environmental Conditions
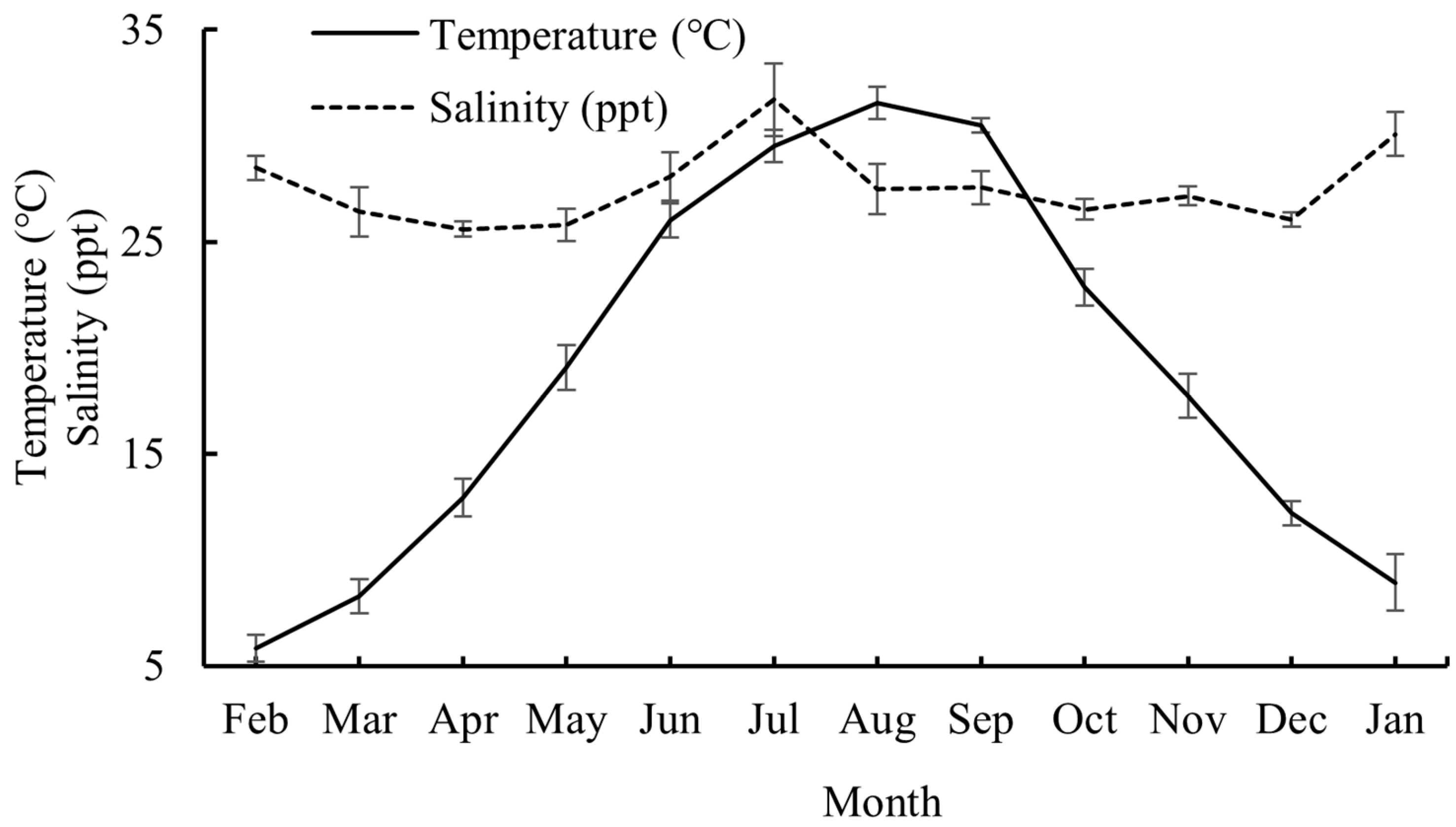
Water temperature exhibited distinct seasonal patterns, with values rising from February to August 2024 (peaking in August) before declining through January 2025. Winter months showed the lowest thermal values. Salinity demonstrated moderate variability, averaging 26.9 ppt between February and June 2024. Elevated levels were recorded in July 2024 and January 2025, while the values stabilized near 27.0 ppt from August to December 2024 (Figure 2).
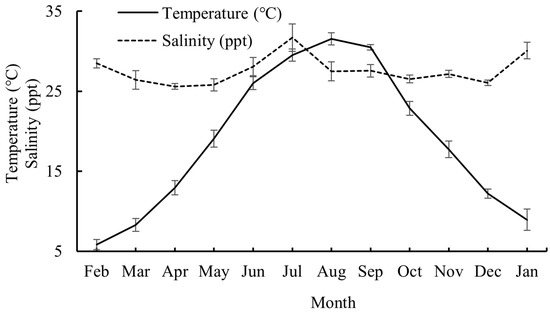
Figure 2.
Mean values of water temperature and salinity from February 2024 to January 2025. Whiskers represent standard errors.
3.2. Community Structure
A total of 1435 fish larvae and juveniles were collected in 288 hauls, belonging to 42 species and 37 genera in 21 families (Table 2). Among them, 49.76% of the total number of individuals were postflexion larvae, followed by juvenile fish (31.99%), flexion larvae (8.78%), and young fish (8.71%). The preflexion larvae were very few (0.77%). Gobiidae, with 14 species, was the most diverse family. Engraulidae had five species, followed by two species of Mugilidae, Clupeidae, Sillaginidae, and Tetraodontidae. The rest of the families had only one species each. There were 24 estuarine species, accounting for 84.04% of the total number of individuals; 15 marine species (15.19%); 3 migration species (0.56%); and 2 freshwater species (0.49). Based on the IRI result, the dominant species was Amoya pflaumi, accounting for 35.33% of the total number of individuals collected from February 2024 to January 2025. The common species were Mugil cephalus, Amblychaeturichthys hexanema, Tridentiger trigonocephalus, Acanthogobius ommaturus, Mugilogobius abei, Thryssa mystax, Periophthalmus modestus, Sillago sihama, and Mugilogobius myxodermus. All other species were classified as rare.

Table 2.
Species composition, index of relative importance (IRI) values, body length range, developmental stage (Pre: preflexion larvae; F: flexion larvae; Post: postflexion larvae; J: juveniles; Y: young), ecological type (Es: estuarine fish; Fr: freshwater fish; Ma: marine fish; Mi: migration fish), and occurring month of fish larvae and juveniles in the surf zone along the coast of Jiangsu Province from February 2024 to January 2025.
3.3. Temporal Variations
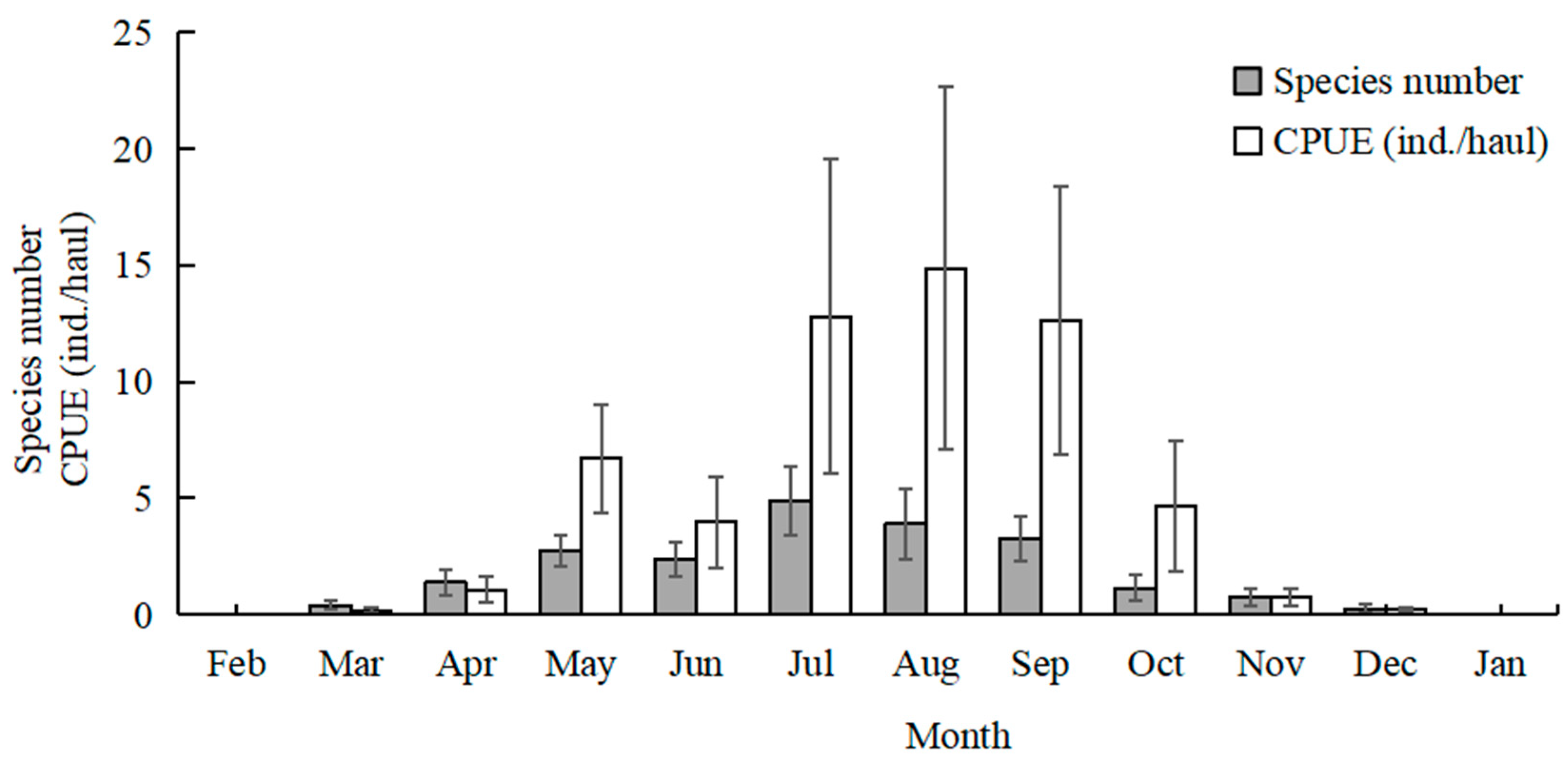
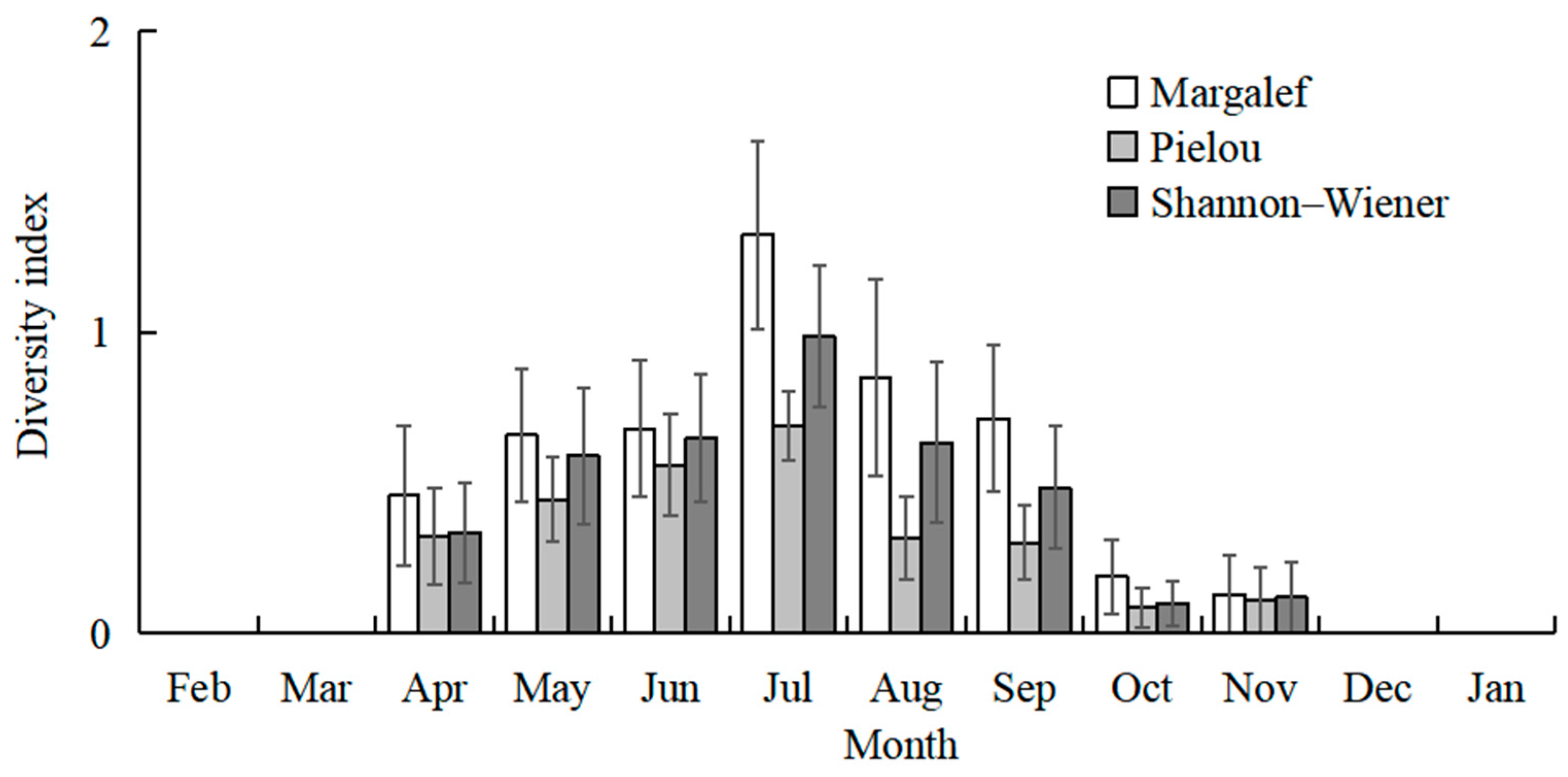
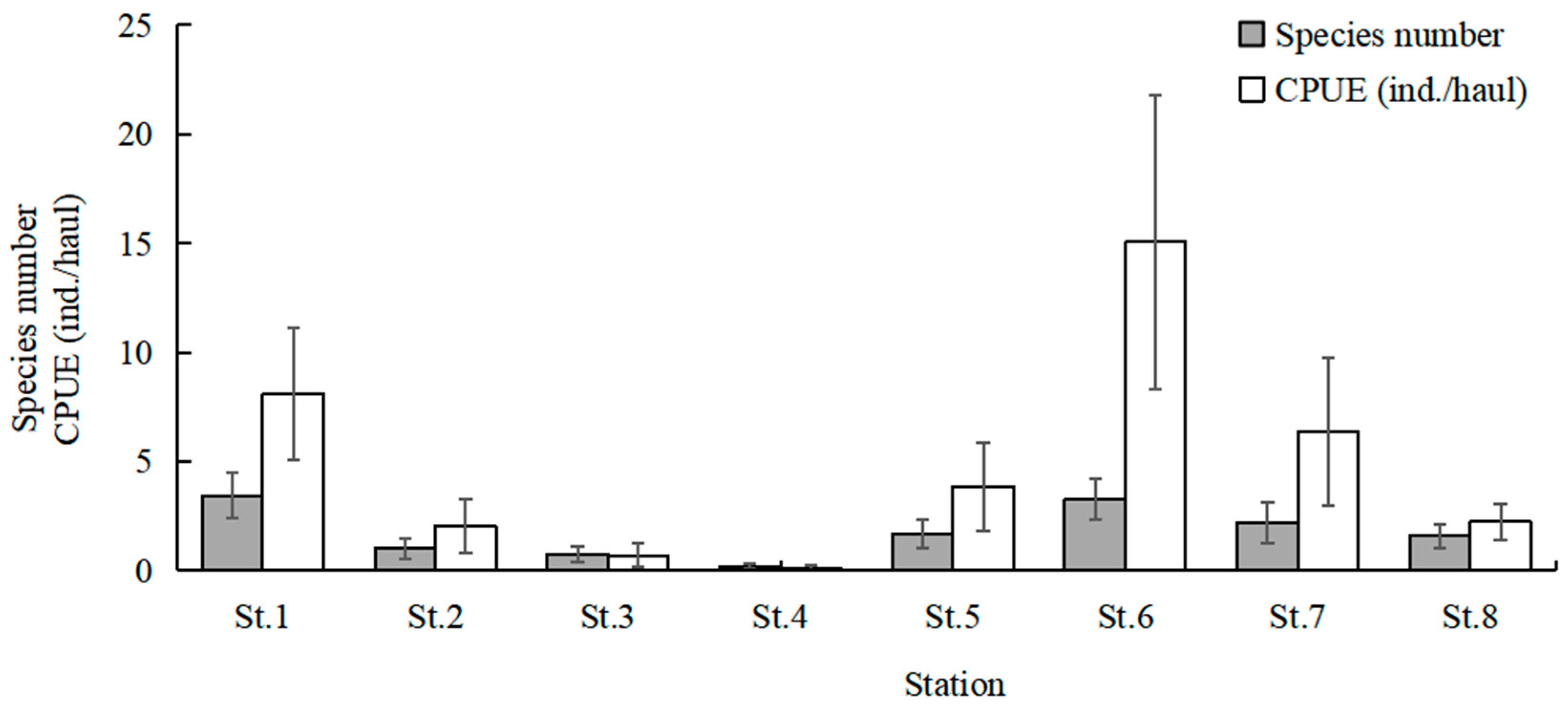
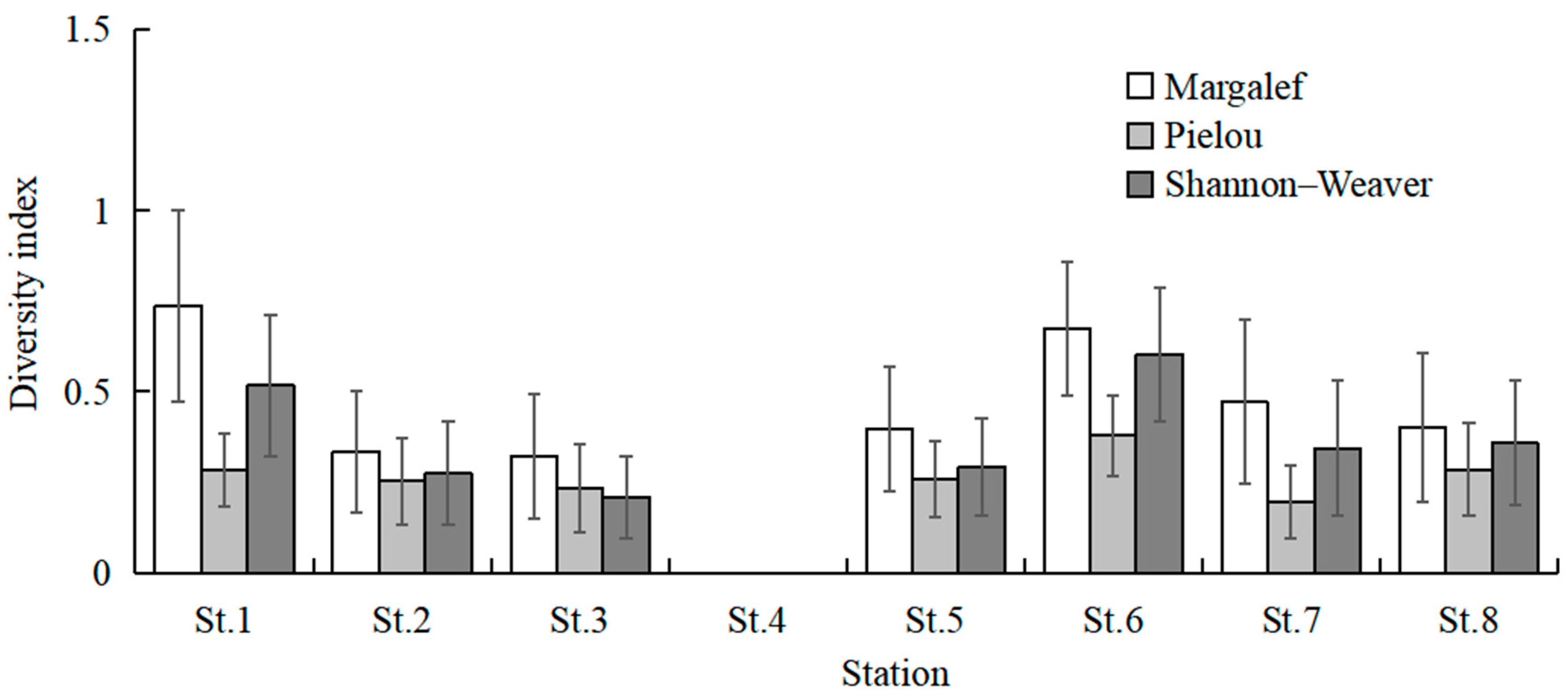
The highest total species number was recorded in July (20 species), followed by August (15 species), and June and September (12 species). No fish larvae or juveniles were collected in February 2024 and January 2025. July and August had higher mean values of species number than the other sampling months (Kruskal–Wallis test, p < 0.001; Figure 3). The mean values of CPUE in July, August, and September were higher than in the other months (Kruskal–Wallis test, p < 0.001; Figure 3). The mean values of Margalef’s richness index in July and August were higher than the other months (Kruskal–Wallis test, p < 0.001; Figure 4). The mean values of Pielou’s evenness index were higher in June and July compared with the other months (Kruskal–Wallis test, p < 0.001; Figure 4). The mean values of Shannon–Wiener’s diversity index were higher in June, July, and August compared with the other months (Kruskal–Wallis test, p < 0.001; Figure 4). Similar trends and significant differences could be found for each parameter among the sampling months.
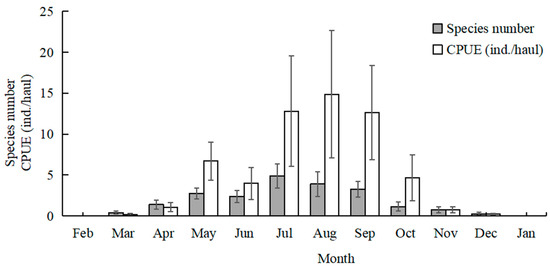
Figure 3.
Temporal variations in mean values of species number and catch per unit effort (CPUE). Whiskers represent standard errors.
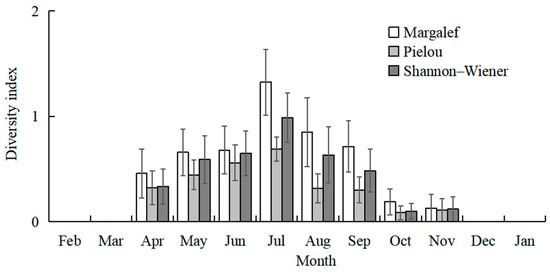
Figure 4.
Temporal variations in mean values of Margalef’s richness index, Shannon–Wiener’s diversity index (H′), and Pielou’s evenness index (J). Whiskers represent standard errors.
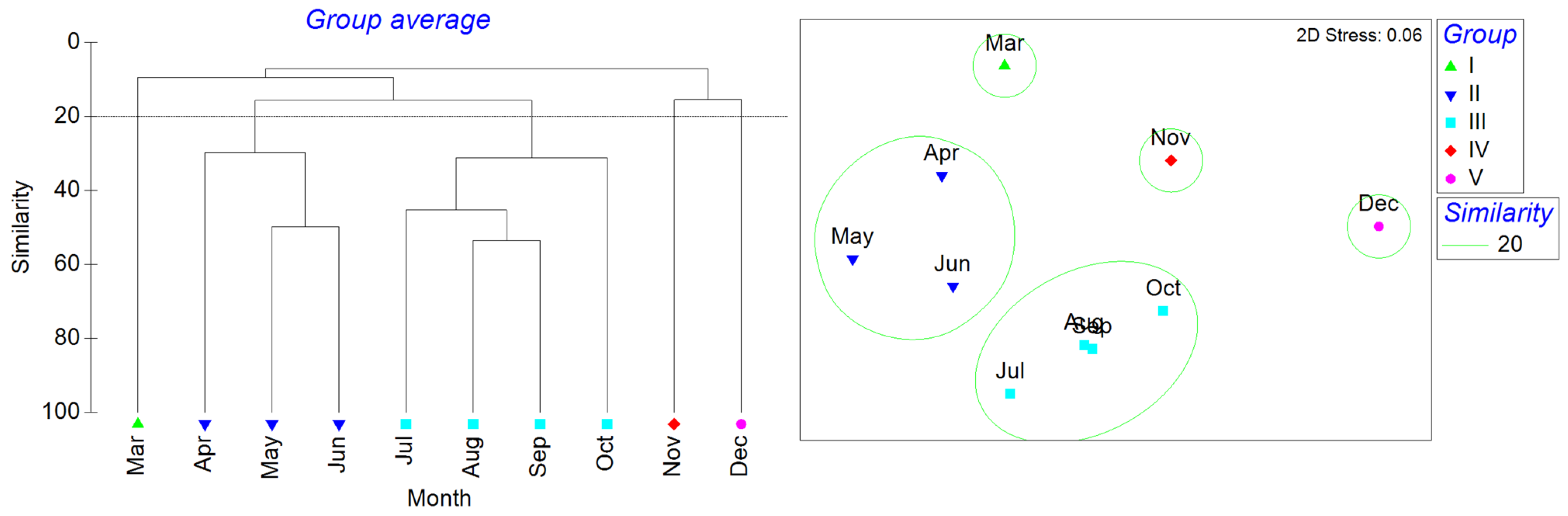
As no fish were collected in February 2024 and January 2025, data from these months were excluded in the monthly clustering analysis to prevent rare data from affecting the overall results. The cluster analysis of the remaining 10 months identified five distinct groups based on a similarity threshold of 20 (Figure 5). March was clustered as group I, April–June was group II, July–October was group III, November was group IV, and December was group V. The n-MDS showed that the stress was 0.06. The ANOSIM analysis values, R = 0.957 and P = 0.01, indicated that the differences between the five groups were significant. The results from the SIMPER analysis revealed that group II had an average similarity of 36.44%, with Amblychaeturichthys hexanema contributing 24.21%, Mugil cephalus contributing 23.88%, and Mugilogobius abei contributing 17.50%. Group III had an average similarity of 39.61%, with Amoya pflaumi contributing 54.63%. Groups I, IV, and V had only one month.
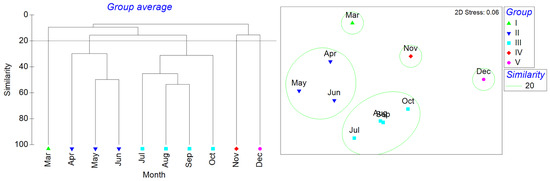
Figure 5.
Temporal similarity clustering analysis based on an ANOSIM of fish communities from March to December 2024.
3.4. Spatial Variations
The highest total species number was observed at St.6 (23 species), followed by St.1 (20 species) and St.7 (18 species). St.1 and St.6 had higher mean values of species number than the other stations (Kruskal–Wallis test, p = 0.022; Figure 6). The mean values of CPUE at St.1, St.6, and St.7 were higher than the other stations (Kruskal–Wallis test, p = 0.020; Figure 6). The mean values of Margalef’s richness index at St.1 and St.6 were higher than the other stations (Kruskal–Wallis test, p = 0.135; Figure 7). The mean values of Pielou’s evenness index were higher at St.1 and St.6 compared with the other stations (Kruskal–Wallis test, p = 0.254; Figure 7). The mean values of Shannon–Wiener’s diversity index were higher at St.1 and St.6 compared with the other stations (Kruskal–Wallis test, p = 0.125; Figure 7). Similar trends were discernible for each parameter, and significant differences were found for the species number and CPUE among sampling stations.
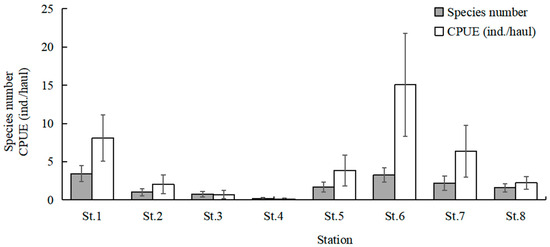
Figure 6.
Spatial variations in mean values of species number and catch per unit effort (CPUE). Whiskers represent standard errors.
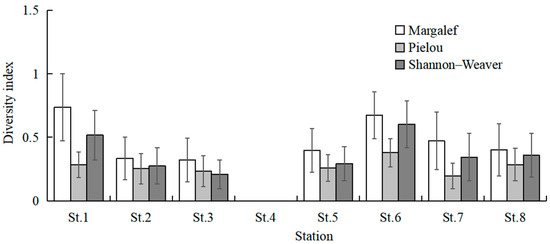
Figure 7.
Spatial variations in mean values of three diversity indexes. Whiskers represent standard errors.
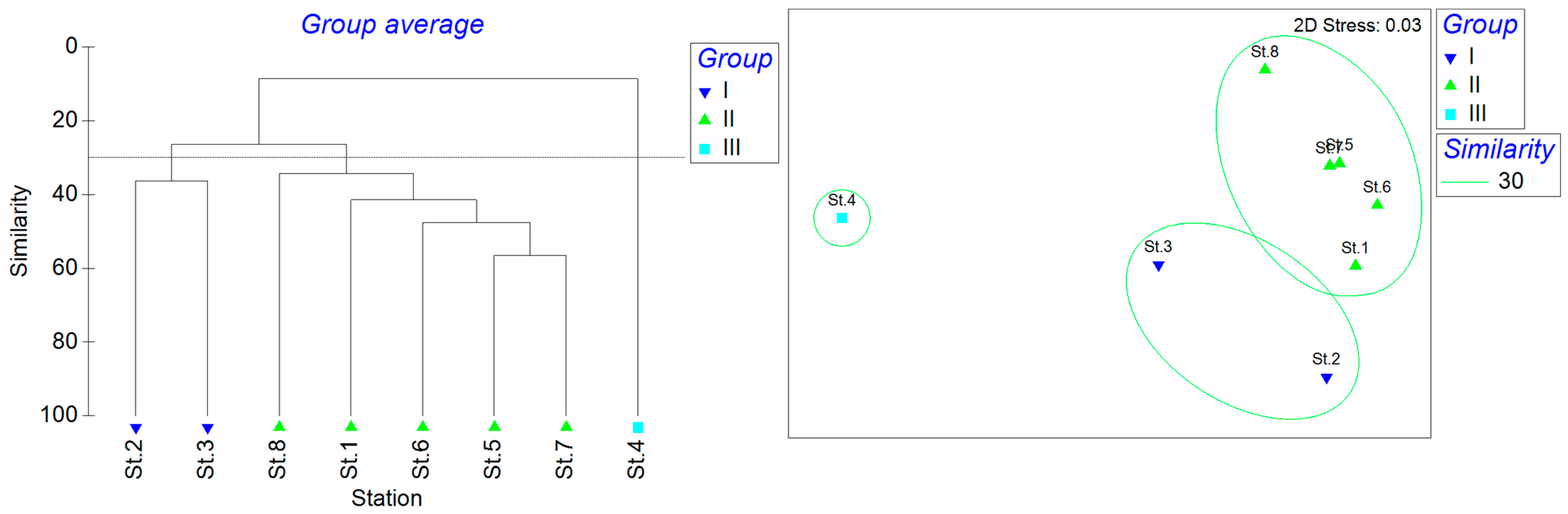
The cluster analysis of the eight stations identified three distinct groups based on a similarity threshold of 30 (Figure 8). St.2 and St.3 were clustered as group I. St.1 and Sts.5–8 were group II, and St.4 was group III. The n-MDS showed that the stress was 0.03. The ANOSIM analysis showed that R = 0.882 and P = 0.6, indicating that the differences between the three groups were not significant. The SIMPER analysis showed that the average similarity within group I was 36.30%, with Amblychaeturichthys hexanema contributing 54.83%, Acanthogobius ommaturus contributing 28.64%, and Tridentiger trigonocephalus contributing 16.53%. The average similarity within Group II was 41.40%, with Amoya pflaumi contributing 26.87%, Mugilogobius abei contributing 13.20%, and Periophthalmus modestus contributing 10.13%. Group III had only one station.
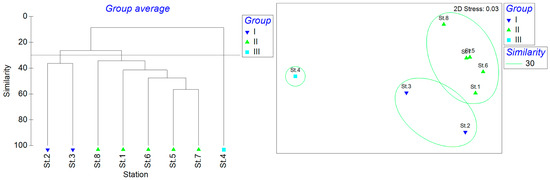
Figure 8.
Spatial similarity clustering analysis based on an ANOSIM of fish communities.
3.5. The Relationships Between Environmental and Biotic Variables
The species number, CPUE, Margalef’s richness index, Pielou’s evenness index, and Shannon–Wiener’s diversity index showed positive correlations with temperature (p < 0.01; Table 3), while no biotic factors showed substantial correlations with salinity (p > 0.05; Table 3).

Table 3.
Matrix showing the Pearson correlation coefficients for environmental and biotic variables.
4. Discussion
4.1. Species Composition
The Jiangsu coastline receives freshwater inputs from multiple rivers [5,6], with all sampling stations influenced by estuarine discharge and aquaculture effluents. This explains the predominance of estuarine species (84.04% abundance) over marine taxa (15.19%) in the surf zone along the coast of Jiangsu Province. The fish larvae and juveniles in this area were mostly gobies, which were distributed from March to December at almost all sampling stations (except for St.1), such as the dominant species, Amoya pflaumi, and other important species, like Amblychaeturichthys hexanema, Tridentiger trigonocephalus, Acanthogobius ommaturus, Mugilogobius abei, Periophthalmus modestus, and Mugilogobius myxodermus. The abundance of gobies most probably related to the adaptation of gobies to the brackish, high turbidity, and wavy characteristics of the waters in the surf zone [25]. The gobies are both a predator of many benthic organisms and a bait organism for a variety of high-trophic-level fish, playing an important role in marine food webs and ecosystems [26].
Compared with neighboring waters throughout one year, first, the surf zone in the Yangtze River Estuary, it was heavily influenced by freshwater discharge from the Yangtze River [18], resulting in a dominance of migratory species such as Coilia nasus and various freshwater species, including cyprinid species. Second, in the surf zone of Sijiao Island, located near Jiangsu Province, there were 46 species from 28 families collected previously. Engraulis japonicus was the most dominant species, and marine fish accounted for the majority of the species composition [27]. Third, the species composition in this study was similar to that in the surf zone of the north coast of Hangzhou Bay. Amoya pflaumi was the dominant species and the estuarine fish were foremost, but there was higher species diversity and CPUE due to the wider range of salinity variations (0-26 ppt) [28]. Therefore, this could be an indication that salinity is the main factor contributing to variations in fish larvae and juvenile communities in these areas.
4.2. Spatial–Temporal Variations
There has been little research regarding the early stages of fish species along the coast of Jiangsu Province. The present study has shown that fish larvae and juveniles in the surf zone are not randomly distributed, but show significant seasonal variability. The mean values of species number and CPUE were low in autumn and winter and high in spring and summer. Three diversity indices showed similar seasonal variations. The seasonal trends observed were consistent with the findings of previous studies, indicating that the primary spawning period for fish in the surf zones of temperate regions is spring [18,27,28,29,30].
The main contributing species in the surf zone also had obvious differences in the occurring period due to the different spawning seasons of various species. Amblychaeturichthys hexanema dominated from March to June. Yang et al. also reported that A. hexanema was the most dominant species from April to July in the coastal waters of the southern Shandong Peninsula [31], while there were only a few juveniles in the Yangtze River Estuary in June [18] and no larvae or juveniles in Hangzhou Bay [28].
Mugil cephalus and Mugilogobius abei were contributing species from April to June. M. cephalus has been reported to spawn from October to February, and the juveniles and young could be captured in April and May along the coast of Jiangsu Province [6]. Kanabashira et al. reported that the eggs of M. abei were observed during the period from April to July in Japan, with a peak in May [32]. The larvae were also found in the surf zone of the Yangtze River Estuary from June to August [16].
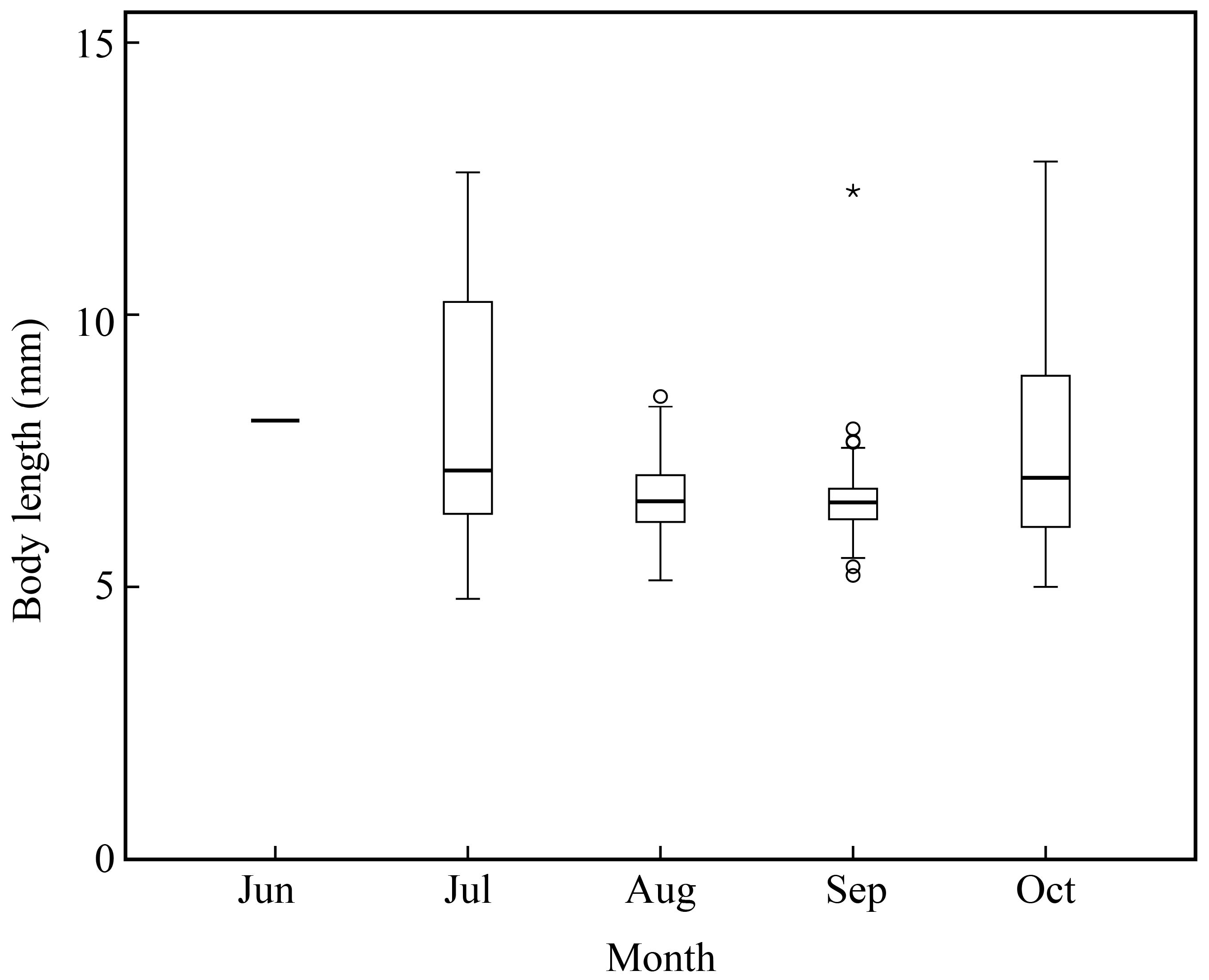
Amoya pflaumi larvae and juveniles occurred in the surf zone from June to October and were the most contributing species from July to October (Table 1; Figure 5 and Figure 9). Chen et al. also reported that this species could be collected from July to November and was the most dominant species in Hangzhou Bay [28]. Liu et al. found that this species mainly spawned in Shandong Province in one month (June), while a much longer spawning period occurred in Japan and Korea [33]. The present study also indicated a longer spawning period for A. pflaumi. The difference in their spawning period might be due to the extremely lower temperature in Jiaozhou Bay than in other waters. Additionally, the salinity, current conditions, day length, and prey and predator density would also affect the reproduction of A. pflaumi [33].
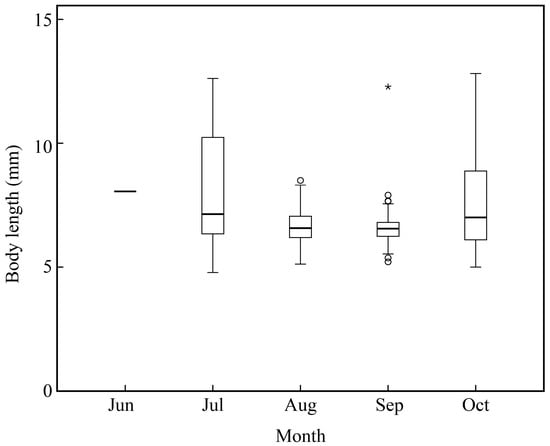
Figure 9.
Body length distribution of Amoya pflaumi in the surf zone along the coast of Jiangsu Province. Open circles represent potential outliers, asterisk represents extreme value.
The highest species number and CPUE occurred at St.6, where there is an aquaculture tidal flat for seaweed. Stimulated by the higher concentrations of nutrients in the waters of aquaculture tidal flats, the productivity and biomass of phytoplankton have increased, which, in turn, enhances the abundance of zooplankton [34]. These planktonic organisms can serve as food for fish larvae and juveniles, which might improve the species’ abundance and diversity. There was no significant difference between fish larvae and juvenile communities per station. This is related to the fact that these sampling stations are mostly muddy mudflats with similar environments.
Among the collected fish larvae and juveniles, there were two scarce species, Elops machnata and Trachidermus fasciatus. E. machnata is a warm-water species and is distributed in the East China Sea and South China Sea in China [22]. The larvae are leptocephalus-like with a caudal fin [21]. The most northern distribution of this species’ larvae was reported along the northern coast of the Yangtze River Estuary [35]. In the present study, a larva (28.61 mm) was found at St.7 in June, a new distribution record with a more northern distribution than the previous study. T. fasciatus is a warm temperate species distributed in the rivers near the Yellow Sea and East China Sea in China. It is one of the most famous catadromous and endangered species [22]. Five flexion larvae (8.39-9.83 mm) and one juvenile (25.87 mm) were collected at St.1 in April and May, respectively. This is the first record of a larval distribution of T. fasciatus in Jiangsu and could indicate that there was a nursery ground of T. fasciatus near St.1, and needs more attention in this area.
5. Conclusions
In this study, a total of 1435 fish larvae and juveniles belonging to 42 species were collected from eight surf zone stations along the coast of Jiangsu Province, of which over 35% were Amoya pflaumi. Almost half of them were postflexion larvae. The species number, CPUE, and three diversity indices showed seasonal variations, being higher in summer. The sampling station near an aquaculture tidal flat for seaweed had the highest biotic parameter. A new record of larval distribution of Elops machnata and Trachidermus fasciatus was found in Jiangsu Province. Given the diverse yet often overlooked fish occurrence in the surf zone, further conservation measures are urgently needed. As the present study was limited to the surf zone area, additional surveys are required in adjacent waters of Jiangsu Province to corroborate the spawning and migration patterns of fish. Moreover, future research should encompass a broader range of physical, chemical, and biological parameters to explore the intricate relationships between the type of environment and structure of the associated fish community.
Author Contributions
Conceptualization: J.Z.; methodology: J.Z.; software: Y.C.; validation: B.Q.; formal analysis: X.W. and T.Z.; investigation: X.W., B.Q., T.Z., J.S. and C.W.; resources: J.Z. and J.S.; data curation: Y.C. and T.Z.; writing—original draft preparation: X.W. and C.W.; writing—review and editing: X.W., J.Z. and C.W.; visualization: T.Z.; supervision: J.Z. and C.W.; project administration: J.Z. and J.S.; funding acquisition: C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Central Agricultural Industry Development Fund Project 2024: Jiangsu Offshore Fishery Resources Survey.
Institutional Review Board Statement
This study complies with the Specifications for Oceanographic Survey—Part 6: Marine biological survey and laws of China. Fish larvae and juveniles were collected using a seine net as this sampling method results in the mortality of the sampled fish; they were not reared or maintained in a laboratory. According to the above guidelines, ethical approval was not needed for this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.
Acknowledgments
We extend our sincere gratitude to the students in our lab and the staff from the Fisheries offices for their generous assistance with the sample collection. We also want to thank Roland Passmore for improving the English of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, B.X.; Liu, Y.X.; Wang, L.; Liu, Y.C.; Sun, C.; Fagherazzi, S. Stability evaluation of tidal flats based on time-series satellite images: A case study of the Jiangsu central coast, China. Estuar. Coast. Shelf Sci. 2022, 264, 107697. [Google Scholar] [CrossRef]
- Zhuang, L.L.; Huang, L.R.; Zhao, C.Y.; Zheng, G.H.; Tang, W.; Zhou, D.A.; Zhu, J.T. Identification and assessment of the mudflat ecological vulnerability dominated by coastline evolution in Jiangsu. Ecol. Indic. 2024, 168, 112761. [Google Scholar] [CrossRef]
- Zhao, B.X.; Liu, Y.X.; Wang, L. Evaluation of the stability of muddy coastline based on satellite imagery: A case study in the central coasts of Jiangsu, China. Remote Sens. 2023, 15, 3323. [Google Scholar] [CrossRef]
- Li, L.J.; Li, G.S.; Du, J.Q.; Wu, J.; Cui, L.L.; Chen, Y.H. Effects of tidal flat reclamation on the stability of coastal wetland ecosystem services: A case study in Jiangsu Coast, China. Ecol. Indic. 2022, 145, 109697. [Google Scholar] [CrossRef]
- Xu, N.; Wang, Y.Q.; Huang, C.H.; Jiang, S.; Jia, M.M.; Ma, Y. Monitoring coastal reclamation changes across Jiangsu Province during 1984–2019 using landsat data. Mar. Policy 2022, 136, 104887. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, H.L. Fishes of Jiangsu Provinc; China Agriculture Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Gao, S.K.; Chen, Z.; Lu, Y.N.; Li, Z.; Zhang, S.; Yu, W. Comparison of Marine Ecosystems of Haizhou Bay and Lvsi Fishing Ground in China Based on the Ecopath Model. Wate 2022, 14, 1397. [Google Scholar] [CrossRef]
- Song, X.J.; Hu, F.; Xu, M.; Zhang, Y.; Jin, Y.; Gao, X.D.; Liu, Z.L.; Ling, J.Z.; Li, S.F.; Cheng, J.H. Spatiotemporal Distribution and Dispersal Pattern of Early Life Stages of the Small Yellow Croaker (Larimichthys polyactis) in the Southern Yellow Sea. Diversity 2024, 16, 521. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Tian, H.; Liu, S.H.; Zu, K.W.; Xia, X.M. Impact of climate change on long-term variations of small yellow croaker (Larimichthys polyactis) winter fishing grounds. Front. Mar. Sci. 2022, 9, 915765. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xu, B.D.; Ji, Y.P.; Zhang, C.L.; Ren, Y.P.; Xue, Y. Comparison of habitat models in quantifying the spatio-temporal distribution of small yellow croaker (Larimichthys polyactis) in Haizhou Bay, China. Estuar. Coast. Shelf Sci. 2021, 261, 107512. [Google Scholar] [CrossRef]
- Ge, H.; Shi, J.J.; Wang, C.Q.; Wang, Y.P.; Yan, X.; Wu, L.; Zhu, H.C.; Tang, J.H. Analysis of marine catch structure and input factor change in Jiangsu Province based on statistical data. Fish. Inf. Strtg. 2022, 37, 157–167, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.D.; Zhao, D.B.; Liu, Q.A.; Lu, T.Y.; Zhong, J.S.; Chen, W.D.; Xie, S.W.; Chen, S. Species composition of fish larvae and juveniles in the Nanji Islands, China. Fishes 2024, 9, 421. [Google Scholar] [CrossRef]
- Bruno, D.O.; Riccialdelli, L.; Acha, E.M.; Fernández, D.A. Seasonal variation of autochthonous and allochthonous carbon sources for the first levels of the Beagle Channel food web. J. Mar. Syst. 2023, 239, 103859. [Google Scholar] [CrossRef]
- Ren, P.; Schmidt, B.V.; Fang, D.A.; Xu, D.P. Spatial distribution patterns of fish egg and larval assemblages in the lower reach of the Yangtze River: Potential implications for conservation and management. Aquat. Conserv. 2021, 31, 1929–1944. [Google Scholar] [CrossRef]
- Dai, P.; Zhou, Y.; Ren, P.; Wang, Y.P.; Xu, P.; Liu, K. Spatial and temporal distributions of fish larvae and juveniles in Lake Wuli, Lake Taihu. Acta Hydrobio. Sin. 2020, 44, 577–586, (In Chinese with English Abstract). [Google Scholar]
- Xu, M.; Liu, Z.L.; Song, X.J.; Wang, F.; Wang, Y.H.; Yang, L.L.; Otaki, T.; Shen, J.B.; Komatsu, T.; Cheng, J.H. Tidal variations of fish larvae measured using a 15-day continuous ichthyoplankton survey in subei shoal: Management implications for the red-crowned crane (Grus japonensis) population in Yancheng Nature Reserve. Animals 2023, 13, 3088. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, Z.L.; Wang, Y.H.; Jin, Y.; Yuan, X.W.; Zhang, H.; Song, X.J.; Otaki, T.; Yang, L.L.; Cheng, J.H. Larval spatiotemporal distribution of six fish species: Implications for sustainable fisheries management in the East China Sea. Sustainability 2022, 14, 14826. [Google Scholar] [CrossRef]
- Jiang, R.J.; Zhong, J.S.; Zhang, D.L.; Fu, C.Z. Species composition and diversity of fish larvae and juveniles in the surf zone of the Yangtze River Estuary. Zool. Res. 2008, 29, 297–304, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xia, W.T.; Miao, Z.B.; Wang, S.; Chen, K.; Liu, Y.L.; Xie, S.G. Influence of tidal and diurnal rhythms on fish assemblages in the surf zone of sandy beaches. Fish. Oceanogr. 2023, 32, 448–460. [Google Scholar] [CrossRef]
- Xia, W.T.; Miao, Z.B.; Chen, K.; Lu, Y.; Wang, S.; Zhu, J.Y.; Xie, S.G. Seasonal patterns of juvenile fish assemblages in the surf zones of tropical sandy beaches along Gaolong Bay, Hainan Island, China. Divers. Distrib. 2024, 30, e13913. [Google Scholar] [CrossRef]
- Okiyama, M. An Atlas of Early Stage Fishes in Japan, 2nd ed.; Donghai University Publishing Association: Kanagawa, Japan, 2014. (In Japanese) [Google Scholar]
- Wu, H.L.; Zhong, J.S. Key to Marine and Estuarial Fishes of China; China Agriculture Press: Beijing, China, 2021. (In Chinese) [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER: Getting Started with V6; PRIMER-E Ltd.: Plymouth, UK, 2005. [Google Scholar]
- Arbuckle, J.L. IBM SPSS Amos 19 User’s Guide; Amos Development Corporation: Crawfordville, FL, USA, 2010. [Google Scholar]
- Wu, H.L.; Zhong, J.S.; Chen, I.S.; Yong, N.; Chong, D.W.; Shao, K.T. Fauna Sinica Ostichthyes Perciformes (V) Gobioidei; Science Press: Beijing, China, 2008. (In Chinese) [Google Scholar]
- Li, M.H.; An, C.T.; Li, A.; Liu, K.Y.; Liu, S.F.; Zhuang, Z.M. DNA barcodes enable higher taxonomic assignments in goby in the Bohai Sea and Yellow Sea of China. J. Fish. Sci. China 2022, 29, 1179–1188, (In Chinese with English Abstract). [Google Scholar]
- Mao, C.Z.; Zhong, J.S.; Fang, T.Q.; Ge, C.G.; Yang, P.H.; Chen, Y.G.; Chen, X.J. Tempo-spatial variation of larval and juveniles fish communities in a sandy surf zone on Sijiao Island. Acta Ecol. Sin. 2016, 36, 286–294, (In Chinese with English Abstract). [Google Scholar]
- Chen, Y.G.; Zhang, Y.; Zhong, J.S.; Ge, K.K.; Mao, C.Z.; Fang, Y.Q. Comparison in fish larvae and juvenile assemblages between the surf zones of south branch of Yangtze River Estuary and north coast of Hangzhou Bay. J. Shanghai Ocean Univ. 2011, 20, 688–696, (In Chinese with English Abstract). [Google Scholar]
- Senta, T.; Kinoshita, I. Larval and juvenile fishes occurring in the surf zones of western Japan. Trans. Am. Fish. Soc. 1985, 11, 609–618. [Google Scholar] [CrossRef]
- Lin, N.; Shen, C.C.; Zhong, J.S. Composition of fish larvae and juveniles in surf zone of Jiulong River estuary. J. Shanghai Ocean Univ. 2009, 18, 686–694, (In Chinese with English Abstract). [Google Scholar]
- Yang, Y.Y.; Zhu, M.M.; Xu, B.Q.; Feng, Y.Y.; Wang, X.X.; Zhang, X.M.; Li, F.; Chen, J.Q. Community structure of ichthyoplankton and its relationship with environmental factors in coastal waters of southern Shandong Peninsula. Ecol. Environ. Sci. 2021, 30, 995–1004, (In Chinese with English Abstract). [Google Scholar]
- Kanabashira, Y.; Sakai, H.; Yasuda, F. Early development and reproductive behavior of the gobiid fish, Mugilogobius abei. Jpn. J. Ichthyol. 1980, 27, 191–198. [Google Scholar]
- Liu, Y.W.; Xue, Y.; Ji, L.M.; Ren, Y.P. Preliminary study on biological characteristics of Amoya pflaumi in Jiaozhou Bay. Period. Ocean Univ. China 2013, 43, 38–43, (In Chinese with English Abstract). [Google Scholar]
- Shen, H.; Wan, X.H.; He, P.M. Review of research on bioremediation in the eutrophication of intertidal flats. Mar. Sci. 2016, 40, 160–169. [Google Scholar]
- Liu, L.; Lin, N.; Zhong, J.S.; Jiang, R.J.; Zhang, D.L. Occurrences on the fish larvae of three warm water species in the surf zone of the Yangtze Estuary. Mar. Fish. 2008, 30, 62–66, (In Chinese with English Abstract). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).