The Effect of the Marine Environment on the Distribution of Sthenoteuthis oualaniensis in the East Equatorial Indian Ocean
Abstract
:1. Introduction
2. Materials and Methods
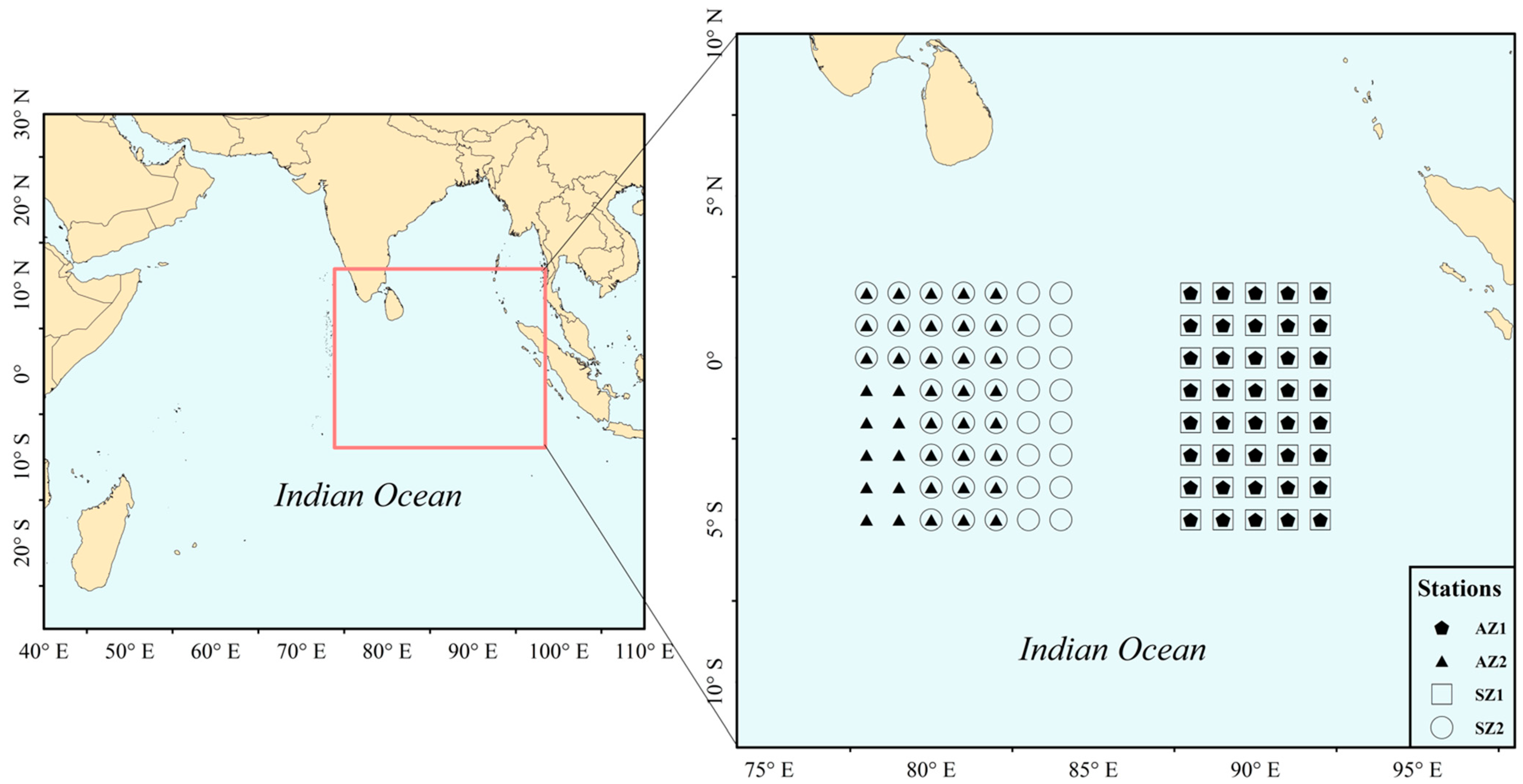
2.1. Fishery Data
2.2. Environment Data
2.3. Data Analyses
3. Results
3.1. The Distribution of CPUE of S. oualaniensis
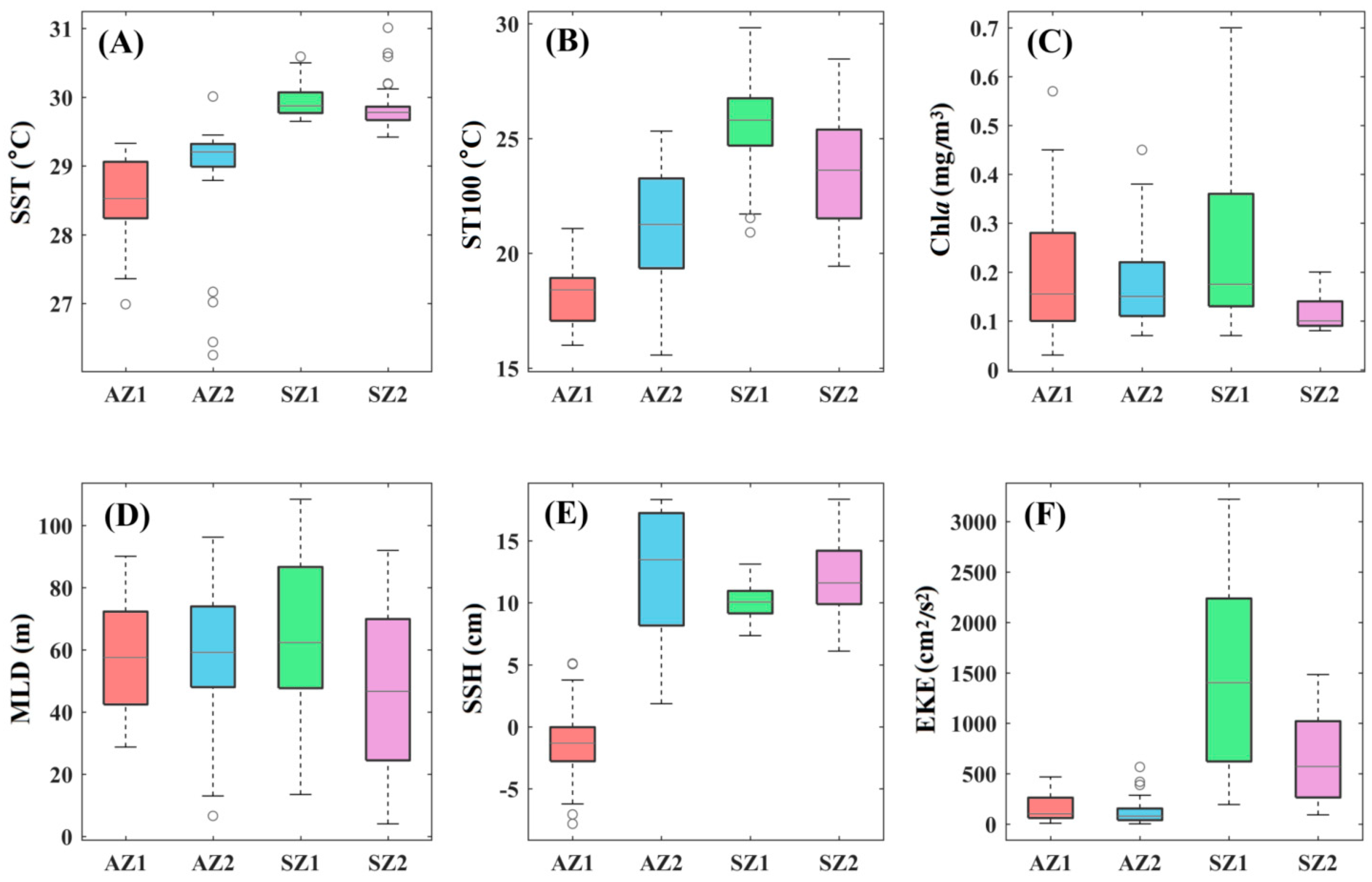
3.2. Environmental Factors at Survey Stations
3.3. GAM Analysis
4. Discussion
4.1. The Distribution of S. oualaniensis
4.2. The Effect of Environmental Factors on the Distribution of S. oualaniensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunning, M.C. A Review of Systematics, Distribution and Biology of the Arrow Squid Genera Ommastrephes d’Orbigny, 1835, Sthenoteuthis Verrill, 1880 and Ornithoteuthis Okada, 1927 (Cephalopoda: Ommastrephidae); Voss, N.A., Vecchione, M., Toll, R.B., Sweeney, M.J., Eds.; Systematics and Biogeography of Cephalopods; Smithsonian University Press: Washington, DC, USA, 1998; pp. 425–433. [Google Scholar]
- Chen, X.; Liu, B.; Tian, S.; Qian, W.; Zhao, X. Fishery Biology of Purpleback Squid, Sthenoteuthis oualaniensis, in the Northwest Indian Ocean. Fish. Res. 2007, 83, 98–104. [Google Scholar]
- Trotsenko, B.G.; Pinchukov, M.A. Mesoscale distribution features of the purpleblack squid Sthenoteuthis oualaniensis with reference to the structure of the upper quasi-homogeneous layer in theWest India Ocean. Oceanology 1994, 34, 380–385. [Google Scholar]
- Kurichithara, K.K.; Najmudeen, T.M.; Ragesh, N.; Jeena, N.S.; Rahuman, S.; Sunil, K.S.; Sasikumar, G.; Mohamed, K.S. Characterisation of an individual of the giant form of the purpleback flying squid Sthenoteuthis oualaniensis (Cephalopoda: Ommastriphidae) in the Arabian Sea and its biological descriptors. Molluscan Res. 2021, 41, 275–284. [Google Scholar]
- Young, R.E.; Hirota, J. Review of the ecology of Sthenoteuthis oualaniensis near the Hawaiian Archipelago. In Contributed Papers to International Symposium on Large Pelagic Squids; Japan Marine Fishery Resources Research Center: Tokyo, Japan, 1998; pp. 113–143. [Google Scholar]
- Fan, J.; Chen, Z.; Feng, X.; Yu, W. Climate-related changes in seasonal habitat pattern of Sthenoteuthis oualaniensis in the South China Sea. Ecosyst. Health Sustain. 2021, 7, 1926338. [Google Scholar] [CrossRef]
- Nigmatullin, C.M. Resources and perspectives of the fisheries of nektonic epipelagic squids in the World Ocean. In Abstracts of the All-Union Conference on Reserve Food Biological Resources of the Open Ocean and Seas of the USSR, Kaliningrad, 1990; MIK: Moscow, Russia, 1990; pp. 11–13. [Google Scholar]
- Mohamed, K.S.; Sasikumar, G.; Koya, K.P.S.; Venkatesan, V.; Kripa, V.; Durgekar, N.R.; Joseph, M.; Alloycious, P.S.; Mani, R.; Vijay, D. Know… The Master of the Arabian Sea—Purple-Back Flying Squid Sthenoteuthis oualaniensis; ICAR—Central Marine Fisheries Research Institute: Kochi, India, 2011. [Google Scholar]
- Zhang, J.; Chen, Z.; Chen, G.; Qiu, Y.; Liu, S.; Yao, S. Hydroacoustic Detection and Estimation Techniques of Squid Sthenoteuthis oualaniensis in the South China Sea. South China Fish. Sci. 2014, 10, 1–11. [Google Scholar]
- Gong, Y.; Zhan, F.; Yang, Y.; Zhang, P.; Kong, X.; Jiang, Y.; Chen, Z. Feeding habits of Symplectoteuthis oualaniensis in the South China Sea. South China Fish. Sci. 2016, 12, 80–87. [Google Scholar]
- André, J.; Haddon, M.; Pecl, G.T. Modelling climate-change-induced nonlinear thresholds in cephalopod population dynamics. Glob. Change Biol. 2010, 16, 2866–2875. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, Y.; Fu, C.; Wang, B.; Li, J.; Ren, Y.; Wan, R. Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 2018, 208, 22–33. [Google Scholar] [CrossRef]
- Ma, S.; Cheng, J.; Li, J.; Liu, Y.; Wan, R.; Tian, Y. Interannual to decadal variability in the catches of small pelagic fishes from China seas and its responses to climatic regime shifts. Deep-Sea Res. II Top. Stud. Oceanogr. 2019, 159, 112–129. [Google Scholar] [CrossRef]
- Han, H.; Jiang, B.; Shi, Y.; Jiang, P.; Zhang, H.; Shang, C.; Sun, Y.; Li, Y.; Xiang, D. Response of the Northwest Indian Ocean purpleback flying squid (Sthenoteuthis oualaniensis) fishing grounds to marine environmental changes and its prediction model construction based on multi-models and multi-spatial and temporal scales. Ecol. Indic. 2023, 154, 110809. [Google Scholar] [CrossRef]
- Su, L.; Chen, Z.; Zhang, P. Reproductive biology of purpleback flying squid (Symplectoteuthis oualaniensis) in the south-central South China Sea in spring and autumn. South China Fish. Sci. 2016, 12, 96–102. [Google Scholar]
- Zhou, W.; Xu, H.; Li, A.; Cui, X.; Chen, G. Comparison of habitat suitability index models for purpleback flying squid (Sthenoteuthis oualaniensis) in the open South China Sea. Appl. Ecol. Env. Res. 2019, 17, 4903–4913. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, W.; Ling, W.; Zhao, C.; Feng, B.; Yan, Y. Relationship between CPUE of Sthenoteuthis oualaniensis and environmental factors in the southeastern Coast of Hainan in Spring. J. Guangdong Ocean Univ. 2020, 40, 63–70. [Google Scholar]
- Tian, S.; Chen, X.; Yang, X. Study on the Fishing Ground Distribution of Symlectoteuthis oualaniensis and Its Relationship with the Environmental Factors in the High Sea of the Northern Arabian Sea. Trans. Oceanogr. Limnol. 2006, 1, 51–57. [Google Scholar]
- Xie, E.; Zhou, Y.; Feng, F.; Wu, Q. Catch per unit effort (CPUE) standardization of purpleback flying squid Sthenoteuthis oualaniensis for Chinese large-scale lighting net fishery in the open sea of South China Sea. J. Dalian Ocean Univ. 2020, 35, 439–446. [Google Scholar]
- Mugo, R.; Saitoh, S.I.; Nihira, A.; Kuroyama, T. Habitat characteristics of skipjack tuna (Katsuwonus pelamis) in the western North Pacific: A remote sensing perspective. Fish. Oceanogr. 2010, 19, 382–396. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Chen, Y.; Yi, Q.; Zhang, Y. Effects of environmental variations on the abundance of western winter-spring cohort of neon flying squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Acta Oceanol. Sin. 2015, 34, 43–51. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Li, J.; Cao, C.; Tian, H.; Li, W.; Tian, Y.; Watanabe, Y.; Lin, L.; Li, Y. Effects of oceanographic environment on the distribution and migration of Pacific saury (Cololabis saira) during main fishing season. Sci. Rep. 2022, 12, 13585. [Google Scholar] [CrossRef]
- Yu, W.; Wen, J.; Chen, X.; Gong, Y.; Liu, B. Trans-Pacific multidecadal changes of habitat patterns of two squid species. Fish. Res. 2021, 233, 105762. [Google Scholar] [CrossRef]
- Shi, P.; Du, Y.; Wang, D.; Gan, Z. Annual cycle of mixed layer in South China Sea. J. Trop. Oceanogr. 2001, 20, 10–17. [Google Scholar]
- Yentsch, C.S.; Menzel, D.W. A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep Sea Res. Oceanogr. Abstr. 1963, 10, 221–231. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R. Generalized additive models: Some applications. J. Am. Stat. Assoc. 1987, 82, 371–386. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Stock, C.A.; John, J.G.; Rykaczewski, R.R.; Asch, R.G.; Cheung, W.W.; Dunne, J.P.; Friedland, K.D.; Lam, V.W.Y.; Sarmiento, J.L.; Watson, R.A. Reconciling fisheries catch and ocean productivity. Proc. Natl. Acad. Sci. USA 2017, 114, E1441–E1449. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, Q.; Tang, D.; Zhao, H.; Chen, P. Response of Sthenoteuthis oualaniensis to marine environmental changes in the north-central South China Sea based on satellite and in situ observations. PLoS ONE 2019, 14, e0211474. [Google Scholar] [CrossRef]
- Xing, Q.; Yu, H.; Liu, Y.; Li, J.; Tian, Y.; Bakun, A.; Cao, C.; Tian, H.; Li, W. Application of a fish habitat model considering mesoscale oceanographic features in evaluating climatic impact on distribution and abundance of Pacific saury (Cololabis saira). Prog. Oceanogr. 2022, 201, 102743. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Wang, R.; Miao, X.; Zhang, R.; Chen, S.; Song, P.; Lin, L. Effects of Vertical Water Column Temperature on Distribution of Juvenile Tuna Species in the South China Sea. Fishes 2023, 8, 135. [Google Scholar] [CrossRef]
- Killworth, P.D.; Dieterich, C.; Le Provost, C.; Oschlies, A.; Willebrand, J. Assimilation of altimetric data and mean sea surface height into an eddy-permitting model of the North Atlantic. Prog. Oceanogr. 2001, 48, 313–335. [Google Scholar] [CrossRef]
- Xing, Q.; Yu, H.; Wang, H.; Ito, S.-I.; Chai, F. Mesoscale eddies modulate the dynamics of human fishing activities in the global midlatitude ocean. Fish and Fish. 2023, 24, 527–543. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Zhou, Y.; Tian, S. A marine remote sensing-based preliminary analysis on the fishing ground of purple flying squid Sthenoteuthis oualaniensis in the northwest Indian Ocean. J. Fish. China 2006, 30, 669–675. [Google Scholar]
- Fu, Y.; Wu, X.; Jin, P.; Yu, W. Impacts of mesoscale eddies on the spatial and temporal distribution of Sthenoteuthis oualaniensis in the Northwest Indian Ocean. J. Fish. Sci. China 2024, 31, 454–464. [Google Scholar]
- Bakun, A. Ocean eddies, predator pits and bluefin tuna: Implications of an inferred ‘low risk–limited payoff’ reproductive scheme of a (former) archetypical top predator. Fish Fish. 2013, 14, 424–438. [Google Scholar] [CrossRef]
- Shao, F.; Chen, X. Relationship between fishing ground of Symlectoteuthis oualaniensis and sea surface height in the northwest Indian ocean. Mar. Sci. 2008, 32, 88–92. [Google Scholar]
- Appen, W.J.V.; Baumann, T.M.; Janout, M.; Koldunov, N.; Lenn, Y.D.; Pickart, R.S.; Scott, R.B.; Wang, Q. Eddies and the Distribution of Eddy Kinetic Energy in the Arctic Ocean. Oceanogr. 2022, 35, 42–51. [Google Scholar] [CrossRef]
- Wang, T.; Du, Y.; Adeagbo, Z.O.S. Influence of rossby wave in southern Indian Ocean on the low frequency variability of eddy kinetic energy within agulhas current system. Deep-Sea Res. I Oceanogr. Res. Pap. 2024, 203, 104218. [Google Scholar] [CrossRef]
- Syah, A.F.; Saitoh, S.-I.; Alabia, I.D.; Hirawake, T. Detection of potential fishing zone for Pacific saury (Cololabis saira) using generalized additive model and remotely sensed data. IOP Conf. Ser. Earth Environ. Sci. 2017, 54, 012074. [Google Scholar] [CrossRef]
- Lan, K.W.; Lee, M.A.; Nishida, T.; Lu, H.J.; Weng, J.-S.; Chang, Y. Environmental effects on yellowfin tuna catch by the Taiwan longline fishery in the Arabian Sea. Int. J. Remote Sens. 2012, 33, 7491–7506. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Zhang, R.; Tian, Y.; Zhang, J.; Lin, L. Relationship between the resource distribution of Scomber japonicus and seawater temperature vertical structure of Northwestern Pacific Ocean. Period. Ocean Univ. China 2019, 49, 29–38. [Google Scholar]
- Yan, L.; Zhang, P.; Yang, B.; Chen, S.; Li, Y.; Li, Y.; Song, P.; Lin, L. Relationship between the catch of Symplectoteuthis oualaniensis and surface temperature and the vertical temperature structure in the South China Sea. J. Fish. Sci. China 2016, 23, 469–477. [Google Scholar]
- Wei, J.; Cui, G.; Xuan, W.; Tao, Y.; Su, S.; Zhu, W. Effects of SST and Chl-a on the spatiotemporal distribution of Sthenoteuthis oualaniensis fishing ground in the Northwest Indian Ocean. J. Fish. Sci. China 2022, 29, 388–397. [Google Scholar]
- Qian, W.G.; Chen, X.J.; Liu, B.L.; Tian, S.; Ye, X. The relationship between fishing ground distribution of Symlectoteuthis oualaniensis and zooplankton in the northwestern Indian Ocean in autumn. Mar. Fish. 2006, 28, 265–271. [Google Scholar]




| Model | Covariant | Degree of Freedom | AIC Value | Variance Explanation % | R-Sq. (adj) |
|---|---|---|---|---|---|
| 1 | SST | 4.15 | 247.07 | 13.90 | 0.11 |
| 2 | ST100 | 1.00 | 242.00 | 13.20 | 0.12 |
| 3 | Chla | 1.00 | 255.32 | 5.81 | 0.04 |
| 4 | MLD | 2.32 | 259.22 | 5.08 | 0.03 |
| 5 | SSH | 5.59 | 214.23 | 30.10 | 0.37 |
| 6 | EKE | 3.15 | 247.00 | 12.80 | 0.10 |
| 7 | SSH + Chla | 6.91 | 214.73 | 31.70 | 0.28 |
| 8 | SSH + MLD | 7.92 | 215.04 | 32.40 | 0.28 |
| 9 | SSH + EKE | 8.35 | 209.72 | 34.90 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, L.; Lian, P.; Kang, J.; Song, P.; Miao, X.; Lin, L.; Wang, R.; Li, Y. The Effect of the Marine Environment on the Distribution of Sthenoteuthis oualaniensis in the East Equatorial Indian Ocean. Fishes 2025, 10, 184. https://doi.org/10.3390/fishes10040184
Liu S, Zhang L, Lian P, Kang J, Song P, Miao X, Lin L, Wang R, Li Y. The Effect of the Marine Environment on the Distribution of Sthenoteuthis oualaniensis in the East Equatorial Indian Ocean. Fishes. 2025; 10(4):184. https://doi.org/10.3390/fishes10040184
Chicago/Turabian StyleLiu, Shigang, Liyan Zhang, Peng Lian, Jianhua Kang, Puqing Song, Xing Miao, Longshan Lin, Rui Wang, and Yuan Li. 2025. "The Effect of the Marine Environment on the Distribution of Sthenoteuthis oualaniensis in the East Equatorial Indian Ocean" Fishes 10, no. 4: 184. https://doi.org/10.3390/fishes10040184
APA StyleLiu, S., Zhang, L., Lian, P., Kang, J., Song, P., Miao, X., Lin, L., Wang, R., & Li, Y. (2025). The Effect of the Marine Environment on the Distribution of Sthenoteuthis oualaniensis in the East Equatorial Indian Ocean. Fishes, 10(4), 184. https://doi.org/10.3390/fishes10040184







