Dietary Puerarin Enhances Growth, Immune Function, Antioxidant Capacity, and Disease Resistance in Farmed Largemouth Bass, Micropterus salmoides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Acclimatization, Chronic Exposure and Sampling
2.3. Immune-Related Enzymes Detection
2.4. Oxidative Stress Detection
2.5. Detection of Immune-Related Genes Expression
2.6. Effects of Puerarin on Resistance to Aeromonas hydrophila Infection
2.7. Statistical Analysis
3. Results
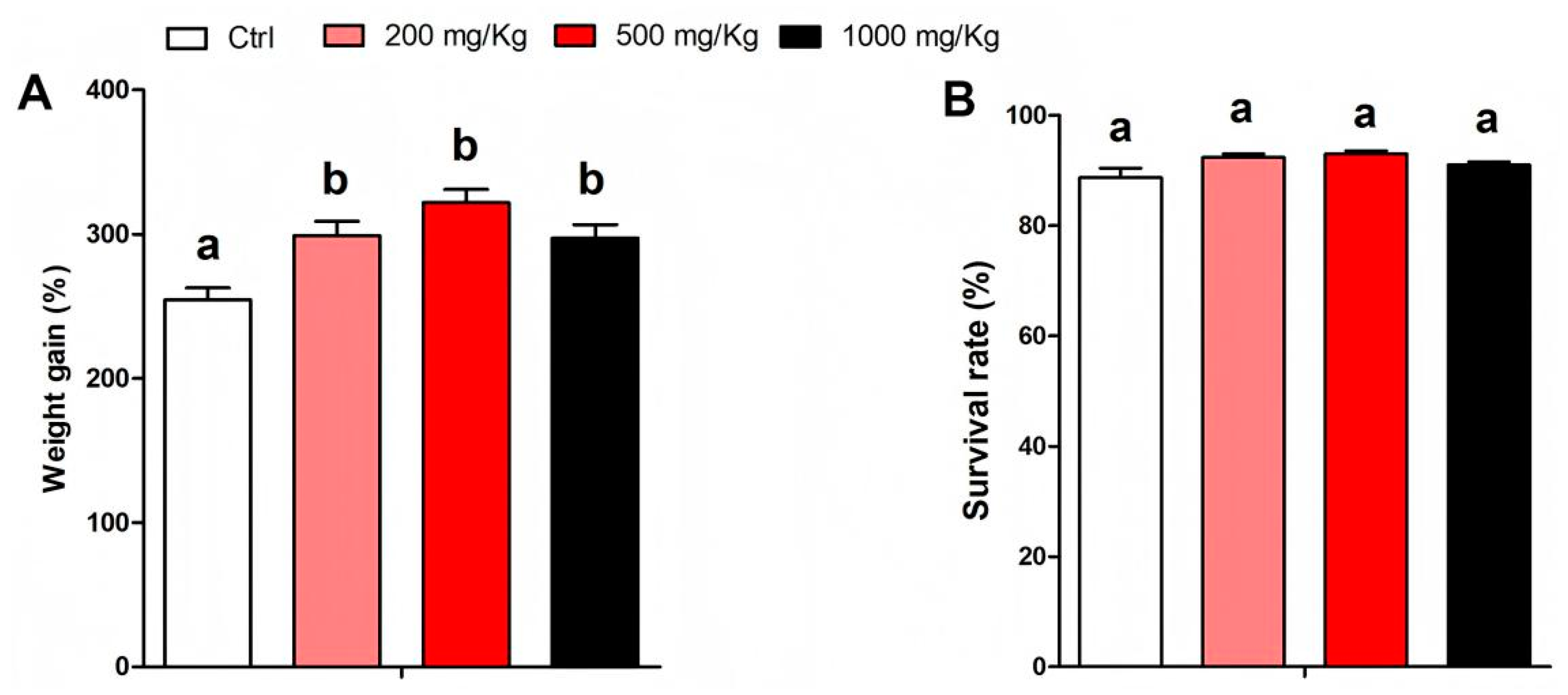
3.1. Effects of Puerarin on Growth Performance of M. salmoides
3.2. Effects of Puerarin on Immunological and Antioxidative Status of M. salmoides
3.3. Expression of Immune-Related Genes
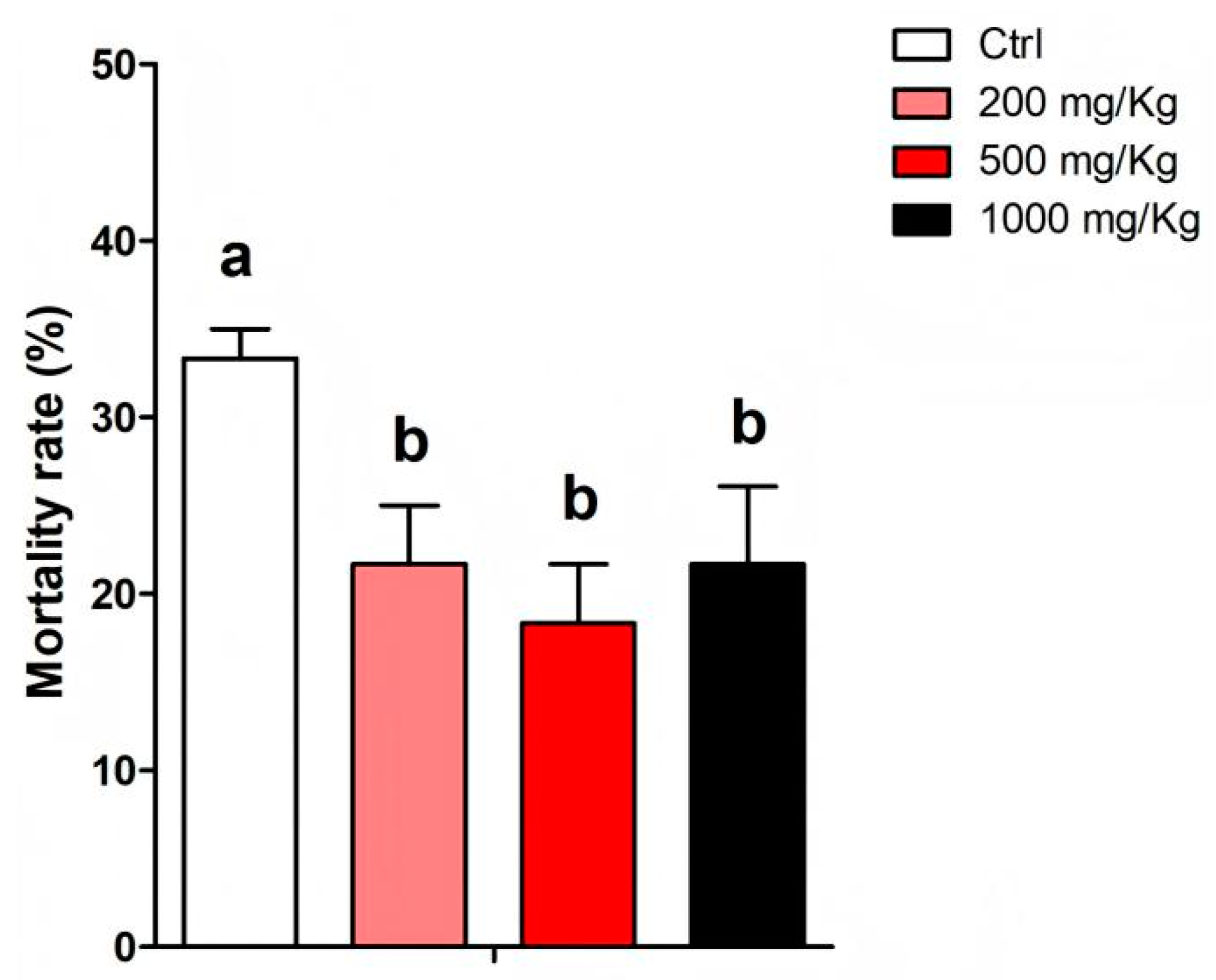
3.4. Effects of Puerarin on Resistance to A. hydrophila Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Zhang, H.; Peng, C. Puerarin: A Review of Pharmacological Effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, L.; Wang, M. Effects of puerarin on chronic inflammation: Focus on the heart, brain, and arteries. AGING Med. 2021, 4, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Shi, S.; Zhang, B.; Xu, X.; Zheng, H.; Li, Y.; Cui, X.; Wu, H.; Song, Q. Role of puerarin in pathological cardiac remodeling: A review. Pharmacol. Res. 2022, 178, 106152. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, M.; Liu, M.; Zhang, W.; Zhi, S.; Qu, L.; Xiong, J.; Wang, L.; Qin, C.; Nie, G. Effects of Genistein on Lipid Metabolism, Antioxidant Activity, and Immunity of Common Carp (Cyprinus carpio L.) Fed with High-Carbohydrate and High-Fat Diets. Aquac. Nutr. 2023, 2023, 9555855. [Google Scholar] [CrossRef]
- Girard, C. A Revision of the North American Astaci, with Observations on Their Habits and Geographical Distribution; Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1852. [Google Scholar]
- Marín-Juez, R.; Jong-Raadsen, S.; Yang, S.; Spaink, H.P. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J. Endocrinol. 2014, 222, 229–241. [Google Scholar] [CrossRef]
- Bai, Y.-l.; Han, L.-l.; Qian, J.-h.; Wang, H.-z. Molecular Mechanism of Puerarin Against Diabetes and its Complications. Front. Pharmacol. 2022, 12, 780419. [Google Scholar] [CrossRef]
- Xu, D.-X.; Guo, X.-X.; Zeng, Z.; Wang, Y.; Qiu, J. Puerarin improves hepatic glucose and lipid homeostasis in vitro and in vivo by regulating the AMPK pathway. Food Funct. 2021, 12, 2726–2740. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, M.; Qi, J.; Song, R.; Wang, L.; Li, J.; Zhou, X.; Chang, D.; Huang, Q.; Li, L.; et al. Puerarin inhibited oxidative stress and alleviated cerebral ischemia-reperfusion injury through PI3K/Akt/Nrf2 signaling pathway. Front. Pharmacol. 2023, 14, 1134380. [Google Scholar] [CrossRef]
- Lu, X.L.; Liu, J.X.; Wu, Q.; Long, S.M.; Zheng, M.Y.; Yao, X.L.; Ren, H.; Wang, Y.G.; Su, W.W.; Fai Cheung, R.T.; et al. Protective effects of puerarin against Aß40-induced vascular dysfunction in zebrafish and human endothelial cells. Eur. J. Pharmacol. 2014, 732, 76–85. [Google Scholar] [CrossRef]
- Bai, J.; Li, S. Development of Largemouth Bass (Micropterus salmoides) Culture. In Aquaculture in China: Success Stories and Modern Trends, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 421–429. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Tian, T.; Du, J.; Lei, C.; Zhu, T.; Han, L.; Song, H. Effects of 17α-methyltestosterone and letrozole on growth and gonadal development in largemouth bass (Micropterus salmodies). Front. Physiol. 2024, 15, 1444918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, C. Largemouth Bass Production in China; CABI Books; CABI: Wallingford, UK, 2019; pp. 37–47. [Google Scholar] [CrossRef]
- Ma, J.; Kong, L.; Huang, Z.; Wang, X.; Quan, F.; Zhao, X.; Yi, Z.; Lin, H.; Liu, L.; Zhao, Y.; et al. Effects of dietary puerarin on growth, digestive enzyme, antioxidant capacity, immune and liver health of Acanthopagrus latus. Aquac. Rep. 2024, 37, 102261. [Google Scholar] [CrossRef]
- Kong, L.; Ma, J.; Zhou, S.; Huang, Z.; Wang, X.; Quan, F.; Zhao, X.; Yi, Z.; Lin, H.; Liu, L.; et al. Effects of puerarin on growth, liver immunity and antioxidant capacity of yellowfin seabream (Acanthopagrus latus) under oxidized fish oil stress. Aquac. Rep. 2024, 37, 102212. [Google Scholar] [CrossRef]
- Hossain, M.R.; Mustafa, A. Puerarin Promotes Overall Health Through Blood Flow and Immune Response within Chinook Salmon (Oncorhynchus tshawytscha) Fingerlings. J. Appl. Aquac. 2014, 26, 119–131. [Google Scholar] [CrossRef]
- Furna, D.J. Combating Stress: The Use of Isoflavones as Neutraceuticals to Improve Immunity and Growth in Nile Tilapia (Oreochromis niloticus). Master’s Thesis, Purdue University, Fort Wayne, India, 2019. [Google Scholar]
- Liu, B.; Fei, F.; Li, X.; Wang, X.; Huang, B. Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquac. Int. 2019, 27, 1599–1612. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, H.; Hu, Y.; Zhou, C.; Wu, J.; Wu, Y.; Wang, H.; Lenahan, C.; Huang, L.; Nie, S.; et al. Puerarin Attenuates Oxidative Stress and Ferroptosis via AMPK/PGC1α/Nrf2 Pathway after Subarachnoid Hemorrhage in Rats. Antioxidants 2022, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Wenjing, C.; Xiandong, X.; Haixin, Z.; Liyun, D.; Zhiyong, Z.; Sheng, Y.; Jin, Y. The effects of puerarin on non-specific immune function and hepatopancreatic tissue structure in grass carp. Bull. Chin. Agric. Sci. 2017, 33, 136–140. [Google Scholar]
- Xie, S.; Xu, J.; Chen, L.; Qi, Y.; Yang, H.; Tan, B. Single-Cell Transcriptomic Analysis Revealed the Cell Population Changes and Cell-Cell Communication in the Liver of a Carnivorous Fish in Response to High-Carbohydrate Diet. J. Nutr. 2024, 154, 2381–2395. [Google Scholar] [CrossRef]
- Wade, N.M.; Trenkner, L.H.; Viegas, I.; Tavares, L.C.; Palma, M.; Skiba-Cassy, S.; Dias, K.; Vachot, C.; Araújo, B.C.; Bourne, N.; et al. Dietary starch promotes hepatic lipogenesis in barramundi (Lates calcarifer). Br. J. Nutr. 2020, 124, 363–373. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Ge, X.; Niu, J.; Wang, J.; Wang, Y.; Chen, L.; Huang, Z.; Yu, W.; Tan, X. The effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2015, 43, 158–166. [Google Scholar] [CrossRef]
- Cao, S.; Xiong, D.; Luo, W.; Tang, J.; Qu, F.; Zhou, Y.; He, Z.; Xie, S.; Liu, Z. Effects of dietary soy isoflavones on growth, antioxidant status, immune response and resistance of juvenile grass carp (Ctenopharyngodon idella) to Aeromonas hydrophila challenge. Aquac. Res. 2020, 51, 2472–2482. [Google Scholar] [CrossRef]
- Chromcova, L.; Stepanova, S.; Plhalova, L.; Praskova, E.; Svobodova, Z. Effect of four selected carrier solvents on embryonal stages of Danio rerio. Neuro Endocrinol. Lett. 2012, 33 (Suppl. S3), 60–65. [Google Scholar] [PubMed]
- Powell, M.E.A.; Smith, M.J.H. The Determination of Serum Acid and Alkaline Phosphatase Activity with 4-Aminoantipyrine (A.A.P.). J. Clin. Pathol. 1954, 7, 245. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Mörsky, P. Turbidimetric determination of lysozyme with Micrococcus lysodeikticus cells: Reexamination of reaction conditions. Anal. Biochem. 1983, 128, 77–85. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free. Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Malondialdehyde (MDA) by Thiobarbituric Acid (TBA) Assay. In Plant-Microbe Interactions: Laboratory Techniques; Senthilkumar, M., Amaresan, N., Sankaranarayanan, A., Eds.; Springer: New York, NY, USA, 2021; pp. 103–105. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Chen, X.-f.; Wang, L.; Wu, Y.-z.; Song, S.-y.; Min, H.-y.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 2018, 8, 1. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, L.; Tang, X.; Liang, J.; Liu, Q.; Luo, W.; Adam, A.A.; Luo, J.; Li, Z.; Yang, S.; et al. High-carbohydrate diet altered conversion of metabolites, and deteriorated health in juvenile largemouth bass. Aquaculture 2022, 549, 737816. [Google Scholar] [CrossRef]
- Zheng, G.; Lin, L.; Zhong, S.; Zhang, Q.; Li, D. Effects of Puerarin on Lipid Accumulation and Metabolism in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e0122925. [Google Scholar] [CrossRef] [PubMed]
- İnal, M.E.; Kanbak, G.; Sunal, E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta 2001, 305, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, X.; Dong, M. Puerarin suppresses MPP+/MPTP-induced oxidative stress through an Nrf2-dependent mechanism. Food Chem. Toxicol. 2020, 144, 111644. [Google Scholar] [CrossRef]
- Georganas, C.; Liu, H.; Perlman, H.; Hoffmann, A.; Thimmapaya, B.; Pope, R.M. Regulation of IL-6 and IL-8 Expression in Rheumatoid Arthritis Synovial Fibroblasts: The Dominant Role for NF-κB But Not C/EBPβ or c-Jun1. J. Immunol. 2000, 165, 7199–7206. [Google Scholar] [CrossRef] [PubMed]
- Savan, R.; Sakai, M. Genomics of fish cytokines. Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 89–101. [Google Scholar] [CrossRef]
- Bayne, C.J.; Gerwick, L. The acute phase response and innate immunity of fish. Dev. Comp. Immunol. 2001, 25, 725–743. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Yuan, M.-H.; Zhang, C.-Y.; Liu, H.-M.; Liu, J.-R.; Wei, A.-L.; Ye, Q.; Zeng, B.; Li, M.-F.; Guo, Y.-P.; et al. Puerariae Lobatae radix flavonoids and puerarin alleviate alcoholic liver injury in zebrafish by regulating alcohol and lipid metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef]
- Evans, C.G.; Chang, L.; Gestwicki, J.E. Heat Shock Protein 70 (Hsp70) as an Emerging Drug Target. J. Med. Chem. 2010, 53, 4585–4602. [Google Scholar] [CrossRef]
- Kang, A.-W.; Sun, C.; Li, H.-T.; Zhong, K.; Zeng, X.-H.; Gu, Z.-F.; Li, B.-Q.; Zhang, X.-N.; Gao, J.-L.; Chen, T.-X. Puerarin extends the lifespan of Drosophila melanogaster by activating autophagy. Food Funct. 2023, 14, 2149–2161. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, X.; Bao, S.; Wu, Q.; Li, J.; Wang, Y.; Liu, B. The consumption of fermented Chinese herbs has resulted in better intestinal health and increased resistance to Aeromonas hydrophila in juvenile largemouth bass (Micropterus salmoides). Front. Mar. Sci. 2023, 10, 1199910. [Google Scholar] [CrossRef]
- Semwal, A.; Kumar, A.; Kumar, N. A review on pathogenicity of Aeromonas hydrophila and their mitigation through medicinal herbs in aquaculture. Heliyon 2023, 9, e14088. [Google Scholar] [CrossRef]
- Wu, M.; Yi, D.; Zhang, Q.; Wu, T.; Yu, K.; Peng, M.; Wang, L.; Zhao, D.; Hou, Y.; Wu, G. Puerarin enhances intestinal function in piglets infected with porcine epidemic diarrhea virus. Sci. Rep. 2021, 11, 6552. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, X.; Xu, F.; Wang, F.; Wu, H.; Bai, Y.; Li, W.; Zhang, G.; Yuan, J.; Pang, Q. Protective effects of puerarin on liver tissue in Salmonella-infected chicks: A proteomic analysis. Poult. Sci. 2024, 103, 103281. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ge, S.; Zhang, H.; Lu, W.; Bao, X.; Pan, S.; Pang, Q. Metabolomic and microbiome analysis of the protective effects of Puerarin against Salmonella Enteritidis Infection in chicks. BMC Vet. Res. 2023, 19, 242. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.A.K.; Huo, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef]


| Nutritional Composition | Contents (%) | Nutrient Level | Contents (%) |
|---|---|---|---|
| Fish meal | 48 | Crude protein | 46.7 |
| Soybean meal | 8 | Crude lipid | 11.5 |
| Flour | 11 | Crude ash | 9.2 |
| Soybean oil | 6 | Moisture | 10.4 |
| Chicken powder | 10 | ||
| Cassava starch | 8 | ||
| Ca(H2PO4)2 | 1.5 | ||
| Squid ointment | 4 | ||
| Gluten | 2 | ||
| Premix * | 1.5 | ||
| Total | 100 |
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| TNF-α Fw | CTTCGTCTACAGCCAGGCATCG |
| TNF-α Rv | TTTGGCACACCGACCTCACC |
| IL-6 Fw | GGACCGCTTTGAAACTCT |
| IL-6 Rv | GCTCCCTGTAACGCTTGT |
| IL-8 Fw | CGTTGAACAGACTGGGAGAGATG |
| IL-8 Rv | AGTGGGATGGCTTCATTATCTTGT |
| Nrf2 Fw | CAGACAGTTCCTTTGCAGGC |
| Nrf2 Rv | AGGGACAAAAGCTCCATCCA |
| HSP70 Fw | GCAGACGCAGACCTTCACCA |
| HSP70 Rv | TGCGCTTCCAGACCTCCAAC |
| β-actin Fw | AAAGGGAAATCGTGCGTGAC |
| β-actin Rv | AAGGAAGGCTGGAAGAGGG |
| Treatment Group (mg/kg Puerarin) | Initial Fish (n) | Growth Assessment (n) | Biochemical/ Molecular Analyses (n) | Bacterial Challenge (n) | PBS-Injected Controls (n) | A. hydrophila-Injected (n) |
|---|---|---|---|---|---|---|
| 0 (Control) | 30 | 30 | 9 | 20 | 15 * | 5 * |
| 200 | 30 | 30 | 9 | 20 | 15 * | 5 * |
| 500 | 30 | 30 | 9 | 20 | 15 * | 5 * |
| 1000 | 30 | 30 | 9 | 20 | 15 * | 5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Ma, W.; Zhang, D.; Chen, X.; Huang, Z.; Hong, Y. Dietary Puerarin Enhances Growth, Immune Function, Antioxidant Capacity, and Disease Resistance in Farmed Largemouth Bass, Micropterus salmoides. Fishes 2025, 10, 197. https://doi.org/10.3390/fishes10050197
Huang Y, Ma W, Zhang D, Chen X, Huang Z, Hong Y. Dietary Puerarin Enhances Growth, Immune Function, Antioxidant Capacity, and Disease Resistance in Farmed Largemouth Bass, Micropterus salmoides. Fishes. 2025; 10(5):197. https://doi.org/10.3390/fishes10050197
Chicago/Turabian StyleHuang, Yi, Wenjing Ma, Disen Zhang, Xi Chen, Zhiqiu Huang, and Yuhang Hong. 2025. "Dietary Puerarin Enhances Growth, Immune Function, Antioxidant Capacity, and Disease Resistance in Farmed Largemouth Bass, Micropterus salmoides" Fishes 10, no. 5: 197. https://doi.org/10.3390/fishes10050197
APA StyleHuang, Y., Ma, W., Zhang, D., Chen, X., Huang, Z., & Hong, Y. (2025). Dietary Puerarin Enhances Growth, Immune Function, Antioxidant Capacity, and Disease Resistance in Farmed Largemouth Bass, Micropterus salmoides. Fishes, 10(5), 197. https://doi.org/10.3390/fishes10050197






