No Correlation between Endo- and Exoskeletal Regenerative Capacities in Teleost Species
Abstract
:1. Introduction
2. Results
2.1. Endoskeletal Regeneration in Zebrafish
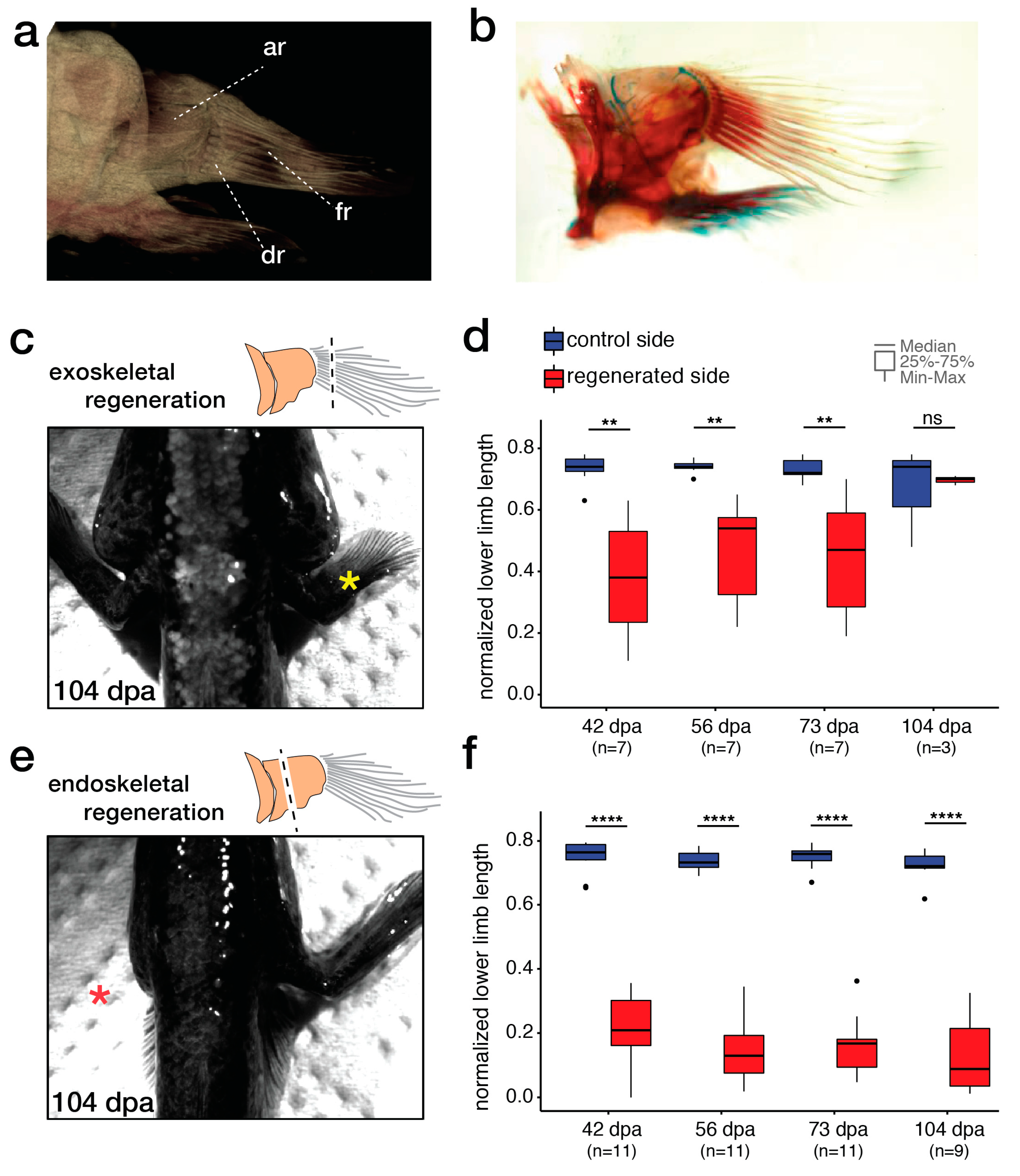
2.2. Comparison of Endo- and Exoskeletal Regeneration in Mudskippers
3. Discussion
4. Materials and Methods
4.1. Fish Maintenance
4.2. Data Acquisition and Analysis
4.3. qRT-PCR Analysis
4.4. Cartilage and Bone Stainings
4.5. Micro-CT Imaging
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Spallanzani, L. An Essay on Animal Reproductions, 1769.
- Tsonis, P.A.; Fox, T.P. Regeneration according to Spallanzani. Dev. Dyn. 2009, 238, 2357–2363. [Google Scholar] [CrossRef]
- Morgan, T.H. Regeneration and liability to injury. Science 1901, 14, 235–248. [Google Scholar] [CrossRef]
- Tanaka, E.M. The Molecular and Cellular Choreography of Appendage Regeneration. Cell 2016, 165, 1598–1608. [Google Scholar] [CrossRef] [Green Version]
- Mokalled, M.H.; Poss, K.D. A regeneration toolkit. Dev. Cell 2018, 47, 267–280. [Google Scholar] [CrossRef]
- Miller, B.M.; Johnson, K.; Whited, J.L. Common themes in tetrapod appendage regeneration: A cellular perspective. EvoDevo 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Taghiyar, L.; Hosseini, S.; Safari, F.; Bagheri, F.; Fani, N.; Stoddart, M.J.; Alini, M.; Eslaminejad, M.B. New insight into functional limb regeneration: A to Z approaches. J. Tissue Eng. Regen. Med. 2018, 12, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.S.; Grotek, B.; Jacinto, A.; Weidinger, G.; Saude, L. The Regenerative Capacity of the Zebrafish Caudal Fin Is Not Affected by Repeated Amputations. PLoS ONE 2011, 6, 22820. [Google Scholar] [CrossRef] [PubMed]
- Nacu, E.; Tanaka, E.M. Limb Regeneration: A New Development? Annu. Rev. Cell Dev. Boil. 2011, 27, 409–440. [Google Scholar] [CrossRef] [PubMed]
- Blum, N.; Begemann, G. Osteoblast de- and redifferentiation are controlled by a dynamic response to retinoic acid during zebrafish fin regeneration. Development 2015, 142, 2894–2903. [Google Scholar] [CrossRef]
- Whitehead, G.G.; Makino, S.; Lien, C.L.; Keating, M.T. fgf20 Is Essential for Initiating Zebrafish Fin Regeneration. Science 2005, 310, 1957–1960. [Google Scholar] [CrossRef]
- Lee, Y.; Grill, S.; Sanchez, A.; Murphy-Ryan, M.; Poss, K.D. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 2005, 132, 5173–5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoick-Cooper, C.L.; Weidinger, G.; Riehle, K.J.; Hubbert, C.; Major, M.B.; Fausto, N.; Moon, R.T. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development 2007, 134, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehner, D.; Cizelsky, W.; Vasudevaro, M.D.; Özhan, G.; Haase, C.; Kagermeier-Schenk, B.; Roder, A.; Dorsky, R.I.; Moro, E.; Argenton, F.; et al. Wnt/β-Catenin Signaling Defines Organizing Centers that Orchestrate Growth and Differentiation of the Regenerating Zebrafish Caudal Fin. Cell Rep. 2014, 6, 467–481. [Google Scholar] [CrossRef]
- Münch, J.; Gonzalez-Rajal, A.; De La Pompa, J.L. Notch regulates blastema proliferation and prevents differentiation during adult zebrafish fin regeneration. Development 2013, 140, 1402–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, M.; Sass, M.; Papp, D.; Takacs-Vellai, K.; Kobolak, J.; Dinnyes, A.; Klionsky, D.J.; Vellai, T. Autophagy is required for zebrafish caudal fin regeneration. Cell Death Differ. 2014, 21, 547–556. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakajima, T.; Ishida, T.; Kudo, A.; Kawakami, A. A diffusible signal derived from hematopoietic cells supports the survival and proliferation of regenerative cells during zebrafish fin fold regeneration. Dev. Biol. 2014, 399, 80–90. [Google Scholar] [CrossRef]
- Pfefferli, C.; Jaźwińska, A. The careg element reveals a common regulation of regeneration in the zebrafish myocardium and fin. Nat. Commun. 2017, 8, 15151. [Google Scholar] [CrossRef]
- Kang, J.; Hu, J.; Karra, R.; Dickson, A.L.; Tornini, V.A.; Nachtrab, G.; Gemberling, M.; Goldman, J.A.; Black, B.L.; Poss, K.D. Modulation of tissue repair by regeneration enhancer elements. Nature 2016, 532, 201–206. [Google Scholar] [CrossRef]
- Tu, S.; Johnson, S.L. Fate restriction in the growing and regenerating zebrafish fin. Dev. Cell 2011, 20, 725–732. [Google Scholar] [CrossRef]
- Knopf, F.; Hammond, C.; Chekuru, A.; Kurth, T.; Hans, S.; Weber, C.W.; Mahatma, G.; Fisher, S.; Brand, M.; Schulte-Merker, S.; et al. Bone Regenerates via Dedifferentiation of Osteoblasts in the Zebrafish Fin. Dev. Cell 2011, 20, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.P.; Holdway, J.E.; Poss, K.D. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev. Cell 2012, 22, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Nachtrab, G.; Czerwinski, M.; Poss, K.D. Sexually dimorphic fin regeneration in zebrafish controlled by androgen/GSK3 signaling. Curr. Boil. 2011, 21, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Nachtrab, G.; Kikuchi, K.; Tornini, V.A.; Poss, K.D. Transcriptional components of anteroposterior positional information during zebrafish fin regeneration. Development 2013, 140, 3754–3764. [Google Scholar] [CrossRef] [Green Version]
- Grandel, H.; Schulte-Merker, S. The development of the paired fins in the Zebrafish (Danio rerio). Mech. Dev. 1998, 79, 99–120. [Google Scholar] [CrossRef]
- Yano, T.; Tamura, K. The making of differences between fins and limbs. J. Anat. 2013, 222, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.C. The Deep Homology of the Autopod: Insights from Hox Gene Regulation. Integr. Comp. Boil. 2013, 53, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M. Fins into limbs: Autopod acquisition and anterior elements reduction by modifying gene networks involving 5’Hox, Gli3, and Shh. Dev. Boil. 2016, 413, 1–7. [Google Scholar] [CrossRef]
- Shubin, N.; Tabin, C.; Carroll, S. Fossils, genes and the evolution of animal limbs. Nature 1997, 388, 639–648. [Google Scholar] [CrossRef]
- Nakamura, T.; Gehrke, A.R.; Lemberg, J.; Szymaszek, J.; Shubin, N.H. Digits and fin rays share common developmental histories. Nature 2016, 537, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Akimenko, M.A.; Marí-Beffa, M.; Becerra, J.; Géraudie, J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 2003, 226, 190–201. [Google Scholar] [CrossRef]
- Iovine, M.K. Conserved mechanisms regulate outgrowth in zebrafish fins. Nat. Methods 2007, 3, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, E.; Van Mai, H.; Ishimatsu, A.; Tanaka, M. Modification of pectoral fins occurs during the larva-to-juvenile transition in the mudskipper (Periophthalmus modestus). Zoöl. Lett. 2018, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, R.; Hernández-Martínez, R.; Chimal-Monroy, J.; Merchant-Larios, H.; Covarrubias, L. Full regeneration of the tribasal Polypterus fin. Proc. Natl. Acad. Sci. USA 2012, 109, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Baumgartner, W.; Brinkman, E.; Devries, R.J.; Stewart, H.A.; Aboagye, D.L.; Ramee, S.W.; Ciaramella, M.A.; Culpepper, C.M.; Petrie-Hanson, L. Fin healing and regeneration in sturgeon. J. Fish Boil. 2018, 93, 917–930. [Google Scholar] [CrossRef]
- Wendler, S.; Hartmann, N.; Hoppe, B.; Englert, C. Age-dependent decline in fin regenerative capacity in the short-lived fish Nothobranchius furzeri. Aging Cell 2015, 14, 857–866. [Google Scholar] [CrossRef]
- Rajaram, S.; Patel, S.; Uggini, G.K.; Desai, I.; Balakrishnan, S. BMP signaling regulates the skeletal and connective tissue differentiation during caudal fin regeneration in sailfin molly (Poecilia latipinna). Dev. Growth Differ. 2017, 59, 629–638. [Google Scholar] [CrossRef]
- Stockdale, W.T.; Lemieux, M.E.; Killen, A.C.; Zhao, J.; Hu, Z.; Riepsaame, J.; Hamilton, N.; Kudoh, T.; Riley, P.R.; van Aerle, R.; et al. Heart regeneration in the Mexican cavefish. Cell Rep. 2018, 25, 1997–2007. [Google Scholar] [CrossRef]
- Katogi, R.; Nakatani, Y.; Shin-I, T.; Kohara, Y.; Inohaya, K.; Kudo, A. Large-scale analysis of the genes involved in fin regeneration and blastema formation in the medaka, Oryzias latipes. Mech. Dev. 2004, 121, 861–872. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Wang, L.; Chen, W.; Chang, Z. Gene expression profiles of fin regeneration in loach (Paramisgurnus dabryanu). Gene Expr. Patterns 2017, 25–26, 124–130. [Google Scholar] [CrossRef]
- Wagner, G.P.; Misof, B.Y.; Wagner, G. Evolutionary modification of regenerative capability in vertebrates: A comparative study on teleost pectoral fin regeneration. J. Exp. Zoöl. 1992, 261, 62–78. [Google Scholar] [CrossRef]
- Lu, S.; Yang, L.; Jiang, H.; Chen, J.; Yu, G.; Chen, Z.; Xia, X.; He, S. Bichirs employ similar genetic pathways for limb regeneration as are used in lungfish and salamanders. Gene 2018, 690, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, A.I.; Golichenkov, V.A. Characteristics of the reparative regeneration of fins in the polypterid fish (Polypteridae, Actinopterygii). Russ. J. Dev. Biol. 2012, 43, 115–120. [Google Scholar] [CrossRef]
- Darnet, S.; Dragalzew, A.C.; Amaral, D.B.; Sousa, J.F.; Thompson, A.W.; Cass, A.N.; Lorena, J.; Pires, E.S.; Costa, C.M.; Sousa, M.P.; et al. Deep evolutionary origin of limb and fin regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 15106–15115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Qian, X.; Zhang, C.; Xu, Z. Fin regeneration from tail segment with musculature, endoskeleton, and scales. J. Exp. Zoöl. Part B Mol. Dev. Evol. 2009, 312, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Kutschera, U.; Elliott, J.M. Do mudskippers and lungfishes elucidate the early evolution of four-limbed vertebrates? Evol. Educ. Outreach 2013, 6, 8. [Google Scholar] [CrossRef]
- Pace, C.M.; Gibb, A.C. Sustained periodic terrestrial locomotion in air-breathing fishes. J. Fish Boil. 2014, 84, 639–660. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Costa, C.M.; Lorena, J.; Moreira, R.N.; Frota-Lima, G.N.; Furtado, C.; Robinson, M.; Amemiya, C.T.; Darnet, S.; Schneider, I. Tetrapod limb and sarcopterygian fin regeneration share a core genetic programme. Nat. Commun. 2016, 7, 13364. [Google Scholar] [CrossRef] [Green Version]
- Goss, R.J. Principles of Regeneration; Academic Press, Inc.: New York, NY, USA, 1970. [Google Scholar]
- Lin, G.; Chen, Y.; Slack, J.M.W. Imparting regenerative capacity to limbs by progenitor cell transplantation. Dev. Cell 2013, 24, 41–51. [Google Scholar] [CrossRef]
- Aztekin, C.; Hiscock, T.W.; Marioni, J.C.; Gurdon, J.B.; Simons, B.D.; Jullien, J. Identification of a regeneration-organizing cell in the Xenopus tail. Science 2019, 364, 653–658. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book, 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer Publishing: New York, NY, USA, 2016. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pápai, N.; Kagan, F.; Csikós, G.; Kosztelnik, M.; Vellai, T.; Varga, M. No Correlation between Endo- and Exoskeletal Regenerative Capacities in Teleost Species. Fishes 2019, 4, 51. https://doi.org/10.3390/fishes4040051
Pápai N, Kagan F, Csikós G, Kosztelnik M, Vellai T, Varga M. No Correlation between Endo- and Exoskeletal Regenerative Capacities in Teleost Species. Fishes. 2019; 4(4):51. https://doi.org/10.3390/fishes4040051
Chicago/Turabian StylePápai, Nóra, Ferenc Kagan, György Csikós, Mónika Kosztelnik, Tibor Vellai, and Máté Varga. 2019. "No Correlation between Endo- and Exoskeletal Regenerative Capacities in Teleost Species" Fishes 4, no. 4: 51. https://doi.org/10.3390/fishes4040051
APA StylePápai, N., Kagan, F., Csikós, G., Kosztelnik, M., Vellai, T., & Varga, M. (2019). No Correlation between Endo- and Exoskeletal Regenerative Capacities in Teleost Species. Fishes, 4(4), 51. https://doi.org/10.3390/fishes4040051





