Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA
Abstract
:1. Introduction
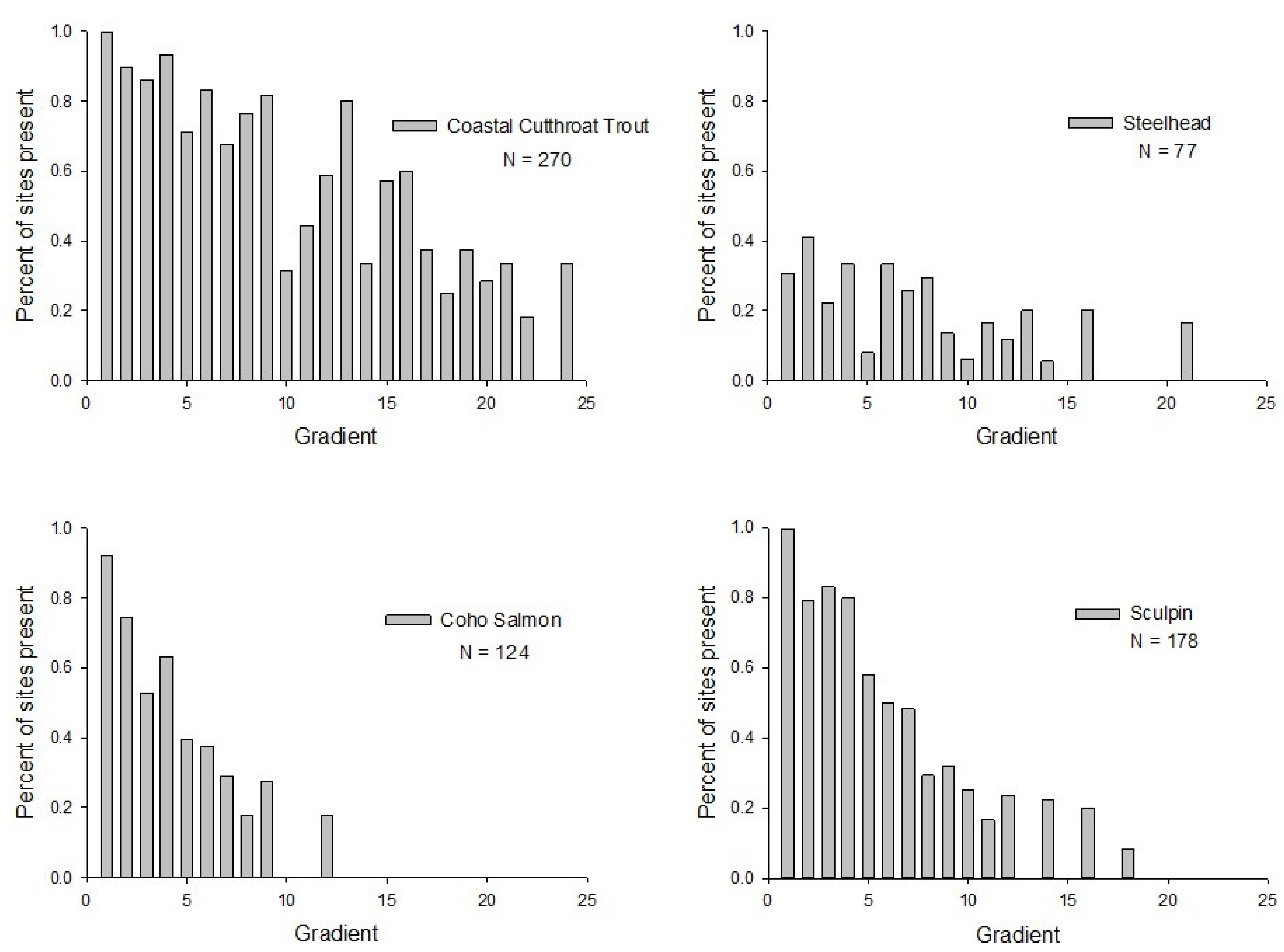
2. Results
3. Discussion
3.1. Co-Occurrence among Species
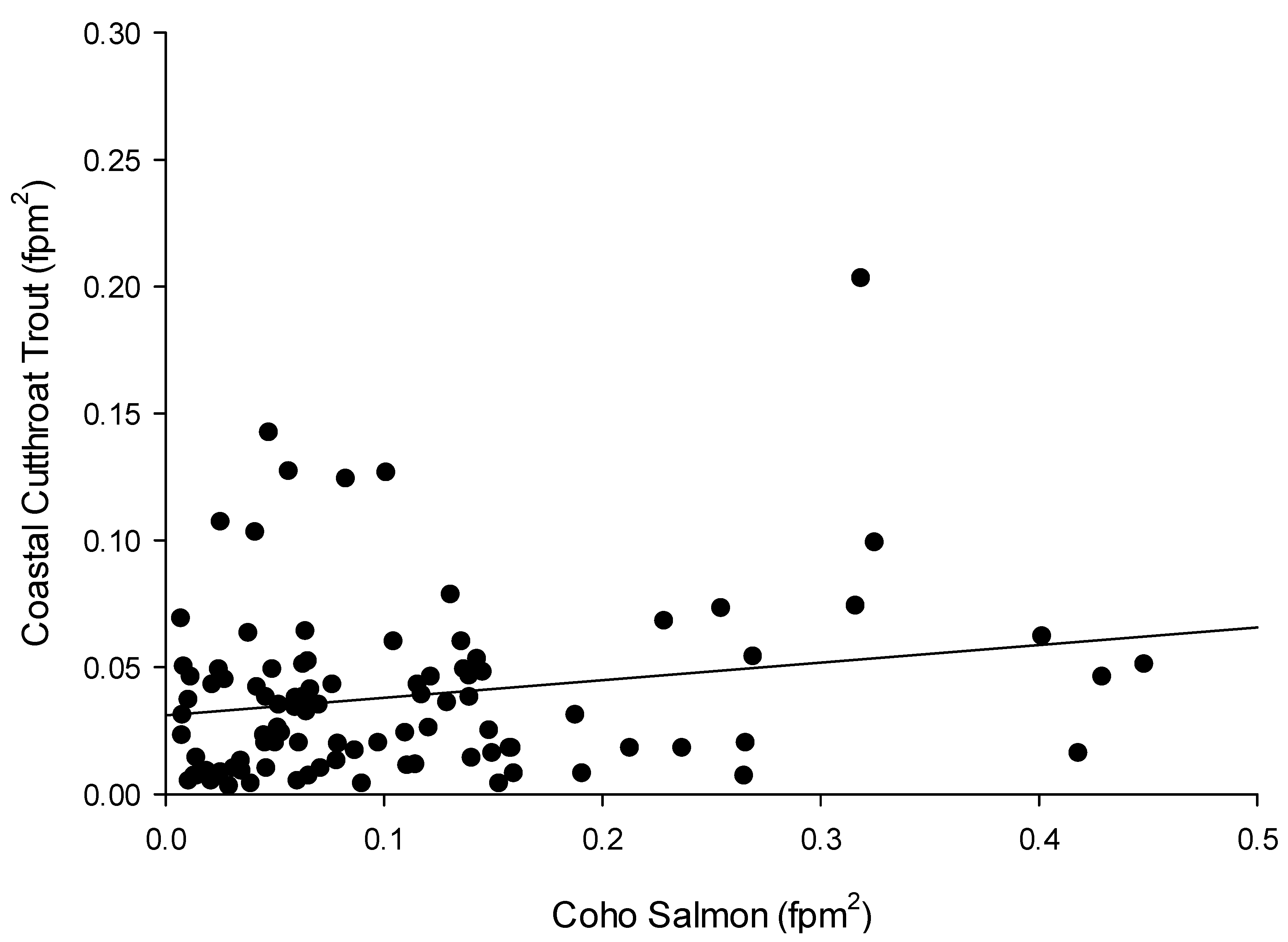
3.2. Density of Coastal Cutthroat Trout and Coho Salmon
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model | Fish density | Avg. Body Size (ABS) * | Wetted Width (WW) | Stream gradient (GRAD) | Predicted Density |
|---|---|---|---|---|---|
| Cutthroat Models | |||||
| CTT~WW + GRAD | -- | -- | 2 m | 3% | 0.0517 fpm2 |
| -- | -- | 3 m | 3% | 0.0395 fpm2 | |
| -- | -- | 2 m | 4% | 0.0550 fpm2 | |
| CTT~COH + WW + GRAD | 0.100 fpm2 | -- | 2 m | 3% | 0.0630 fpm2 |
| 0.200 fpm2 | -- | 2 m | 3% | 0.0550 fpm2 | |
| Coho models | |||||
| Coho~ABS + WW + GRAD | -- | 80 mm | 2 m | 3% | 0.0845 fpm2 |
| -- | 80 mm | 2 m | 2% | 0.0913 fpm2 | |
| -- | 100 mm | 2 m | 3% | 0.0717 fpm2 | |
| -- | 80 mm | 3 m | 3% | 0.0823 fpm2 | |
| Coho~CTT + ABS + WW + GRAD | 0.100 fpm2 | 100 mm | 2 m | 3% | 0.0630 fpm2 |
| 0.200 fpm2 | 100 mm | 2 m | 3% | 0.0447 fpm2 |
References
- Grossman, G.D.; Dowd, J.F.; Crawford, M. Assemblage stability in stream fishes: A review. Environ. Manag. 1990, 14, 661–671. [Google Scholar] [CrossRef]
- Gido, K.B.; Jackson, D.A. (Eds.) Community Ecology of Stream Fishes: Concepts, Approaches, and Techniques; American Fisheries Society: Bethesda, MD, USA, 2010. [Google Scholar]
- Meehan, W.R. Influences of Forest and Rangeland Management on Salmonid Fishes and Their Habitats: Introduction and Overview; American Fisheries Society: Bethesda, MD, USA, 1991. [Google Scholar]
- Northcote, T.G.; Hartman, G.F. (Eds.) Fishes and Forestry: Worldwide Watershed Interactions and Management; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Young, K.A.; Hinch, S.G.; Northcote, T.G. Status of resident coastal cutthroat trout and their habitat twenty-five years after riparian logging. N. Am. J. Fish. Manag. 1999, 19, 901–911. [Google Scholar] [CrossRef]
- De Groot, J.D.; Hinch, S.G.; Richardson, J.S. Effects of logging second-growth forests on headwater populations of coastal cutthroat trout: A 6-year, multistream, before-and-after field experiment. Trans. Am. Fish. Soc. 2007, 136, 211–226. [Google Scholar] [CrossRef]
- Bateman, D.S.; Sloat, M.R.; Gresswell, R.E.; Berger, A.M.; Hockman-Wert, D.P.; Leer, D.W.; Skaugset, A.E. Effects of stream-adjacent logging in fishless headwaters on downstream coastal cutthroat trout. Can. J. Fish. Aquat. Sci. 2016, 73, 1898–1913. [Google Scholar] [CrossRef]
- Reeves, G.H.; Sleeper, J.D.; Lang, D.W. Seasonal changes in habitat availability and the distribution and abundance of salmonids along a stream gradient from headwaters to mouth in coastal Oregon. Trans. Am. Fish. Soc. 2011, 140, 537–548. [Google Scholar] [CrossRef]
- Chelgren, N.D.; Dunham, J.B. Connectivity and conditional models of access and abundance of species in stream networks. Ecol. Appl. 2015, 25, 1357–1372. [Google Scholar] [CrossRef]
- Colvin, S.A.; Sullivan, S.M.P.; Shirey, P.D.; Colvin, R.W.; Winemiller, K.O.; Hughes, R.M.; Fausch, K.D.; Infante, D.M.; Olden, J.D.; Bestgen, K.R.; et al. Headwater streams and wetlands are critical for sustaining fish, fisheries, and ecosystem services. Fisheries 2019, 44, 73–91. [Google Scholar] [CrossRef]
- Hawkins, D.K.; Foote, C.J. Early survival and development of coastal cutthroat trout (Oncorhynchus clarki clarki), steelhead (Oncorhynchus mykiss), and reciprocal hybrids. Can. J. Fish. Aquat. Sci. 1998, 55, 2097–2104. [Google Scholar] [CrossRef]
- Dunson, W.A.; Travis, J. The role of abiotic factors in community organization. Am. Nat. 1991, 138, 1067–1091. [Google Scholar] [CrossRef]
- Crowder, L.B. Community ecology. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 609–627. [Google Scholar]
- Fausch, K.D.; Nakano, S.; Ishigaki, K. Distribution of two congeneric charrs in streams of Hokkaido Island, Japan: Considering multiple factors across scales. Oecologia 1994, 100, 1–12. [Google Scholar] [CrossRef]
- Isaak, D.J.; Hubert, W.A. Are trout populations affected by reach-scale stream slope? Can. J. Fish. Aquat. Sci. 2000, 57, 468–477. [Google Scholar] [CrossRef]
- Dunham, J.B.; Cade, B.S.; Terrell, J.W. Influences of spatial and temporal variation on fish-habitat relationships defined by regression quantiles. Trans. Am. Fish. Soc. 2002, 131, 86–98. [Google Scholar] [CrossRef]
- Rosenfeld, J. Assessing the habitat requirements of stream fishes: An overview and evaluation of different approaches. Trans. Am. Fish. Soc. 2003, 132, 953–968. [Google Scholar] [CrossRef]
- Meyer, J.L.; Strayer, D.L.; Wallace, J.B.; Eggert, S.L.; Helfman, G.S.; Leonard, N.E. The Contribution of Headwater Streams to Biodiversity in River Networks. Jawra J. Am. Water Resour. Assoc. 2007, 43, 86–103. [Google Scholar] [CrossRef] [Green Version]
- Markle, D.F. A Guide to Freshwater Fishes of Oregon; Oregon State University Press: Corvalis, OR, USA, 2016. [Google Scholar]
- Ptolemy, R.A. Predictive models for differentiating habitat use of Coastal Cutthroat Trout and steelhead at the reach and landscape scale. N. Am. J. Fish. Manag. 2013, 33, 1210–1220. [Google Scholar] [CrossRef]
- Rosenfeld, J.; Porter, M.; Parkinson, E. Habitat factors affecting the abundance and distribution of juvenile cutthroat trout (Oncorhynchus clarkii) and Coho Salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 2000, 57, 766–774. [Google Scholar] [CrossRef]
- Johnson, S.W.; Heifetz, J.; Koski, K.V. Effects of logging on the abundance and seasonal distribution of juvenile steelhead in some southeastern Alaska streams. N. Am. J. Fish. Manag. 1986, 6, 532–537. [Google Scholar] [CrossRef]
- Kendall, N.W.; McMillan, J.R.; Sloat, M.R.; Buehrens, T.W.; Quinn, T.P.; Pess, G.R.; Kuzishchin, K.V.; McClure, M.M.; Zabel, R.W. Anadromy and residency in steelhead and rainbow trout (Oncorhynchus mykiss): A review of the processes and patterns. Can. J. Fish. Aquat. Sci. 2015, 72, 319–342. [Google Scholar] [CrossRef] [Green Version]
- Buehrens, T.W.; Kiffney, P.; Pess, G.R.; Bennett, T.R.; Naman, S.M.; Brooks, G.; Quinn, T.P. Increasing juvenile Coho Salmon densities during early recolonization have not affected resident Coastal Cutthroat Trout growth, movement, or survival. N. Am. J. Fish. Manag. 2014, 34, 892–907. [Google Scholar] [CrossRef] [Green Version]
- Ford, M.J.; Albaugh, A.; Barnas, K.; Cooney, T.D.; Cowen, J.; Hard, J.J.; Kope, R.G.; McClure, M.M.; McElhany, P.; Myers, J.M.; et al. Status Review Update for Pacific Salmon and Steelhead Listed under the Endangered Species Act: Pacific Northwest; National Oceanic and Atmospheric Administration: Seattle, WA, USA, 2011; NOAA Tech. Memo. NMFS-NWFSC-113.
- Roni, P. Habitat use by fishes and Pacific giant salamanders in small western Oregon and Washington streams. Trans. Am. Fish. Soc. 2002, 131, 743–761. [Google Scholar] [CrossRef]
- Buffington, J.M.; Montgomery, D.R. Geomorphic classification of rivers. In Treatise on Geomorphology; Fluvial Geomorphology; Shroder, J., Wohl, E., Eds.; Academic Press: San Diego, CA, USA, 2013; Volume 9, pp. 730–767. [Google Scholar]
- Isaak, D.J.; Wenger, S.J.; Peterson, E.E.; Ver Hoef, J.M.; Nagel, D.E.; Luce, C.H.; Hostetler, S.W.; Dunham, J.B.; Roper, B.B.; Wollrab, S.P.; et al. The NorWeST summer stream temperature model and scenarios for the western US: A crowd-sourced database and new geospatial tools foster a user community and predict broad climate warming of rivers and streams. Water Resour. Res. 2017, 53, 9181–9205. [Google Scholar] [CrossRef] [Green Version]
- Glova, G.J. Management implications of the distribution and diet of sympatric populations of juvenile coho salmon and coastal cutthroat trout in small streams in British Columbia, Canada. Progress. Fish-Cult. 1984, 46, 269–277. [Google Scholar] [CrossRef]
- Bisson, P.A.; Sullivan, K.; Nielsen, J.L. Channel hydraulics, habitat use, and body form of juvenile coho salmon, steelhead, and cutthroat trout in streams. Trans. Am. Fish. Soc. 1988, 117, 262–273. [Google Scholar] [CrossRef]
- Heggenes, J.; Northcote, T.G.; Peter, A. Seasonal habitat selection and preferences by cutthroat trout (Oncorhynchus clarki) in a small coastal stream. Can. J. Fish. Aquat. Sci. 1991, 48, 1364–1370. [Google Scholar] [CrossRef]
- Andersen, H.V. Transferability of Models to Predict Selection of Cover by Coastal Cutthroat Trout in Small Streams in Western Oregon, USA. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2008. [Google Scholar]
- Gonzalez, R.; Dunham, J.; Lightcap, S.; McEnroe, J. Large Wood and Instream Habitat for Juvenile Coho Salmon and Larval Lampreys in a Pacific Northwest Stream. N. Am. J. Fish. Manag. 2017, 37, 683–699. [Google Scholar] [CrossRef]
- Glova, G.J. Interaction for food and space between experimental populations of juvenile Coho Salmon (Oncorhynchus kisutch) and Coastal Cutthroat Trout (Salmo clarki) in a laboratory stream. Hydrobiologia 1986, 131, 155–168. [Google Scholar] [CrossRef]
- Tague, C.; Grant, G.E. A geological framework for interpreting the low-flow regimes of Cascade streams, Willamette River Basin, Oregon. Water Resour. Res. 2004, 40. [Google Scholar] [CrossRef]
- Clark, S.C.; Tanner, T.L.; Sethi, S.A.; Bentley, K.T.; Schindler, D.E. Migration timing of adult Chinook Salmon into the Togiak River, Alaska, watershed: Is there evidence for stock structure? Trans. Am. Fish. Soc. 2015, 144, 829–836. [Google Scholar] [CrossRef]
- Flitcroft, R.L.; Burnett, K.M.; Reeves, G.H.; Ganio, L.M. Do network relationships matter? Comparing network and instream habitat variables to explain densities of juvenile coho salmon (Oncorhynchus kisutch) in mid-coastal Oregon, USA. Aquat. Conserv. Mar. Freshw. Ecosyst. 2012, 22, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Falke, J.A.; Dunham, J.B.; Jordan, C.E.; McNyset, K.M.; Reeves, G.H. Spatial ecological processes and local factors predict the distribution and abundance of spawning by steelhead (Oncorhynchus mykiss) across a complex riverscape. PLoS ONE 2013, 11, e79232. [Google Scholar] [CrossRef]
- Elliott, J.M. Quantitative Ecology and the Brown Trout; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Atlas, W.I.; Buehrens, T.W.; McCubbing, D.J.; Bison, R.; Moore, J.W. Implications of spatial contraction for density dependence and conservation in a depressed population of anadromous fish. Can. J. Fish. Aquat. Sci. 2015, 72, 1682–1693. [Google Scholar] [CrossRef] [Green Version]
- Sabo, J.L.; Pauley, G.B. Competition between stream-dwelling cutthroat trout (Oncorhynchus clarki) and Coho Salmon (Oncorhynchus kisutch): Effects of relative size and population origin. Can. J. Fish. Aquat. Sci. 1997, 54, 2609–2617. [Google Scholar] [CrossRef]
- Young, K.A. Asymmetric competition, habitat selection, and niche overlap in juvenile salmonids. Ecology 2004, 85, 134–149. [Google Scholar] [CrossRef]
- Naeem, S. Experimental validity and ecological scale as criteria for evaluating research programs. In Scaling Relations in Experimental Ecology; Columbia University Press: New York, NY, USA, 2001. [Google Scholar]
- Keeley, E.R.; Grant, J.W. Prey size of salmonid fishes in streams, lakes, and oceans. Can. J. Fish. Aquat. Sci. 2001, 58, 1122–1132. [Google Scholar] [CrossRef]
- McIntyre, J.K.; Baldwin, D.H.; Beauchamp, D.A.; Scholz, N.L. Low-level copper exposures increase visibility and vulnerability of juvenile Coho Salmon to cutthroat trout predators. Ecol. Appl. 2012, 22, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 1980, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Wootton, J.T. The nature and consequences of indirect effects in ecological communities. Annu. Rev. Ecol. Syst. 1994, 443–466. [Google Scholar] [CrossRef]
- Power, M.E.; Dietrich, W.E. Food webs in river networks. Ecol. Res. 2002, 17, 451–471. [Google Scholar] [CrossRef]
- Baxter, C.V.; Fausch, K.D.; Saunders, C.W. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 2005, 50, 201–220. [Google Scholar] [CrossRef]
- Rose, K.A.; Cowan, J.H., Jr.; Winemiller, K.O.; Myers, R.A.; Hilborn, R. Compensatory density dependence in fish populations: Importance, controversy, understanding and prognosis. Fish Fish. 2001, 2, 293–327. [Google Scholar] [CrossRef] [Green Version]
- Leasure, D.R.; Wenger, S.J.; Chelgren, N.D.; Neville, H.M.; Dauwalter, D.C.; Bjork, R.; Fesenmyer, K.A.; Dunham, J.B.; Peacock, M.M.; Luce, C.H.; et al. 2019. Hierarchical multi-population viability analysis. Ecology 2019, 100, e02538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, D.L.; Falke, L.P.; Henderson, J.S.; Novak, M. Food-web interaction strength distributions are conserved by greater variation between than within predator–prey pairs. Ecology 2019, 100, e02816. [Google Scholar] [CrossRef] [PubMed]
- Houze, R.A.; McMurdie, L., Jr.; Peterson, W.; Schwaller, M.; Baccus, W.; Lundquist, J.; Mass, C.; Nijssen, B.; Rutledge, S.; Hudak, D.; et al. Olympic Mountains Experiment (OLYMPEX). Bull. Am. Meteorol. Soc. 2017, 2167–2188. [Google Scholar] [CrossRef] [PubMed]
- Strahler, A.N. Quantitative analysis of watershed geomorphology. EosTrans. Am. Geophys. Union 1957, 38, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Martens, K.D. Washington State Department of Natural Resources’ Riparian Validation Monitoring Program for salmonids on the Olympic Experimental State Forest—Study Plan; Washington State Department of Natural Resources, Forest Resources Division: Olympia, WA, USA, 2016.
- Washington State Department of Natural Resources (WADNR). Final Habitat Conservation Plan; Washington State Department of Natural Resources: Olympia, WA, USA, 1997.
- Bolsinger, C.L.; Waddell, K.L. Area of Old-Growth Forests in California, Oregon, and Washington; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Corvalis, OR, USA, 1993; Resource Bulletin PNW-RB-197.
- Connolly, P.J. Resident cutthroat trout in the central Coast Range of Oregon: Logging effects, habitat associations, and sampling protocols. Doctoral Thesis, Oregon State University, Corvallis, OR, USA, 1996. [Google Scholar]
- Martens, K.D.; Connolly, P.J. Juvenile anadromous salmonid production in Upper Columbia River side channels with different levels of hydrological connection. Trans. Am. Fish. Soc. 2014, 143, 757–767. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 29 January 2021).
- Sály, P.; Erős, T.; Takács, P.; Specziár, A.; Kiss, I.; Bíró, P. Assemblage level monitoring of stream fishes: The relative efficiency of single-pass vs. double-pass electrofishing. Fish. Res. 2009, 99, 226–233. [Google Scholar]
- Vehanen, T.; Sutela, T.; Jounela, P.; Huusko, A.; Mäki-Petäys, A. Assessing electric fishing sampling effort to estimate stream fish assemblage attributes. Fish. Manag. Ecol. 2013, 20, 10–20. [Google Scholar] [CrossRef]
- Teixeira-de Mello, F.; Kristensen, E.A.; Meerhoff, M.; González-Bergonzoni, I.; Baattrup-Pedersen, A.; Iglesias, C.; Kristensen, P.B.; Mazzeo, N.; Jeppesen, E. Monitoring fish communities in wadeable lowland streams: Comparing the efficiency of electrofishing methods at contrasting fish assemblages. Environ. Monit. Assess. 2014, 186, 1665–1677. [Google Scholar] [CrossRef]
- Burnett, K.M.; Reeves, G.H.; Miller, D.J.; Clarke, S.; Vance-Borland, K.; Christiansen, K. Distribution of salmon-habitat potential relative to landscape characteristics and implications for conservation. Ecol. Appl. 2007, 17, 66–80. [Google Scholar] [CrossRef]
- McMillan, J.R.; Liermann, M.C.; Starr, J.; Pess, G.R.; Augerot, X. Using a stream network census of fish and habitat to assess models of juvenile salmonid distribution. Trans. Am. Fish. Soc. 2013, 142, 942–956. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Burnham, K.; Anderson, D. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]


| CTT | COH | STH | CHN | SCP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNF | OESF | SNF | OESF | SNF | OESF | SNF | OESF | SNF | OESF | |
| Min | 0.3 | 1.3 | 0.3 | 1.3 | 0.7 | 1.7 | 0.8 | NP | 0.3 | 1.3 |
| Mean | 6.8 | 5.7 | 3.7 | 3.6 | 5.2 | 6.7 | 2.8 | NP | 5.2 | 3.9 |
| Median | 6.8 | 4.5 | 3.2 | 3.2 | 4.3 | 4.5 | 1.7 | NP | 3.7 | 3.3 |
| 95TH % | 17.8 | 16.3 | 8.9 | 7.1 | 12.5 | --a | --a | NP | 11.9 | 8.5 |
| Max | 23.9 | 21.1 | 11.9 | 7.1 | 15.4 | 21.1 | 11.1 | NP | 17.6 | 16.1 |
| Occurrence (%) | 97 | 93 | 42 | 51 | 29 | 18 | 8 | NP | 61 | 73 |
| Model | K | AICc | ΔAICc | AICcWT | Cum WT | LL |
|---|---|---|---|---|---|---|
| CTT = WW + GRAD | 4 | −744.58 | 0.00 | 0.59 | 0.59 | 376.39 |
| CTT = COH + WW + GRAD | 5 | −743.33 | 1.25 | 0.32 | 0.90 | 376.82 |
| CTT = COH*ABS + WW + GRAD | 7 | −740.70 | 3.88 | 0.08 | 0.99 | 377.65 |
| CTT = WW | 3 | −736.61 | 7.97 | 0.01 | 1.00 | 371.37 |
| CTT = GRAD | 3 | −712.08 | 32.50 | 0.00 | 1.00 | 359.80 |
| CTT = COH*ABS | 3 | −707.28 | 37.30 | 0.00 | 1.00 | 358.80 |
| CTT = COH | 3 | −694.05 | 50.53 | 0.00 | 1.00 | 350.09 |
| Model | K | AICc | ΔAICc | AICcWT | Cum WT | LL |
|---|---|---|---|---|---|---|
| COH = ABS + WW + GRAD | 5 | −397.50 | 0.00 | 0.24 | 0.24 | 203.91 |
| COH = CTT + ABS + WW + GRAD | 6 | −396.55 | 0.95 | 0.15 | 0.39 | 204.49 |
| COH = GRAD | 3 | −396.38 | 1.12 | 0.14 | 0.53 | 201.25 |
| COH = WW+ GRAD | 4 | −396.28 | 1.22 | 0.13 | 0.66 | 202.24 |
| COH = CTT + ABS | 4 | −396.05 | 1.45 | 0.12 | 0.78 | 202.13 |
| COH = ABS | 3 | −395.89 | 1.61 | 0.11 | 0.88 | 201.01 |
| COH = CTT + WW + GRAD | 5 | 395.03 | 2.47 | 0.07 | 0.95 | 202.67 |
| COH = CTT | 3 | −392.85 | 4.65 | 0.02 | 0.98 | 199.49 |
| COH = WW | 3 | −392.77 | 4.73 | 0.02 | 1.00 | 199.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, K.D.; Dunham, J. Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA. Fishes 2021, 6, 4. https://doi.org/10.3390/fishes6010004
Martens KD, Dunham J. Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA. Fishes. 2021; 6(1):4. https://doi.org/10.3390/fishes6010004
Chicago/Turabian StyleMartens, Kyle D., and Jason Dunham. 2021. "Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA" Fishes 6, no. 1: 4. https://doi.org/10.3390/fishes6010004
APA StyleMartens, K. D., & Dunham, J. (2021). Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA. Fishes, 6(1), 4. https://doi.org/10.3390/fishes6010004







