Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms
Abstract
:1. Introduction
- (a)
- Fish growth and welfare parameters, including effects on fish growth and feed conversion parameters, gut microbiota and anatomy, immunity, and resistance to pathogens.
- (b)
- Environmental parameters, including fishponds and/or tanks (water quality, diversity of aquatic microbiota).
2. Optimal Feeding Regimes and Improved Feed Conversion Are Prerequisites for Reducing the Environmental Impact Caused by Freshwater Fish Farms
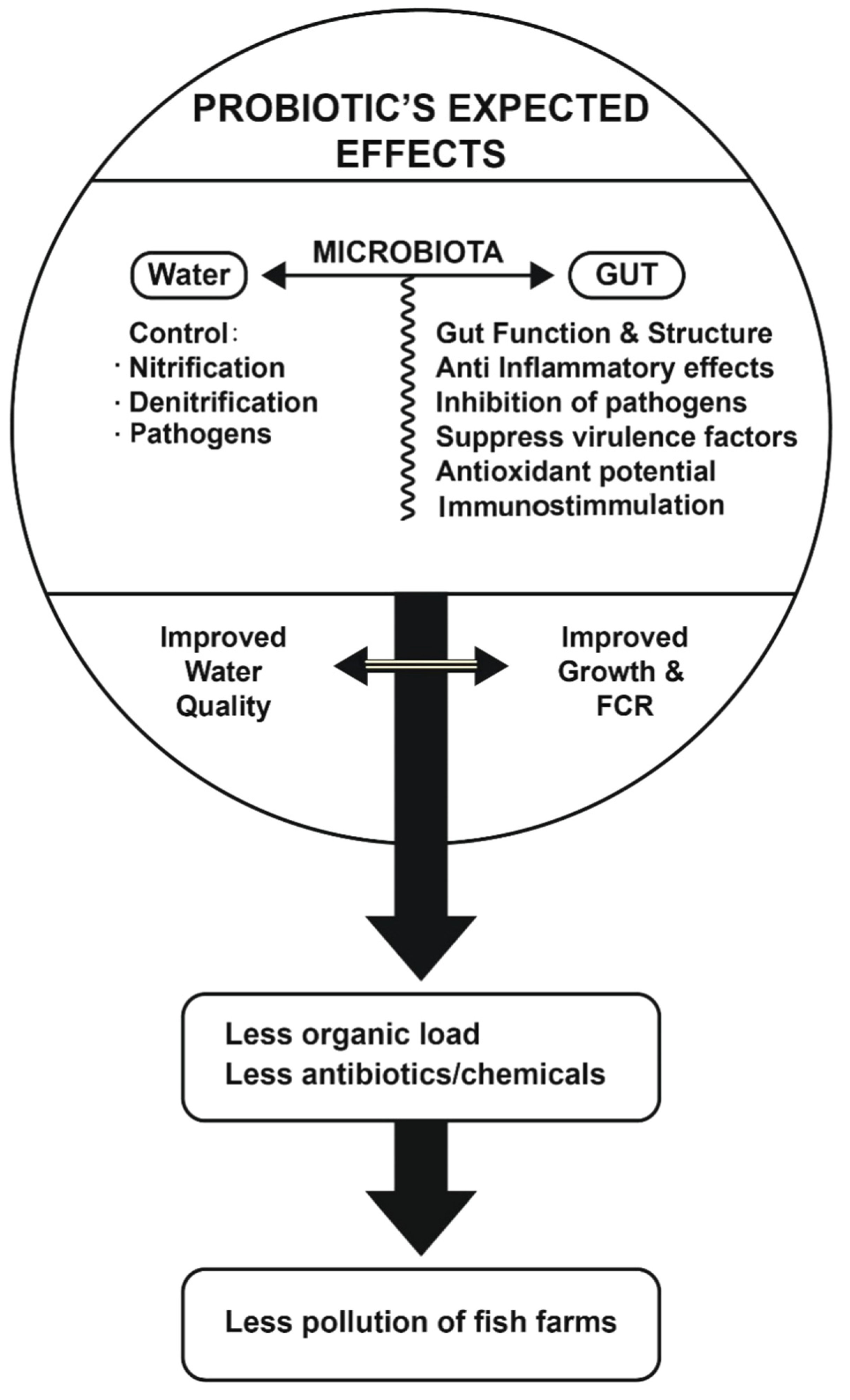
3. How Probiotics Can Improve FCR, Fish Health, and Fish Growth and Help Reduce the Environmental Impact Caused by Freshwater Fish Farms
3.1. Probiotics Can Improve the Digestion of Fish Diets and Support the Replacement of Fish Oils and Fish Proteins as Ingredients of Fish Feeds
3.2. Probiotics Can Reduce Subacute Intestinal Pathological Problems, Improve Feed Conversion, and Reduce Disease Outbreaks, Mortality, and Antibiotic Usage
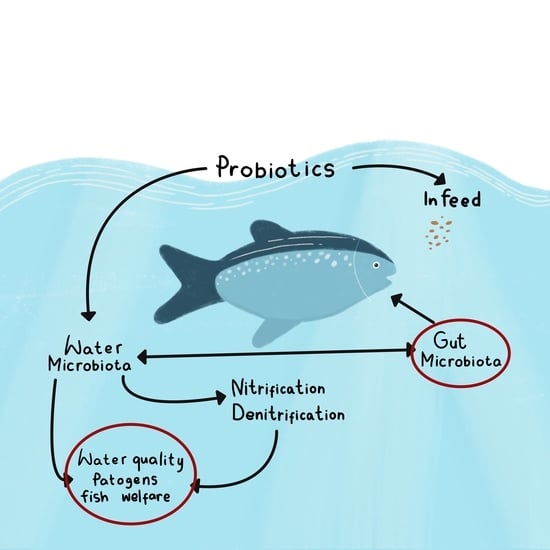
3.3. Probiotics Can Improve Water Quality of Freshwater Fishponds and Help Reduce the Environmental Impact of Freshwater Fish Farms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockus, A.B.; Rawles, S.D.; Sealey, W.M.; Conley, Z.B.; Gaylord, T.G. Effects of elevated temperature and dietary additives Thermal Care™, Bio-Mos®, and GroBiotic® A on rainbow trout (Oncorhynchus mykiss) performance. Aquaculture 2021, 544, 737084. [Google Scholar] [CrossRef]
- Soltani, M.; Ghosh, K.; Hoseinifar, S.H.; Kumar, V.; Lymbery, A.; Roy, S.; Ringø, E. Genusbacillus, promising probiotics in aquaculture: Aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev. Fish. Sci. Aquac. 2019, 27, 331–379. [Google Scholar] [CrossRef] [Green Version]
- Paray, B.A.; El-Basuini, M.F.; Alagawany, M.; Albeshr, M.F.; Farah, M.A.; Dawood, M.A.O. Yucca schidigera Usage for Healthy Aquatic Animals: Potential Roles for Sustainability. Animals 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.H.S.; Jourdehi, A.Y.; Zelti, A.H.; Masouleh, A.S.; Lakani, F.B. Effects of commercial superzist probiotic on growth performance and hematological and immune indices in fingerlings Acipenser baerii. Aquac. Int. 2020, 28, 377–387. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.; Dawood, M.A. The enrichment of diet with beneficial bacteria (single-or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
- Mohammadi, G.; Adorian, T.J.; Rafiee, G. Beneficial effects of Bacillus subtilis on water quality, growth, immune responses, endotoxemia and protection against lipopolysaccharide-induced damages in Oreochromis niloticus under biofloc technology system. Aquac. Nutr. 2020, 26, 1476–1492. [Google Scholar] [CrossRef]
- Putra, A.N.; Mustahal, M.; Syamsunarno, M.B.; Hermawan, D.; Fatimah, D.G.; Putri, P.B.; Sevia, S.; Isnaeni, R.; Herjayanto, M. Dietary Bacillus NP5 supplement impacts on growth, nutrient digestibility, immune response, and resistance to Aeromonas hydrophila infection of African catfish, Clarias gariepinus. Biodiversitas J. Biol. Divers. 2021, 22, 253–261. [Google Scholar] [CrossRef]
- Al-Dohail, M.A.; Hashim, R.; Aliyu-Paiko, M. Effects of the probiotic, Lactobacillus acidophilus, on the growth performance, haematology parameters and immunoglobulin concentration in African Catfish (Clarias gariepinus, Burchell 1822) fingerling. Aquac. Res. 2009, 40, 1642–1652. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S.H.; Tongsiri, S.; Chitmanat, C.; Musthafa, M.S.; El-Haroun, E.; Ringo, E. Modulation of growth, innate immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) culture under biofloc system by supplementing pineapple peel powder and Lactobacillus plantarum. Fish Shellfish. Immunol. 2021, 115, 212–220. [Google Scholar] [CrossRef]
- Yanbo, W.; Zirong, X. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim. Feed. Sci. Technol. 2006, 127, 283–292. [Google Scholar] [CrossRef]
- Modanloo, M.; Soltanian, S.; Akhlaghi, M.; Hoseinifar, S.H. The effects of single or combined administration of galactooligosaccharide and Pediococcus acidilactici on cutaneous mucus immune parameters, humoral immune responses and immune related genes expression in common carp (Cyprinus carpio) fingerlings. Fish Shellfish. Immunol. 2017, 70, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, D.L.; Carnevali, O. Probiotic modulation of the gut microbiota of fish. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; Wiley: Hoboken, NJ, USA, 2014; pp. 185–222. [Google Scholar]
- Sugimura, Y.; Hagi, T.; Hoshino, T. Correlation between in vitro mucus adhesion and the in vivo colonization ability of lactic acid bacteria: Screening of new candidate carp probiotics. Biosci. Biotechnol. Biochem. 2011, 75, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrifield, D.; Bradley, G.; Baker, R.; Davies, S. Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment. Aquac. Nutr. 2010, 16, 496–503. [Google Scholar] [CrossRef]
- Hai, N.V. The use of probiotics in aquaculture. J. Appl. Microbiol. 2015, 119, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Melo-Bolívar, J.F.; Pardo, R.Y.R.; Hume, M.E.; Díaz, L.M.V. Multistrain probiotics use in main commercially cultured freshwater fish: A systematic review of evidence. Rev. Aquac. 2021, 13, 1758–1780. [Google Scholar] [CrossRef]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic Bacteria as Biological Control Agents in Aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, S.; Ali, M.L.; Alam, M.A.; Rahman, M.M.; Jørgensen, N.O. Effect of probiotic and sand filtration treatments on water quality and growth of tilapia (Oreochromis niloticus) and pangas (Pangasianodon hypophthalmus) in earthen ponds of southern Bangladesh. J. Appl. Aquac. 2016, 28, 199–212. [Google Scholar] [CrossRef]
- Langlois, L.; Akhtar, N.; Tam, K.C.; Dixon, B.; Reid, G. Fishing for the right probiotic: Host–microbe interactions at the interface of effective aquaculture strategies. FEMS Microbiol. Rev. 2021, 45, fuab030. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Mavraganis, T.; Thorarensen, H.; Tsoumani, M.; Nathanailides, C. On the Environmental Impact of Freshwater Fish Farms in Greece and in Iceland. Annu. Res. Rev. Biol. 2017, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Imsland, A.K.D.; Gunnarsson, S.; Thorarensen, H. Impact of environmental factors on the growth and maturation of farmed Arctic charr. Rev. Aquac. 2020, 12, 1689–1707. [Google Scholar] [CrossRef]
- Azevedo, P.A.; Cho, C.Y.; Leeson, S.; Bureau, D.P. Effects of feeding level and water temperature on growth, nutrient and energy utilization and waste outputs of rainbow trout (Oncorhynchus mykiss). Aquat. Living Resour. 1998, 11, 227–238. [Google Scholar] [CrossRef]
- Brett, J. Environmental factors and growth. In Fish Physiology; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; Volume 8, pp. 599–675. [Google Scholar]
- Myrick, C.; Cech, J.J. Temperature influences on California rainbow trout physiological performance. Fish Physiol. Biochem. 2000, 22, 245–254. [Google Scholar] [CrossRef]
- Penn, M.H.; Bendiksen, E.; Campbell, P.; Krogdahl, Å. High level of dietary pea protein concentrate induces enteropathy in Atlantic salmon (Salmo salar L.). Aquaculture 2011, 310, 267–273. [Google Scholar] [CrossRef]
- Kumar, V.; Fawole, F.J.; Romano, N.; Hossain, S.; Labh, S.N.; Overturf, K.; Small, B.C. Insect (black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish Shellfish. Immunol. 2021, 109, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, J.E.; Cho, C.Y. Minimising waste through bioenergetically and behaviourally based feeding strategies. Water Sci. Technol. 1995, 31, 29–40. [Google Scholar] [CrossRef]
- Bureau, D.P.; Hua, K. Towards effective nutritional management of waste outputs in aquaculture, with particular reference to salmonid aquaculture operations. Aquac. Res. 2010, 41, 777–792. [Google Scholar] [CrossRef]
- Lambert, Y.; Dutil, J.-D. Food intake and growth of adult Atlantic cod (Gadus morhua L.) reared under different conditions of stocking density, feeding frequency and size-grading. Aquaculture 2001, 192, 233–247. [Google Scholar] [CrossRef]
- Cho, C.Y.; Bureau, D.P. Reduction of waste output from salmonid aquaculture through feeds and feeding. Prog. Fish Cult. 1997, 59, 155–160. [Google Scholar] [CrossRef]
- Aguado-Giménez, F. Effect of Feed Delivery Rate and Pellet Size on Rearing Performance, Feed Wastage and Economic Profitability in Gilthead Seabream (Sparus aurata) Ongrowing. Water 2020, 12, 954. [Google Scholar] [CrossRef] [Green Version]
- Berillis, P.; Mente, E. Histology of Goblet Cells in the Intestine of the Rainbow Trout Can Lead to Improvement of the Feeding Management. J. Fish. 2017, 11, 32–33. [Google Scholar] [CrossRef] [Green Version]
- Mavraganis, T.; Tsoumani, M.; Kolygas, M.; Chatziefstathiou, M.; Nathanailides, C. Using seasonal variability of water quality parameters to assess the risk of aquatic pollution from rainbow trout fish farms in Greece. Int. J. Energy Water Resour. 2021, 5, 379–389. [Google Scholar] [CrossRef]
- Rafiee, G.; Saad, C.R. Nutrient cycle and sludge production during different stages of red tilapia (Oreochromis sp.) growth in a recirculating aquaculture system. Aquaculture 2005, 244, 109–118. [Google Scholar] [CrossRef]
- Mavraganis, T.; Constantina, C.; Kolygas, M.; Vidalis, K.; Nathanailides, C. Environmental issues of aquaculture development. Egypt. J. Aquat. Biol. Fish. 2020, 24, 441–450. [Google Scholar] [CrossRef]
- Burrells, C.; Williams, P.D.; Southgate, P.J.; Crampton, V.O. Immunological, physiological and pathological responses of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Veter. Immunol. Immunopathol. 1999, 72, 277–288. [Google Scholar] [CrossRef]
- Mente, E.; Carter, C.; Barnes, R.; Vlahos, N.; Nengas, I. Post-Prandial Amino Acid Changes in Gilthead Sea Bream. Animals 2021, 11, 1889. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.D.; Galián, C.; Fernández, V.; Chaves-Pozo, E.; García de la Serrana, D.; Sáez, M.I.; Arizcun, M. Influence of low dietary inclusion of the microalga Nannochloropsis gaditana (Lubián 1982) on performance, fish morphology, and muscle growth in juvenile gilthead seabream (Sparus aurata). Animals 2020, 10, 2270. [Google Scholar] [CrossRef]
- Mousavi, S.; Zahedinezhad, S.; Loh, J.Y. A review on insect meals in aquaculture: The immunomodulatory and physiological effects. Int. Aquat. Res. 2020, 12, 100–115. [Google Scholar]
- Montazeri Parchikolaei, H.; Abedian Kenari, A.; Esmaeili, M. Soya bean-based diets plus probiotics improve the profile of fatty acids, digestibility, intestinal microflora, growth performance and the innate immunity of beluga (Huso huso). Aquac. Res. 2021, 52, 152–166. [Google Scholar] [CrossRef]
- Dawood, M. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Ogunkalu, O. Effects of Feed Additives in Fish Feed for Improvement of Aquaculture. Eurasian J. Food Sci. Technol. 2019, 3, 49–57. [Google Scholar]
- Tahar, A.; Kennedy, A.; Fitzgerald, R.D.; Clifford, E.; Rowan, N. Full Water Quality Monitoring of a Traditional Flow-through Rainbow Trout Farm. Fishes 2018, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Carr, O.; Goulder, R. Fish-farm effluents in rivers—I. effects on bacterial populations and alkaline phosphatase activity. Water Res. 1990, 24, 631–638. [Google Scholar] [CrossRef]
- Nathanailides, C.; Tsoumani, M.; Kakali, F.; Logothetis, P.; Beza, P.; Mayraganis, T.; Kanlis, G.; Delis, G.; Tiligadas, I.; Chatziefstathiou, M. A correlation between alkaline phosphatase and phosphate levels with the biomass of trout farm effluents. In Proceedings of the VI International Conference WATER & FISH, Faculty of Agriculture, Zemun, Serbia, 12–14 June 2015; pp. 170–175. [Google Scholar]
- Rurangwa, E.; Verdegem, M. Microorganisms in recirculating aquaculture systems and their management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Kord, M.I.; Maulu, S.; Srour, T.M.; Omar, E.A.; Farag, A.A.; Nour, A.A.M.; Hasimuna, O.J.; Abdel-Tawwab, M.; Khalil, H.S. Impacts of water additives on water quality, production efficiency, intestinal morphology, gut microbiota, and immunological responses of Nile tilapia fingerlings under a zero-water-exchange system. Aquaculture 2022, 547, 737503. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Noble, C.; Sánchez-Vázquez, F.J. Does feeding time affect fish welfare? Fish Physiol. Biochem. 2012, 38, 143–152. [Google Scholar] [CrossRef]
- Xie, M.; Zhou, W.; Xie, Y.; Li, Y.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Ran, C.; Zhou, Z. Effects of Cetobacterium somerae fermentation product on gut and liver health of common carp (Cyprinus carpio) fed diet supplemented with ultra-micro ground mixed plant proteins. Aquaculture 2021, 543, 736943. [Google Scholar] [CrossRef]
- Ostaszewska, T.; Dabrowski, K.; Kamaszewski, M.; Grochowski, P.; Verri, T.; Rzepkowska, M.; Wolnicki, J. The effect of plant protein-based diet supplemented with dipeptide or free amino acids on digestive tract morphology and PepT1 and PepT2 expressions in common carp (Cyprinus carpio L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Øverland, M.; Sørensen, M.; Storebakken, T.; Penn, M.; Krogdahl, Å.; Skrede, A. Pea protein concentrate substituting fish meal or soybean meal in diets for Atlantic salmon (Salmo salar)—Effect on growth performance, nutrient digestibility, carcass composition, gut health, and physical feed quality. Aquaculture 2009, 288, 305–311. [Google Scholar] [CrossRef]
- Roh, H.; Park, J.; Kim, A.; Kim, N.; Lee, Y.; Kim, B.S.; Vijayan, J.; Lee, M.K.; Park, C.-I.; Kim, D.-H. Overfeeding-Induced Obesity Could Cause Potential Immuno-Physiological Disorders in Rainbow Trout (Oncorhynchus mykiss). Animals 2020, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Serna-Duque, J.A.; Esteban, M. Effects of inflammation and/or infection on the neuroendocrine control of fish intestinal motility: A review. Fish Shellfish. Immunol. 2020, 103, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Udayangani, R.; Dananjaya, S.; Nikapitiya, C.; Heo, G.-J.; Lee, J.; De Zoysa, M. Metagenomics analysis of gut microbiota and immune modulation in zebrafish (Danio rerio) fed chitosan silver nanocomposites. Fish Shellfish. Immunol. 2017, 66, 173–184. [Google Scholar] [CrossRef]
- Azimirad, M.; Meshkini, S.; Ahmadifard, N.; Hoseinifar, S.H. The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish. Immunol. 2016, 54, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef] [Green Version]
- Sealey, W.M.; Conley, Z.B.; Bensley, M. Prebiotic Supplementation has Only Minimal Effects on Growth Efficiency, Intestinal Health and Disease Resistance of Westslope Cutthroat Trout Oncorhynchus clarkii lewisi Fed 30% Soybean Meal. Front. Immunol. 2015, 6, 396. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Duan, X.-D.; Jiang, W.-D.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; et al. Soybean glycinin decreased growth performance, impaired intestinal health, and amino acid absorption capacity of juvenile grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2019, 45, 1589–1602. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, S.; Makol, A.; Benítez-Santana, T.; Caballero, M.J.; Montero, D.; Sweetman, J.; Izquierdo, M. Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Fish Shellfish. Immunol. 2011, 30, 674–681. [Google Scholar] [CrossRef]
- Van den Ingh, T.S.G.A.M.; Krogdahl, Å.; Olli, J.J.; Hendriks, H.G.C.J.M.; Koninkx, J.G.J.F. Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon: A morphological study. Aquaculture 1991, 94, 297–305. [Google Scholar] [CrossRef]
- Shafique, L.; Abdel-Latif, H.M.; Hassan, F.U.; Alagawany, M.; Naiel, M.A.; Dawood, M.A.; Yilmaz, S.; Liu, Q. The feasibility of using yellow mealworms (Tenebrio molitor): Towards a sustainable aquafeed industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Panteli, N.; Mastoraki, M.; Lazarina, M.; Chatzifotis, S.; Mente, E.; Kormas, K.; Antonopoulou, E. Configuration of Gut Microbiota Structure and Potential Functionality in Two Teleosts under the Influence of Dietary Insect Meals. Microorganisms 2021, 9, 699. [Google Scholar] [CrossRef]
- Islam, S.M.; Rohani, F. Shahjahan Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac. Rep. 2021, 21, 100800. [Google Scholar] [CrossRef]
- Kaushik, S.; Oliva-Teles, A. Effect of digestible energy on nitrogen and energy balance in rainbow trout. Aquaculture 1985, 50, 89–101. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Moriarty, D.J. Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 1998, 164, 351–358. [Google Scholar] [CrossRef]
- Sakai, M.; Yoshida, T.; Atsuta, S.; Kobayashi, M. Enhancement of resistance to vibriosis in rainbow trout, Oncorhynchus mykiss (Walbaum), by oral administration of Clostridium butyricum bacterin. J. Fish Dis. 1995, 18, 187–190. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; DeCamp, O.; Dawood, M.; Eltholth, M. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Sunitha, K.; Padmavathi, P. Influence of probiotics on water quality and fish yield in fish ponds. Int. J. Pure Appl. Sci. Technol. 2013, 19, 48. [Google Scholar]
- Deng, Y.; Verdegem, M.C.; Eding, E.; Kokou, F. Effect of rearing systems and dietary probiotic supplementation on the growth and gut microbiota of Nile tilapia (Oreochromis niloticus) larvae. Aquaculture 2022, 546, 737297. [Google Scholar] [CrossRef]
- Putra, D.F.; Fanni, M.; Muchlisin, Z.A.; Muhammadar, A.A. Growth performance and survival rate of climbing perch (Anabas testudineus) fed Daphnia sp. enriched with manure, coconut dregs flour and soybean meal. Aquac. Aquar. Conserv. Legis. 2016, 9, 944–948. [Google Scholar]
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.; Bøgwald, J.; Castex, M.; Ringø, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Zibiene, G.; Zibas, A. Impact of commercial probiotics on growth parameters of European catfish (Silurus glanis) and water quality in recirculating aquaculture systems. Aquac. Int. 2019, 27, 1751–1766. [Google Scholar] [CrossRef]
- El-Kady, A.A.; Magouz, F.I.; Mahmoud, S.A.; Abdel-Rahim, M.M. The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737249. [Google Scholar] [CrossRef]
- Cruz, P.M.; Ibáñez, A.L.; Hermosillo, O.A.M.; Saad, H.C.R. Use of Probiotics in Aquaculture. ISRN Microbiol. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Jafaryan, H.; Taati, M.M.; Jafarzadeh, M. The enhancement of growth parameters in common carp (Cyprinus carpio) larvae using probiotic in rearing tanks and feeding by various Artemia nauplii. Aquac. Aquar. Conserv. Legis. 2011, 4, 511–518. [Google Scholar]
- Morales-Jiménez, J. Effect of bacterial probiotics bio-encapsulated into Artemia franciscana on weight and length of the shortfin silverside (Chirostoma humboldtianum), and PCR-DGGE characterization of its intestinal bacterial community. Lat. Am. J. Aquat. Res. 2017, 45, 1031–1043. [Google Scholar]
- Merrifield, D.L.; Dimitroglou, A.; Bradley, G.; Baker, R.T.M.; Davies, S.J. Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria. Aquac Nutr. 2010, 16, 504–510. [Google Scholar] [CrossRef]
- Begum, N.; Islam, M.; Haque, A.; Suravi, I. Growth and yield of monosex tilapia Oreochromis niloticus in floating cages fed commercial diet supplemented with probiotics in freshwater pond, Sylhet. Bangladesh J. Zool. 2017, 45, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Gao, S.; Zhu, Y.; Wang, G.; Zhang, K.; Li, Z.; Yu, E.; Tian, J.; Xia, Y.; Xie, J.; et al. Effect of the Aerobic Denitrifying Bacterium Pseudomonas furukawaii ZS1 on Microbiota Compositions in Grass Carp Culture Water. Water 2021, 13, 1329. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Dai, Y.-X.; Li, Y.-M.; Qin, J.-G.; Wang, Y. Can Application of Commercial Microbial Products Improve Fish Growth and Water Quality in Freshwater Polyculture? N. Am. J. Aquac. 2016, 78, 154–160. [Google Scholar] [CrossRef]
- Thurlow, C.M.; Williams, M.A.; Carrias, A.; Ran, C.; Newman, M.; Tweedie, J.; Allison, E.; Jescovitch, L.N.; Wilson, A.E.; Terhune, J.S.; et al. Bacillus velezensis AP193 exerts probiotic effects in channel catfish (Ictalurus punctatus) and reduces aquaculture pond eutrophication. Aquaculture 2019, 503, 347–356. [Google Scholar] [CrossRef]
- Sharma, G.K.; Thakur, A. Bioremediation of Farm Ponds for Improving Water Quality and Fish Productivity in Bastar Plateau. Adv. Biores 2018, 9, 81–85. [Google Scholar]
- Iribarren, D.; Dagá, P.; Moreira, M.T.; Feijoo, G. Potential environmental effects of probiotics used in aquaculture. Aquac. Int. 2012, 20, 779–789. [Google Scholar] [CrossRef]
| Probiotic Used | Max Dosage Used | Fresh Water Fish Species Fed with Probiotic Supplements | Reported Effectiveness | Source |
|---|---|---|---|---|
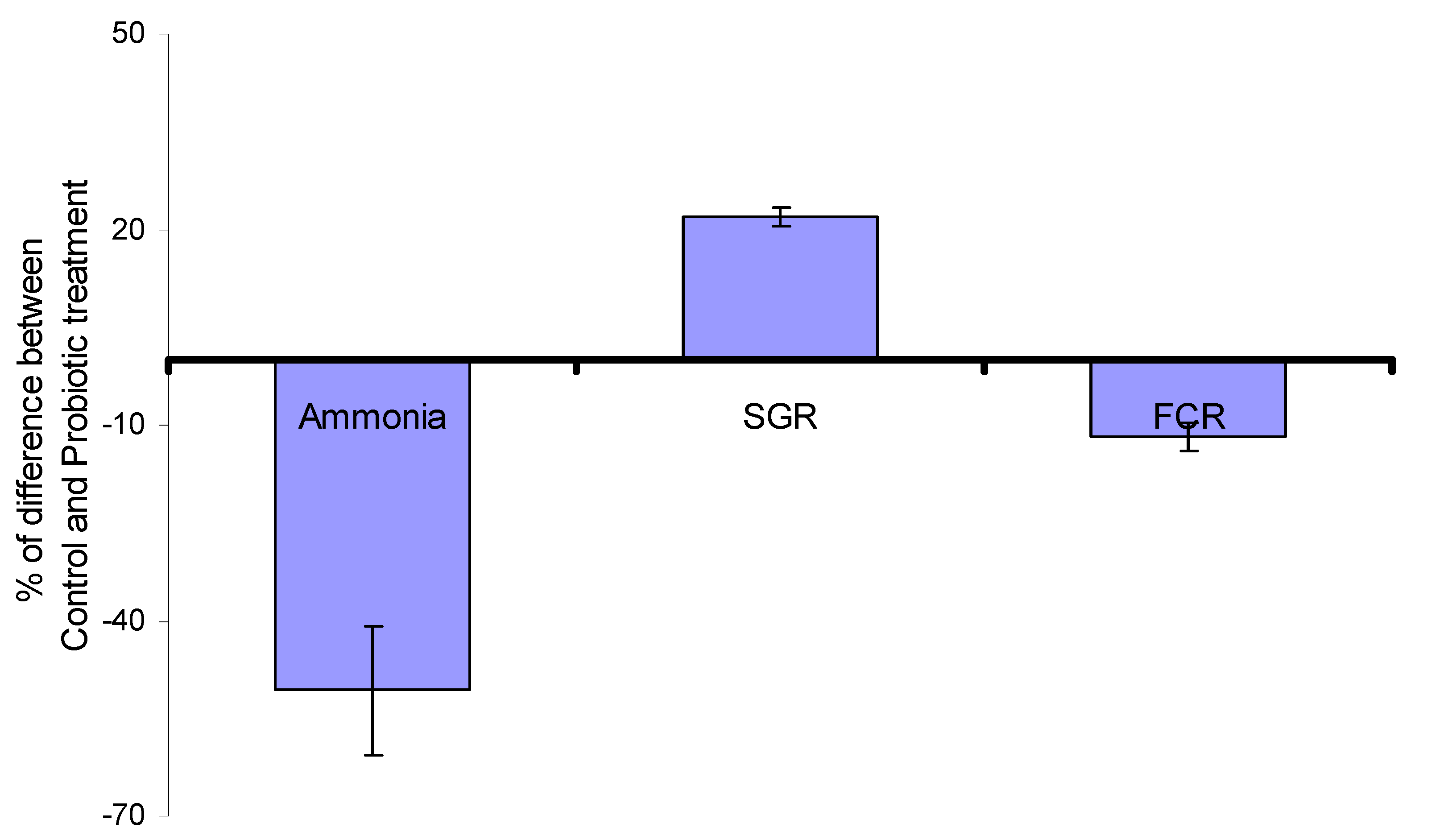
| Incorporated in the Feed, L. acidophilus, | 3.01 × 107 CFU/g of feed | African catfish (Clarias gariepinus) | Increased SGR, improved FCR, reduction of NHx compounds | [8] |
| (Lactobacillus plantarum or Multi-strain (Bacillus subtilis, L. plantarum, L. rhamnosus, L. acidophilus & L. delbrueckii) | 0.2% (of commercial preparation (108 CFU/g); per kg feed | Nile tilapia (Oreochromis niloticus) | SGR increased, FCR improved, NHx compounds decreased, lower mortality rate when challenged with Aeromonas hydrophi | [5] |
| Commercial mixtrue (super biotic; biozyme; zymetin) | incorparated in the feed (no dosage reported) | Nile tilapia O. niloticus | SGR increased, FCR improved, no effect on nitrate and ammonia | [70] |
| Bacillus strains mixture (Sanolife PRO-F) | In feed 0.1–0.2 g/kg | Nile tilapia O. niloticus | SGR increased, FCR improved, NHx compounds reduced | [25] |
| P. furukawaii ZS1 | 4 × 105 CFU m/L. Diluted in ponds | grass carp (Ctenopharyngodon idella) | NHx compounds were reduced | [72] |
| Lactobacillus spp., Bifidobacterium spp., Lactococcus spp., Saccharomyces cerevisiae, and yeast culture | 1 mL of 1.5 × 106 CFU. diluted in 0.6 m3 volume tank (RAS) | European catfish (Silurus glanis) | NHx compounds decreased, improved FCR and SGR. | [73] |
| Commercial products: -Prozym powder Ultra -Microban, -Aquastar, -Sanolife PROW | 0.0010% 0.002 g/m3/day | Nile tilapia O. niloticus | Increased SGR, NHx compounds decreased, decreased Vibrio counts in pond water and fish guts. | [48] |
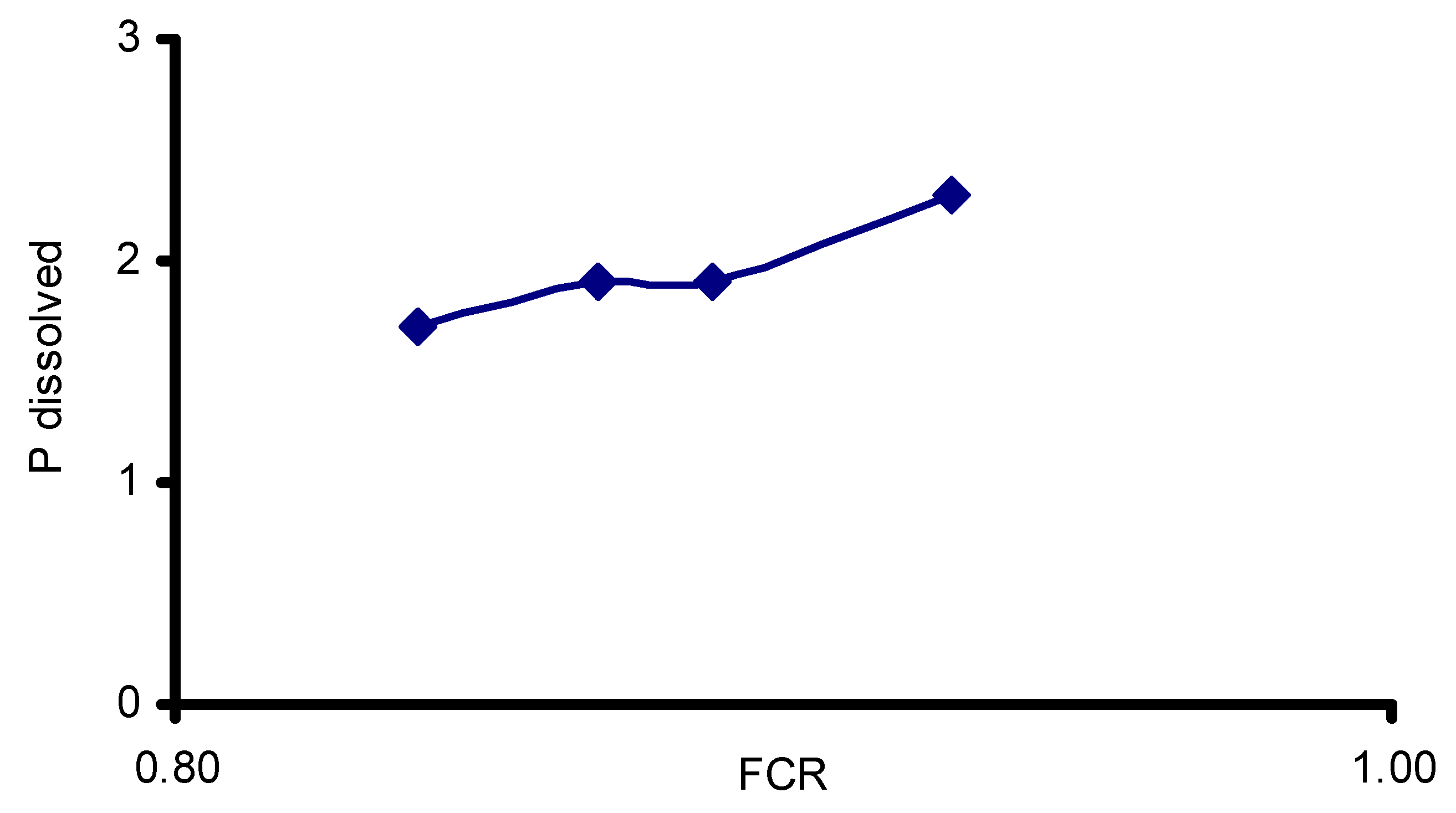
| Commercial Preparations (AquaStar®; EM® MicroPan®) | 2 × 1012 CFU/kg diluted in 1 m3 tanks 0.0015 to 0.002 g m−3 day −1 | Nile tilapia O. niloticus | SGR increased, N and P load decreased AquaStar® and EM® reduced ammonia levels by around 70% and MicroPan® by 55%. | [74] |
| Commercial products: (i) Novozymes pond plus (ii) BIO-AQUA | (i) & (ii) 1010 CFU/g, (iii) 1011 CFU/g | Polyculture (Grass Carp C. idella, Gibel Carp Carassius gibelio and Silver Carp Hypophthalmichthys molitrix) | SGR increased, NS effect on Ammonia | [71] |
| Bacillus velezensis AP193 | 4 × 107 CFU/g | channel catfish (Ictalurus punctatus) | SGR increased, N and P load decreased | [75] |
| Commercial products diluted in earthen ponds: (i) PondPlus® (blend of mainly Bacillus) (ii) AquaPhoto® (Rhodopseudomonas sp. & Bacillus subtili) | -PondPlus: 50 mg per 1000 L water -Aquaphoto:109 CFU mL−1 | Tilapia (Oreochromis niloticus) and pangas (Pangasianodon hypophthalmus) | NS on SGR, reduction of Ammonia | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nathanailides, C.; Kolygas, M.; Choremi, K.; Mavraganis, T.; Gouva, E.; Vidalis, K.; Athanassopoulou, F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes 2021, 6, 76. https://doi.org/10.3390/fishes6040076
Nathanailides C, Kolygas M, Choremi K, Mavraganis T, Gouva E, Vidalis K, Athanassopoulou F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes. 2021; 6(4):76. https://doi.org/10.3390/fishes6040076
Chicago/Turabian StyleNathanailides, Cosmas, Markos Kolygas, Konstantina Choremi, Theodoros Mavraganis, Evangelia Gouva, Kosmas Vidalis, and Fotini Athanassopoulou. 2021. "Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms" Fishes 6, no. 4: 76. https://doi.org/10.3390/fishes6040076
APA StyleNathanailides, C., Kolygas, M., Choremi, K., Mavraganis, T., Gouva, E., Vidalis, K., & Athanassopoulou, F. (2021). Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes, 6(4), 76. https://doi.org/10.3390/fishes6040076