1. Introduction
Consumption patterns in the globe today, as a result of a growing population, put a lot of strain on existing natural resources [
1]. In recent years, meeting the protein requirements of the world’s rising population has been a major concern [
2]. Catfish of the genus
Clarias are extensively dispersed in Africa and Asia and have long been a source of aquaculture interest. Catfish are suitable for stocking in ponds and they tolerate low dissolved oxygen with high-quality protein (15% by weight) and low fat contents compared to other common fish species [
3]. Because of its fast growth rate, disease resistance, and amenability to high-density culture, a variety of
Clarias species and hybrids are produced [
4]. African catfish is regarded as one of the greatest proper choices of fish farming alternatives to tilapia in Africa [
4]. Catfish production yields from ponds may be larger than tilapia yields of the same area due to the ability to use low-grade feed of agricultural by-products [
5]. Fishmeal yield is expected to drop in the future due to decreasing fishing resources, and as a result, the price of fishmeal is steadily rising [
6]. Many developing countries will be unable to rely on fishmeal as a primary protein source in aquafeeds for a long time, particularly in intensive fish farming. Finding less expensive sources of protein that give adequate growth is beneficial to both diet manufacturers and aquaculture producers [
7].
Fewer studies have been done on replacing soybean meal in most fish feeds, except a trial to use peanut meal as an alternative to soybean meal in Nile tilapia feeds [
8]. Soybean meal protein is mostly utilized in African aquaculture diets for omnivorous fish species, such as tilapias (
Oreochromis species) and catfish (
Clarias species). Soybean meal is tasty and inexpensive compared to fishmeal, but, due to increased competition in poultry and livestock feeding, it has become more expensive and scarce [
9]. In addition, antinutritional substances restrict the use of soybean meal in aquafeeds [
10].
Meat and bone meal, blood meal, soybean meal, silk worm pupae, different oil cakes, cotton seed meal, chicken by-product meal, dry brewer’s yeast, hydrolyzed feather meal, maize gluten meal, and fish silage have all been explored as protein sources [
11,
12,
13]. These proteins are referred to as secondary protein sources, and they are typically found in low concentrations in actual fish diets [
14]. Apart from often unbalanced amino acid profiles, endogenous antinutritional factors, palatability, and feed acceptability are among the reasons that limit the utilization of alternative protein sources, particularly plant feedstuffs [
15].
SBp meal has been identified as a possible animal protein source by increasing shrimp production from captures and farms [
16] and could reduce environmental problems resulting from the improper dumping of inedible parts of shrimp waste meal due to its high fibee and ash contents [
17]. Shrimp by-products have been found as a high-potential animal protein source, as well as a significant source of chitin [
18]. Only 65% of the shrimp are edible, with the rest being dumped as waste (cephalothoraxes and exoskeleton). Polymers derived from this waste are promising substitutes of synthetic polymers for the production of bioplastics, which have biodegradable properties [
19]. The ratio of shrimp collected to rejected species in shrimp capture fisheries ranges from 1:5 to 1:15, resulting in shrimpers discarding an estimated 15 million tons of undesired fish [
20]. SBp meal generally contains 8–10% chitin, 30–40% protein, 0–14% lipids, and 10–20% calcium in dry weights [
21]. The acid treatment processing technique of SBp meal using formic acid [
22] or acetic acid [
17] can reduce its ash and fiber contents, but increases its crude protein contents, while the autoclaving processing technique of SBp meal cannot affect its chemical composition except lowering its ether extract content [
23]. These wastes have also been observed to diminish the digestibility of crustacean (
Callianassa) meals in tilapia [
24], decrease lipid absorption, and increase water content in the stools of Atlantic salmon [
25].
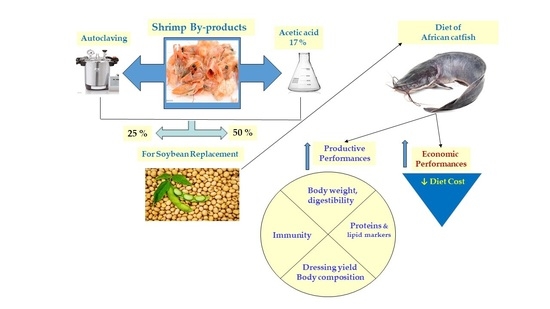
As far as we know, there is an information scarcity on the use of SBp meal as a soybean replacer in the African catfish (Clarias lazera) diet. This study assesses the dietary usage of SBp meal in African catfish through production and nutrient utilization parameters (growth, digestibility, body composition %, dressing %, blood parameters, and immunity) and economic indices.
4. Discussion
The partial replacement of soybean meal with 25% autoclaved and acid-treated SBp had no adverse effect on final body weight, total feed intake/fish, feed conversion ratio, and protein efficiency ratio. Meanwhile, it showed a significant increase (
p < 0.05) in body weight gain, weight gain %, and average daily gain relative to the control group. Moreover, the partial replacement of soybean meal with 50% autoclaved and acid-treated SBp significantly increases (
p < 0.05) the final body weight, body weight gain, WG%, ADG, and PER, and significantly improves FCR values relative to the control group (100% soybean meal). The results of the present study were dissimilar to many authors [
17,
28,
30], who found that the increased levels of SBp, in the form of preserved shrimp head, did not improve the growth of catfish and tilapia. On the other hand, the findings of the present study agreed with Li et al. and Hardy et al. [
20,
48]. They found that the increased levels of SBp and by-catch meal with shrimp trawling at 50% of the dietary protein improve the weight gain and feed efficiency of rainbow trout when compared with those fed the control diet. These effects could be referred to the improvement of digestibility, palatability, and consumption rate of the current treated SBp to enhance catfish growth parameters.
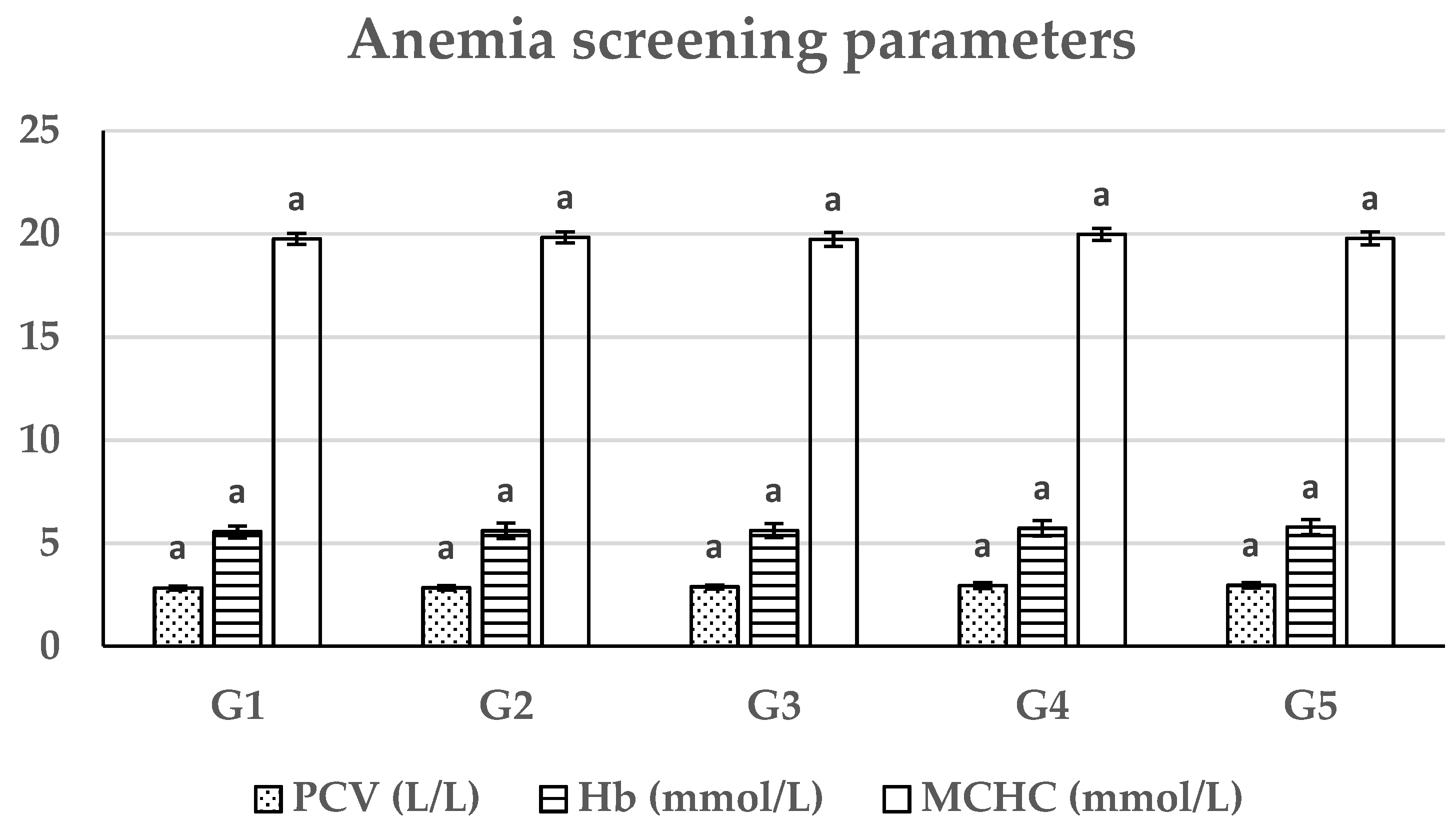
Anemia screening parameters of the existing study showed a non-significant difference between the tested groups for hemoglobin content, PCV, and MCHC. There are no studies seeking the impact of the existing SBp meal feeding on blood profiles of catfish or other fish species. These outcomes could be referred to the contents of autoclaved and acid-treated SBp meals having no adverse effects on the hematopoietic system of the African catfish and keeping the hematological parameters within control group physiological limits.
The protein markers of the present study revealed that the values of total plasma protein were significantly increased in groups 3 and 5 (25% and 50% acid-treated SBp, respectively) compared with the control one. Moreover, there was a significant increase in plasma albumin of group 5 relative to the values of other groups, including the control (G1). Plasma proteins showed a similar trend of improvement in fingerlings fed diets with different levels of fish bio-silage [
49] or fermented soy pulp [
50] as partial or total replacements of fishmeal. In general, the high crude protein % (40.21, 40.26—
Table 1) content with low crude fiber % (13.04, 7.18%—
Table 1) of autoclaved and acid-treated SBp meals, respectively, may reflect on increased levels of plasma protein profile for the replaced groups (G2, G3, G4, and G5).
The present study of plasma triglycerides and cholesterol noted a non-considerable variation between control and SBp-replaced groups. These outcomes matched the Oduguwa et al. [
51] results, who revealed that the replacement of fishmeal with shrimp waste meal in the diet of growing pigs leads to a non-significant difference in serum cholesterol level. The inedible parts of shrimp waste meal may be restricted due to its high fiber and ash contents [
17], which decrease lipid absorption in Atlantic salmon,
Salmo salar L. [
25]. The high fat content and low fiber contents of acid-treated SBp meal might help in lipid absorption into blood and increase lipid profiles relative to autoclaved SBp meal. In contrast, the high fat and fiber contents of autoclaved SBp meal may decrease lipid absorption into blood and decrease the plasma lipid profiles compared with acid-treated SBp meal (
Table 1).
The immunological parameters improvements of the existing study revealed a significant increase in SBp treated groups relative to the control one. Parallel enhancement of phagocytic index and activity were noted in treated groups with fermented earthworms as a feed additive in catfish (
Clarias gariepinus) [
52]. In contrast, fish fed spirulina and/or β-glucan showed a non-significant change in A/G ratio than the control group in African catfish [
53]. Generally, the SBp meals of the current study had good quality proteins and recorded a growth enhancement, which were reflected on immunological parameter augmentation.
African catfish body composition of the present study recorded a major change between treated groups (G2: G5) and the control one (G1). In addition, Leal et al. [
30] found that the crude lipid content of fish body significantly increased by increasing the inclusion levels of shrimp head hydrolysate. On a digestible-protein basis, the body composition of red drum fed shrimp waste meal, shrimp processing waste meal, and by-catch meal connected with shrimp trawling did not change appreciably [
30]. On the other hand, the tilapia body analysis fed different replacement levels of fishmeal with shrimp head silage (33.3%, 66.7%, and 100%) had statistically no appreciable variation in the moisture and protein content [
14]. In the current study, the high fat, protein, and lower fiber contents of acid-treated SBp meal may reflect on ether extract % and crude protein % of African catfish body (chiefly G5). In addition, the high fat, protein, and fiber contents of autoclaved SBp meal were reflected on ether extract % and crude protein % of fish body.
The dressing yield of the current study revealed a significant increase in replacement groups (2, 3, and 5) by SBp in comparison to the control one (G1). The Li et al. [
54] results indicated that the fish fed dietary protein (32% protein diet) had a higher body yield than fish fed the lower protein diets (26% and 28%), regardless of fish strain. Generally, the high fat and protein contents of autoclaved and acid-treated SBp meals help protein and fat deposition in the fish muscle that redirected for the current African catfish dressing yield.
The highest means of chromic oxide and dry matter digestibility percentages were recorded in G5 relative to the control (G1) and SBp-replaced groups (G2, G3, and G4). Fanimo et al. [
16] reported that the nutritional value of shrimp waste meals depends on the amount of shell or exoskeleton contained in the feed. Many studies used shrimp by-product as a protein source in poultry diets, but its practical use seems to be difficult because of the low digestibility of chitin present in SBp [
55]. The outcomes of the present study disagreed with Li et al. [
20]. They found a low apparent digestibility coefficient for organic matter and crude protein in red drum fed shrimp processing waste meal. Additionally, the percent apparent digestibility of protein in by-catch meal associated with shrimp trawling and shrimp processing waste meal was significantly lower than anchovy meal [
48]. However, Hardy and co-authors [
48] showed that amino acid deficiencies and/or imbalances due to low methionine and lysine levels might play a role. Therefore, the shrimp waste meal diet contained large amounts of this indigestible material and low levels of two essential amino acids, resulting in limited utilization by the red drum. Fish fed diets comprising 30% and 40% shrimp head silage had a non-significant improvement in feed digestibility and had a lower digestibility percent [
28]. In our opinion, the high content of easily utilized amino acids and lower crude fiber content of acid-treated SBp meal than the autoclaved one may reflect on the dry matter digestibility percentage of the current study. Digestibility values are an important parameter to consider in the diet formulation and in determining feed utilization. Feedstuffs that are poorly digested would be of limited value to an animal. These results suggested that the apparent protein digestibility of SBp is higher than or equal to the soybean for African catfish. Possible reasons for the enhancement of feed utilization, digestibility, and growth parameters recorded at the highest replacing levels of soybean by SBp (50%) may be related to the low crude fiber (7.85% of acid-treated,
Table 1) of SBp. These causes were reflected on palatability, feed intake, feed conversion ratio, and protein efficiency ratio [
56].
The economic assessment of soybean replacement with SBp can reduce the catfish diet cost that can alleviate the problem of soybean high cost and availability. The fish feed cost (up to 70%) is the largest component in breeders’ total costs [
57]. Feed/production income ratio of fish (weighing over 200g) was 0.64 in seabass (
Dicentrarchus labrax) [
58]. All other costs in the present study were constant. Therefore, the feeding costs required to produce 1 kg weight gain could be used to compare the different experimental groups [
27]. The calculated data showed that the feed cost was reduced in all replacing levels of soybean by SBp, and the replacing level of 50% reduced feeding costs by 7.28% and decreased feed costs/kg weight gain by 14.95% for D5 (
Table 9). Consequently, partial replacement of soybean by SBp significantly reduced cost indices and improved profit level compared to the basal diet.