A 90-Day Subchronic Toxicity Study of Consumption of GH-Transgenic Triploid Carp in Wistar Rats
Abstract
:1. Introduction
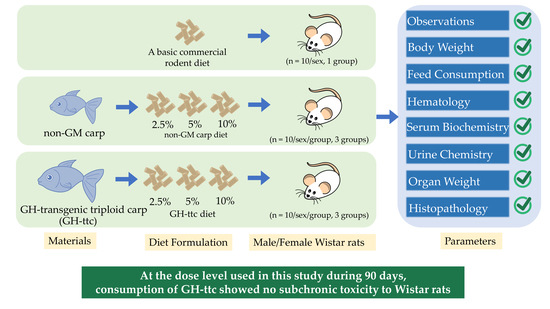
2. Materials and Methods
2.1. Materials and Diet Formulation
2.2. Diet Formulation
2.3. Animals
2.4. Animal Experimental Design
2.5. Clinical Observation, Optic Examination, Body Weight, and Feed Consumption
2.6. Hematology
2.7. Serum Chemistry
2.8. Urine Test
2.9. Organ Weight and Histopathology
2.10. Statistical Analysis
3. Results
3.1. Clinical Evaluations and Optic Examination
3.2. Body Weight and Feed Consumption
3.3. Hematology
3.4. Serum Chemistry Analysis
3.5. Urine Chemistry
3.6. Organ Weight and Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maclean, N. Genetically modified fish and their effects on food quality and human health and nutrition. Trends Food Sci. Technol. 2003, 14, 242–252. [Google Scholar] [CrossRef]
- Zhong, C.; Song, Y.; Wang, Y.; Li, Y.; Liao, L.; Xie, S.; Zhu, Z.; Hu, W. Growth hormone transgene effects on growth performance are inconsistent among offspring derived from different homozygous transgenic common carp (Cyprinus carpio L.). Aquaculture 2012, 356–357, 404–411. [Google Scholar] [CrossRef]
- Zhu, Z. Novel gene transfer into the fertilized eggs of gold fish (Carassius auratus L. 1758). J. Appl. Ichthyol. 1985, 1, 31–34. [Google Scholar] [CrossRef]
- Wei, H.; Zhu, Z.Y. Integration mechanisms of transgenes and population fitness of GH transgenic fish. Sci. China Life Sci. 2010, 53, 401–408. [Google Scholar]
- Burns, J.C.; Heimschuessel, R.; Chatakcndi, N.; Dunham, R.A. Transgenic fish: Ideal models for basic research and biotechnological applications. Zool. Stud. 1995, 34, 215–234. [Google Scholar]
- Fletcher, G.L.; Shears, M.A.; King, M.J.; Davies, P.L.; Hew, C.L. Evidence for antifreeze protein gene transfer in Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1988, 45, 352–357. [Google Scholar] [CrossRef]
- Du, S.J.; Gong, Z.; Fletcher, G.L.; Shears, M.A.; King, M.J.; Idler, D.R.; Hew, C.L. Growth enhancement in transgenic Atlantic salmon by the use of an “all fish” chimeric growth hormone gene construct. Bio/Technology 1992, 10, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Yesaki, T.Y.; Donaldson, E.M.; Du, S.J.; Hew, C.-L. Production of germline transgenic Pacific salmonids with dramatically increased growth performance. Can. J. Fish. Aquat. 1995, 52, 1376–1384. [Google Scholar] [CrossRef]
- Nam, Y.K.; Cho, Y.S.; Cho, H.J.; Kim, D.S. Accelerated growth performance and stable germ-line transmission in androgenetically derived homozygous transgenic mud loach, Misgurnus mizolepis. Aquaculture 2002, 209, 257–270. [Google Scholar] [CrossRef]
- Martınez, R.; Arenal, A.; Estrada, M.P.; Herrera, F.; Huerta, V.; Vazquez, J.; Sanchez, T.; de la Fuente, J. Mendelian transmission, transgene dosage and growth phenotype in transgenic tilapia (Oreochromis hornorum) showing ectopic expression of homologous growth hormone. Aquaculture 1999, 173, 271–283. [Google Scholar] [CrossRef]
- Rahman, M.; Ronyai, A.; Engidaw, B.; Jauncey, K.; Hwang, G.L.; Smith, A.; Roderick, E.; Penman, D.; Varadi, L.; Maclean, N. Growth and nutritional trials on transgenic Nile tilapia containing an exogenous fish growth hormone gene. J. Fish Biol. 2001, 59, 62–78. [Google Scholar] [CrossRef]
- Ledford, H. Salmon approval heralds rethink of transgenic animals. Nature 2015, 527, 417–418. [Google Scholar] [CrossRef]
- Grossman, M.R. Genetically engineered animals in the United States: The AquAdvantage Salmon. Eur. Food Feed L. Rev. 2016, 11, 190. [Google Scholar]
- Smith, M.D.; Asche, F.; Guttormsen, A.G.; Wiener, J.B. Food safety. Genetically modified salmon and full impact assessment. Science 2010, 330, 1052–1053. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. First transgenic salmon sold in Canada. Nature 2017, 548, 148. [Google Scholar] [CrossRef]
- Wang, Y.; Hamid, N.; Jia, P.P.; Pei, D.S. A comprehensive review on genetically modified fish: Key techniques, applications and future prospects. Rev. Aquac. 2021, 13, 1635–1660. [Google Scholar] [CrossRef]
- Wang, Y.; He, L. Retrospect and prospect of transgenic fish breeding in China. Chin. J. Biotechnol. 2016, 32, 851–860. (In Chinese) [Google Scholar]
- Maclean, N.; Laight, R.J. Transgenic fish: An evaluation of benefits and risks. Fish Fish. 2010, 1, 146–172. [Google Scholar] [CrossRef]
- Felip, A.; Piferrer, F.; Zanuy, S.; Carrillo, M. Comparative growth performance of diploid and triploid European sea bass over the first four spawning seasons. J. Fish Biol. 2001, 58, 76–88. [Google Scholar] [CrossRef]
- Suresh, A.V.; Sheehan, R.J. Muscle fibre growth dynamics in diploid and triploid rainbow trout. J. Fish Biol. 1998, 52, 570–587. [Google Scholar] [CrossRef]
- Tiwary, B.K.; Kirubagaran, R.; Ray, A.K. The biology of triploid fish. Rev. Fish Biol. Fish 2004, 14, 391–402. [Google Scholar] [CrossRef]
- Bazaz, A.I.; Kashmir, J.A.; Ahmad, I.I.; Nafath-Ul-Arab, I.; Fatima, A. A review on induction of triploidy in fish using heat, pressure and cold shock treatments. J. Entomol. Zool. Stud. 2020, 8, 381–385. [Google Scholar]
- Parven, M.A. Triploidy induction and growth performance of hybrid catfish (Clarias macrocephalus × C. gariepinus). Int. J. Fish. Aquat. Stud. 2014, 1, 151–162. [Google Scholar]
- Li, F.; Xiang, J.; Zhou, L.; Wu, C.; Zhang, X. Optimization of triploid induction by heat shock in Chinese shrimp Fenneropenaeus chinensis. Aquaculture 2003, 219, 221–231. [Google Scholar] [CrossRef]
- Gheyas, A.; Mollah, M.; Hussain, M.G. Triploidy induction in stinging catfish Heteropneustes fossilis using cold shock. Asian Fish Sci. 2001, 21, 323–332. [Google Scholar] [CrossRef]
- Peruzzi, S.; Chatain, B. Pressure and cold shock induction of meiotic gynogenesis and triploidy in the European sea bass, Dicentrarchus labrax L.: Relative efficiency of methods and parental variability. Aquaculture 2000, 189, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Varadaraj, K.; Pandian, T. Induction of triploids in Oreochromis mossambicus by thermal, hydrostatic pressure and chemical shocks. In Proceedings of the Aquaculture International Congress and Expo, Vancouver, BC, Canada, 6–9 September 1988; pp. 531–535. [Google Scholar]
- Yu, F.; Xiao, J.; Liang, X.; Liu, S.; Zhou, G.; Luo, K.; Liu, Y.; Hu, W.; Wang, Y.; Zhu, Z. Rapid growth and sterility of growth hormone gene transgenic triploid carp. Chin. Sci. Bull. 2011, 56, 1679–1684. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Animals. 2008. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/guidelines/zh/ (accessed on 21 December 2021).
- EC. Commission Implementing Regulation (EU) No 503/2013 of 3 April 2013 on Applications for Authorisation of Genetically Modified Food and Feed in Accordance with Regulation (EC) No 1829/2003 of the European Parliament and of the Council and Amending Commission Regulations (EC) No 641/2004 and (EC) No 1981/2006. OJEU 2013, L157, 1–57. [Google Scholar]
- General Office of the Ministry of Agriculture, China. Notice of the General Office of the Ministry of Agriculture on the Issuance of Guidelines for Safety Assessment of Agricultural GMOs (Plants, Animals and Microorganisms for Animal Use). 2017. Available online: http://www.moa.gov.cn/ztzl/zjyqwgz/sbzn/201703/t20170324_5538055.htm (accessed on 21 December 2021).
- Bai, H.; Wang, Z.; Hu, R.; Kan, T.; Li, Y.; Zhang, X.; Zhang, J.; Lian, L.; Han, H.; Lian, Z. A 90-day toxicology study of meat from genetically modified sheep overexpressing TLR4 in Sprague-Dawley rats. PLoS ONE 2015, 10, e0121636. [Google Scholar] [CrossRef]
- Zou, S.; Tang, M.; He, X.; Cao, Y.; Zhao, J.; Xu, W.; Liang, Z.; Huang, K. A 90-day subchronic study of rats fed lean pork from genetically modified pigs with muscle-specific expression of recombinant follistatin. Regul. Toxicol. Pharmacol. 2015, 73, 620–628. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.X.; Feng, X.L.; Wang, H.L.; Liu, H.B.; Zhi, Y.; Geng, G.Y.; Zhao, J.; Xu, H.B. Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. Food Chem. Toxicol. 2013, 57, 314–321. [Google Scholar] [CrossRef]
- Ahmadnia, A.; Sahari, M.A.; Barzegar, M.; Seyfabadi, S.J.; Abdollahi, M. Vitamins contents of some commercially important fish species from south Caspian Sea. Am. Eur. J. Sustain. Agric. 2008, 2, 285–293. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. 2020. Available online: https://www.fao.org/3/ca9229en/CA9229EN.pdf (accessed on 21 December 2021).
- Khan, M.N.; Shahzad, K.; Chatta, A.; Sohail, M.; Piria, M.; Treer, T. A review of introduction of common carp Cyprinus carpio in Pakistan: Origin, purpose, impact and management. Croat. J. Fish. 2016, 74, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zhang, X.; Wang, X.; Li, J.; Liu, G.; Kuang, Y.; Xu, J.; Zheng, X.; Ren, L.; Wang, G.; et al. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Ma, H.; Wang, Y.; Hu, Y.; Lin, Z.; Zhu, Z.; Hu, W. Vitreoscilla hemoglobin (VHb) overexpression increases hypoxia tolerance in zebrafish (Danio rerio). J. Mar. Biotechnol. 2011, 13, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.L.; Hobbs, R.S.; Evans, R.P.; Shears, M.A.; Hahn, A.L.; Hew, C.L. Lysozyme transgenic Atlantic salmon (Salmo salar L.). Aquac. Res. 2011, 42, 427–440. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Zhu, Z. Introduction of the human lactoferrin gene into grass carp (Ctenopharyngodon idellus) to increase resistance against GCH virus. Aquaculture 2002, 214, 93–101. [Google Scholar] [CrossRef]
- Krasnov, A.; Pitkänen, T.I.; Mölsä, H. Gene transfer for targeted modification of salmonid fish metabolism. Genet. Anal. 1999, 15, 115–119. [Google Scholar] [CrossRef]
- Hallerman, E.M.; McLean, E.; Fleming, I.A. Effects of growth hormone transgenes on the behavior and welfare of aquacultured fishes: A review identifying research needs. Appl. Anim. Behav. Sci. 2007, 104, 265–294. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Wei, H.; Cui, Z.; Zhu, Z.; Yang, J.; Peng, R. Physiological and pathological analysis of the mice fed with “all-fish” gene transferred yellow river carp. High Technol. Lett. 2000, 10, 17–19. (In Chinese) [Google Scholar]
- Kaijian, C.; Huaiyun, Z.; Xuewen, Z.; Tiaoyi, X.; Lixiang, C.; Jianmin, S.; Donggeng, W. A study on the safety of feeding transgenic grass carps to rats. J. Hunan Univ. Nat. Sci. Ed. 2002, 28, 147–149. (In Chinese) [Google Scholar]
- Liu, Y.; Zhang, W.; Yong, L.; Zhao, X.; Jia, X.; Li, N. An assessment of androgenic/anti-androgenic effects of GH transgenic carp by Hershberger assay. Biomed. Environ. Sci. 2011, 24, 445–449. [Google Scholar] [PubMed]
- Yong, L.; Liu, Y.; Jia, X.; Li, N.; Zhang, W. Subchronic toxicity study of GH transgenic carp. Food Chem. 2012, 50, 3920–3926. [Google Scholar] [CrossRef] [PubMed]
- Alimuddin, A.; Murtini, S.; Faridah, N.; Nuryati, S. Behavior, histopathology and physiological responses of rat fed diets containing growth hormone transgenic fish meal. Hayati J. Biosci. 2019, 26, 1. [Google Scholar] [CrossRef]


| Non-GM Carp | GH-ttc | |
|---|---|---|
| Moisture (g/100 g) | 77.6 | 80.1 |
| Ash content (g/100 g) | 1.24 | 1.27 |
| Protein (g/100 g) | 19.7 | 16.5 |
| Fat (g/100 g) | 2.0 | 1.6 |
| Crude fiber (g/100 g) | 0 | 0 |
| Calcium (mg/kg) | 476 | 1677 |
| Phosphorus (mg/kg) | 2064 | 2779 |
| Control Group | Non-GM Carp | GH-ttc | |||||
|---|---|---|---|---|---|---|---|
| 2.5% | 5% | 10% | 2.5% | 5% | 10% | ||
| Ash content (g/100 g) | 6.21 | 5.99 | 5.87 | 5.62 | 5.99 | 5.87 | 5.62 |
| Protein (g/100 g) | 19.65 | 19.36 | 19.36 | 19.36 | 19.28 | 19.20 | 19.04 |
| Fat (g/100 g) | 4.43 | 4.30 | 4.24 | 4.12 | 4.29 | 4.22 | 4.08 |
| Crude fiber (g/100 g) | 3.35 | 4.72 | 4.63 | 4.46 | 4.72 | 4.63 | 4.46 |
| Calcium (g/100 g) | 1.2 | 1.15 | 1.12 | 1.07 | 1.16 | 1.13 | 1.08 |
| Phosphorus (g/100 g) | 0.83 | 0.80 | 0.79 | 0.76 | 0.80 | 0.79 | 0.76 |
| Control Group | Non-GM Carp | GH-ttc | |||||
|---|---|---|---|---|---|---|---|
| 2.5% | 5% | 10% | 2.5% | 5% | 10% | ||
| Males | |||||||
| WBC (109/L) | 7.36 ± 2.19 | 6.57 ± 2.31 | 6.61 ± 2.57 | 4.87 ± 0.84 | 7.36 ± 2.45 | 7.39 ± 1.70 | 7.92 ± 3.46 |
| RBC (1012/L) | 7.61 ± 0.25 | 7.49 ± 1.10 | 6.78 ± 1.36 | 8.33 ± 0.37 | 7.01 ± 1.61 | 7.79 ± 0.37 | 7.69 ± 0.40 |
| HCT (%) | 38.7 ± 1.7 | 36.9 ± 8.2 | 38.2 ± 0.9 | 39.6 ± 3.1 | 40.0 ± 5.8 | 36.0 ± 8.3 | 41.0 ± 3.2 |
| HGB (g/L) | 129 ± 5 | 126 ± 20 | 112 ± 25 | 136 ± 8 | 116 ± 27 | 131 ± 4 | 131 ± 7 |
| PLT (109/L) | 569 ± 68 | 616 ± 91 | 572 ± 104 | 639 ± 135 | 543 ± 96 | 553 ± 111 | 600 ± 48 |
| PT (s) | 9.64 ± 1.12 | 9.26 ± 0.63 | 9.11 ± 0.17 | 9.76 ± 0.71 | 9.50 ± 1.19 | 9.39 ± 0.72 | 9.78 ± 1.38 |
| APTT (s) | 22.33 ± 5.65 | 24.14 ± 6.51 | 20.38 ± 7.36 | 25.76 ± 6.54 | 19.80 ± 2.81 | 21.06 ± 4.85 | 25.28 ± 8.74 |
| NE (109/L) | 2.89 ± 1.12 | 2.71 ± 1.11 | 2.56 ± 0.97 | 2.14 ± 0.49 | 2.63 ± 1.10 | 3.19 ± 0.72 | 3.32 ± 2.00 |
| LY (109/L) | 4.09 ± 1.15 | 3.50 ± 1.26 | 3.73 ± 1.56 | 2.44 ± 0.47 | 4.25 ± 1.24 | 3.76 ± 1.06 | 4.13 ± 1.42 b |
| EO (109/L) | 0.10 ± 0.09 | 0.06 ± 0.06 | 0.03 ± 0.02 | 0.06 ± 0.05 | 0.12 ± 0.12 | 0.11 ± 0.05 b | 0.09 ± 0.10 |
| MO (109/L) | 0.26 ± 0.09 | 0.29 ± 0.16 | 0.28 ± 0.18 | 0.21 ± 0.05 | 0.30 ± 0.14 | 0.31 ± 0.13 | 0.37 ± 0.13 |
| BA (109/L) | 0.03 ± 0.04 | 0.01 ± 0.03 | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.05 ± 0.06 | 0.03 ± 0.02 | 0.01 ± 0.02 |
| Females | |||||||
| WBC (109/L) | 3.91 ± 1.29 | 4.54 ± 2.32 | 4.80 ± 1.42 | 3.83 ± 2.08 | 4.14 ± 1.70 | 5.22 ± 1.18 | 4.05 ± 1.72 |
| RBC (1012/L) | 6.34 ± 1.11 | 6.31 ± 1.39 | 6.41 ± 1.35 | 6.96 ± 1.72 | 6.94 ± 0.36 | 6.27 ± 1.28 | 6.50 ± 1.18 |
| HCT (%) | 35.1 ± 6.3 | 37.4 ± 2.0 | 33.8 ± 7.0 | 36.0 ± 5.8 | 34.3 ± 8.0 | 34.7 ± 7.1 | 37.7 ± 10.0 |
| HGB (g/L) | 113 ± 22 | 112 ± 27 | 113 ± 25 | 122 ± 32 | 124 ± 5 | 111 ± 24 | 120 ± 22 |
| PLT (109/L) | 565 ± 95 | 584 ± 102 | 559 ± 112 | 561 ± 132 | 616 ± 82 | 562 ± 120 | 598 ± 114 |
| PT (s) | 9.29 ± 0.71 | 9.28 ± 0.28 | 9.47 ± 1.00 | 9.33 ± 0.93 | 9.26 ± 0.46 | 9.85 ± 0.95 | 9.33 ± 0.62 |
| APTT (s) | 28.00 ± 5.32 | 30.18 ± 9.22 | 26.20 ± 7.60 | 26.87 ± 10.52 | 26.09 ± 5.05 | 28.28 ± 8.15 | 30.58 ± 7.33 |
| NE (109/L) | 1.30 ± 0.63 | 1.52 ± 0.76 | 1.39 ± 0.54 | 1.12 ± 0.64 | 1.17 ± 0.36 | 1.45 ± 0.50 | 1.27 ± 0.48 |
| LY (109/L) | 2.34 ± 0.63 | 2.78 ± 1.34 | 3.18 ± 0.92 | 2.47 ± 1.38 | 2.74 ± 1.32 | 3.56 ± 0.73 | 2.57 ± 1.11 |
| EO (109/L) | 0.09 ± 0.08 | 0.07 ± 0.10 | 0.03 ± 0.03 | 0.06 ± 0.08 | 0.06 ± 0.05 | 0.03 ± 0.03 | 0.03 ± 0.02 |
| MO (109/L) | 0.14 ± 0.07 | 0.16 ± 0.14 | 0.20 ± 0.08 | 0.16 ± 0.09 | 0.16 ± 0.08 | 0.18 ± 0.08 | 0.16 ± 0.17 |
| BA (109/L) | 0.04 ± 0.05 | 0.03 ± 0.07 | 0.01 ± 0.01 | 0.02 ± 0.03 | 0.02 ± 0.03 | 0.01 ± 0.02 | 0.01 ± 0.01 |
| Control Group | Non-GM Carp | GH-ttc | |||||
|---|---|---|---|---|---|---|---|
| 2.5% | 5% | 10% | 2.5% | 5% | 10% | ||
| Males | |||||||
| ALT (U/L) | 50.7 ± 5.7 | 36.1 ± 7.4 a | 48.2 ± 6.3 a | 50.3 ± 4.9 | 41.5 ± 3.8 a | 38.0 ± 2.9 a | 46.4 ± 4.8 |
| AST (U/L) | 175 ± 30 | 160 ± 21 | 216 ± 37 | 219 ± 53 | 175 ± 13 | 187 ± 8 | 177 ± 20 |
| TP (g/L) | 59.7 ± 6.4 | 50.3 ± 11.0 | 52.6 ± 11.5 | 50.0 ± 11.4 | 48.2 ± 11.1 | 54.5 ± 6.9 | 59.9 ± 2.7 |
| ALB (g/L) | 19.9 ± 2.4 | 19.5 ± 2.5 | 20.5 ± 2.4 | 20.9 ± 1.5 | 20.4 ± 1.1 | 18.9 ± 1.5 | 20.9 ± 1.5 |
| ALP (U/L) | 92.4 ± 14.0 | 73.5 ± 13.6 | 76.3 ± 14.0 | 81.9 ± 21.1 | 73.4 ± 9.8 | 73.6 ± 14.2 | 80.8 ± 8.8 |
| GGT (U/L) | 1.50 ± 0.88 | 2.90 ± 1.19 | 2.26 ± 1.08 | 3.56 ± 1.59 a | 2.87 ± 0.90 | 2.63 ± 1.19 | 2.69 ± 0.90 |
| Glu (mmol/L) | 6.39 ± 1.86 | 5.65 ± 1.66 | 5.24 ± 1.14 | 7.22 ± 1.62 | 6.38 ± 1.30 | 4.65 ± 1.45 | 5.81 ± 1.73 |
| Urea(mmol/L) | 10.17 ± 1.32 | 9.99 ± 1.31 | 9.13 ± 1.48 | 10.43 ± 1.57 | 9.07 ± 1.21 | 9.65 ± 2.13 | 11.20 ± 1.77 |
| Cr (μmol/L) | 38.8 ± 8.0 | 35.4 ± 6.4 | 35.5 ± 4.7 | 42.4 ± 9.5 | 38.5 ± 4.1 | 34.6 ± 5.9 | 39.3 ± 4.4 |
| TC (mmol/L) | 2.76 ± 0.39 | 2.35 ± 0.44 | 2.45 ± 0.59 | 2.56 ± 0.80 | 2.23 ± 0.64 | 1.84 ± 0.36 a | 2.45 ± 0.45 |
| TG (mmol/L) | 0.43 ± 0.08 | 0.44 ± 0.17 | 0.46 ± 0.08 | 0.54 ± 0.13 | 0.47 ± 0.06 | 0.52 ± 0.26 | 0.43 ± 0.09 |
| LDH (U/L) | 2696 ± 625 | 2156 ± 736 | 2635 ± 488 | 2682 ± 540 | 2673 ± 334 | 2630 ± 346 | 2456 ± 627 |
| K (mmol/L) | 4.64 ± 0.50 | 4.45 ± 0.29 | 4.38 ± 0.31 | 4.66 ± 0.26 | 4.23 ± 0.23 | 4.36 ± 0.30 | 4.67 ± 0.22 |
| Na (mmol/L) | 126.8 ± 3.7 | 124.8 ± 4.0 | 128.7 ± 5.3 | 127.5 ± 4.4 | 127.3 ± 3.9 | 125.2 ± 4.5 | 128.7 ± 2.8 |
| Cl (mmol/L) | 117.5 ± 5.3 | 123.2 ± 7.6 | 118.1 ± 5.0 | 120.2 ± 8.1 | 131.1 ± 7.9 | 120.3 ± 9.3 | 121.3 ± 7.3 |
| Females | |||||||
| ALT (U/L) | 70.4 ± 10.3 | 66.6 ± 23.0 | 67.2 ± 13.6 | 57.4 ± 15.5 | 56.3 ± 10.7 | 56.5 ± 16.0 | 68.5 ± 20.3 |
| AST (U/L) | 169 ± 41 | 196 ± 25 | 164 ± 32 | 187 ± 40 | 187 ± 35 | 181 ± 39 | 203 ± 54 |
| TP (g/L) | 79.3 ± 10.3 | 59.5 ± 6.8 a | 61.3 ± 18.7 | 50.2 ± 19.3 a | 68.8 ± 10.0 | 59.7 ± 9.8 a | 66.0 ± 19.0 |
| ALB(g/L) | 29.2 ± 3.5 | 30.1 ± 2.5 | 28.8 ± 5.2 | 25.2 ± 2.6 | 27.7 ± 2.4 | 25.2 ± 1.9 | 29.1 ± 4.7 |
| ALP (U/L) | 67.0 ± 24.6 | 78.9 ± 36.9 | 76.1 ± 24.0 | 73.8 ± 37.6 | 73.4 ± 19.4 | 74.8 ± 26.3 | 66.1 ± 16.7 |
| GGT (U/L) | 3.41 ± 1.03 | 3.23 ± 1.04 | 2.62 ± 0.49 | 2.95 ± 1.21 | 3.62 ± 1.37 | 2.97 ± 1.07 | 2.45 ± 0.89 |
| Glu (mmol/L) | 9.51 ± 2.94 | 9.17 ± 1.94 | 7.64 ± 3.29 | 8.66 ± 2.53 | 8.40 ± 2.29 | 6.44 ± 2.14 | 8.00 ± 1.71 |
| Urea(mmol/L) | 8.18 ± 1.66 | 9.17 ± 1.85 | 9.54 ± 2.32 | 9.71 ± 1.88 | 9.57 ± 1.57 | 8.74 ± 1.89 | 11.70 ± 2.77 |
| Cr (μmol/L) | 63.4 ± 7.8 | 57.8 ± 8.5 | 57.8 ± 10.9 | 58.6 ± 7.3 | 60.2 ± 11.7 | 53.9 ± 8.4 | 68.4 ± 10.9 |
| TC (mmol/L) | 2.88 ± 0.45 | 2.03 ± 0.58 | 2.33 ± 0.90 | 2.12 ± 0.62 | 2.19 ± 0.30 a | 2.27 ± 0.46 | 2.76 ± 0.63 |
| TG (mmol/L) | 0.54 ± 0.11 | 0.51 ± 0.13 | 0.53 ± 0.12 | 0.45 ± 0.11 | 0.44 ± 0.08 | 0.72 ± 0.31 | 0.89 ± 0.29 b |
| LDH (U/L) | 2052 ± 767 | 2380 ± 538 | 1884 ± 629 | 1967 ± 431 | 2314 ± 945 | 2140 ± 487 | 2119 ± 996 |
| K (mmol/L) | 3.56 ± 0.32 | 3.57 ± 0.30 | 3.66 ± 0.34 | 3.79 ± 0.54 | 3.86 ± 0.51 | 3.81 ± 0.36 | 3.65 ± 0.42 |
| Na (mmol/L) | 118.4 ± 5.2 | 123.5 ± 2.8 | 125.7 ± 4.0 a | 118.1 ± 3.2 | 122.5 ± 4.3 | 122.7 ± 4.5 | 120.3 ± 5.1 |
| Cl (mmol/L) | 136.9 ± 19.1 | 132.8 ± 7.8 | 135.0 ± 5.6 | 140.5 ± 16.4 | 131.2 ± 11.5 | 135.0 ± 7.0 | 143.1 ± 7.9 |
| Control Group | Non-GM Carp | GH-ttc | |||||
|---|---|---|---|---|---|---|---|
| 2.5% | 5% | 10% | 2.5% | 5% | 10% | ||
| Males | |||||||
| Glucose (mmol/L) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Specific gravity | 1.019 ± 0.011 | 1.012 ± 0.009 | 1.012 ± 0.008 | 1.014 ± 0.009 | 1.016 ± 0.012 | 1.011 ± 0.004 | 1.009 ± 0.004 |
| Occult blood (Cell/μL) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| pH | 5.35 ± 0.75 | 5.30 ± 0.63 | 5.15 ± 0.47 | 5.25 ± 0.79 | 4.70 ± 1.77 | 5.45 ± 0.72 | 5.15 ± 0.47 |
| Females | |||||||
| Glucose (mmol/L) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Specific gravity | 1.019 ± 0.010 | 1.017 ± 0.010 | 1.010 ± 0.009 | 1.012 ± 0.007 | 1.016 ± 0.013 | 1.011 ± 0.008 | 1.015 ± 0.009 |
| Occult blood (Cell/μL) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| pH | 5.30 ± 0.63 | 5.10 ± 0.32 | 5.30 ± 0.63 | 5.00 ± 0 | 5.85 ± 0.78 | 5.40 ± 0.66 | 5.20 ± 0.42 |
| Control Group | Non-GM Carp | GH-ttc | |||||
|---|---|---|---|---|---|---|---|
| 2.5% | 5% | 10% | 2.5% | 5% | 10% | ||
| Males | |||||||
| Brain | 2.09 ± 0.08 | 2.10 ± 0.10 | 2.08 ± 0.13 | 2.09 ± 0.13 | 2.07 ± 0.08 | 2.10 ± 0.07 | 2.01 ± 0.25 |
| Liver | 12.65 ± 0.55 | 13.34 ± 1.61 | 13.32 ± 1.56 | 12.07 ± 1.33 | 12.44 ± 1.90 | 12.22 ± 1.37 | 13.35 ± 3.99 |
| Spleen | 0.89 ± 0.20 | 0.98 ± 0.14 | 0.94 ± 0.11 | 0.81 ± 0.13 | 0.88 ± 0.15 | 0.97 ± 0.16 | 0.92 ± 0.13 |
| Heart | 1.45 ± 0.12 | 1.43 ± 0.13 | 1.55 ± 0.19 | 1.41 ± 0.13 | 1.55 ± 0.15 | 1.33 ± 0.13 b | 1.38 ± 0.17 |
| Thymus | 0.39 ± 0.09 | 0.47 ± 0.12 | 0.51 ± 0.10 | 0.40 ± 0.08 | 0.44 ± 0.16 | 0.42 ± 0.07 | 0.40 ± 0.10 |
| Kidney | 3.52 ± 0.18 | 3.92 ± 0.52 | 3.87 ± 0.43 | 3.79 ± 0.27 | 3.70 ± 0.48 | 3.77 ± 0.22 | 3.86 ± 0.46 |
| Adrenal gland | 0.064 ± 0.009 | 0.067 ± 0.014 | 0.070 ± 0.016 | 0.062 ± 0.015 | 0.065 ± 0.012 | 0.064 ± 0.017 | 0.063 ± 0.014 |
| Testis | 3.60 ± 0.33 | 3.75 ± 0.41 | 3.64 ± 0.21 | 3.39 ± 0.12 | 3.47 ± 0.31 | 3.72 ± 0.28 | 3.44 ± 0.46 |
| Epididymis | 1.55 ± 0.19 | 1.74 ± 0.46 | 1.63 ± 0.30 | 1.55 ± 0.28 | 1.60 ± 0.22 | 1.65 ± 0.31 | 1.68 ± 0.42 |
| Females | |||||||
| Brain | 1.89 ± 0.06 | 1.87 ± 0.10 | 1.94 ± 0.10 | 1.86 ± 0.09 | 1.82 ± 0.09 | 1.90 ± 0.07 | 1.87 ± 0.10 |
| Liver | 8.16 ± 1.18 | 8.56 ± 0.86 | 8.39 ± 0.97 | 8.28 ± 0.94 | 7.68 ± 0.69 | 7.56 ± 0.62 | 10.41 ± 1.3 a |
| Spleen | 0.59 ± 0.07 | 0.60 ± 0.06 | 0.64 ± 0.06 | 0.58 ± 0.06 | 0.53 ± 0.09 | 0.58 ± 0.07 | 0.65 ± 0.16 |
| Heart | 0.95 ± 0.10 | 0.93 ± 0.05 | 0.98 ± 0.10 | 1.00 ± 0.10 | 0.94 ± 0.09 | 0.95 ± 0.10 | 1.07 ± 0.09 |
| Thymus | 0.31 ± 0.07 | 0.32 ± 0.06 | 0.36 ± 0.12 | 0.30 ± 0.09 | 0.31 ± 0.07 | 0.35 ± 0.07 | 0.36 ± 0.07 |
| Kidney | 1.90 ± 0.09 | 2.05 ± 0.16 | 2.06 ± 0.13 | 2.08 ± 0.15 | 2.05 ± 0.22 | 1.94 ± 0.16 | 2.26 ± 0.25 a |
| Adrenal gland | 0.087 ± 0.012 | 0.112 ± 0.014 a | 0.098 ± 0.009 | 0.096 ± 0.015 | 0.089 ± 0.021 b | 0.089 ± 0.010 | 0.100 ± 0.017 |
| Ovary | 0.16 ± 0.04 | 0.17 ± 0.05 | 0.20 ± 0.03 | 0.16 ± 0.03 | 0.18 ± 0.04 | 0.19 ± 0.05 | 0.17 ± 0.06 |
| Uterus | 0.69 ± 0.23 | 0.74 ± 0.40 | 0.68 ± 0.24 | 0.56 ± 0.12 | 0.63 ± 0.25 | 0.67 ± 0.22 | 0.64 ± 0.19 |
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| Control Group | 10% Non-GM Carp Group | 10% GH-ttc Group | Control Group | 10% Non-GM Carp Group | 10% GH-ttc Group | ||
| Heart | Focal inflammation | 1/10 | 1/10 | 1/10 | 1/10 | 0/10 | 0/10 |
| Kidney | Chronic progressive nephropathy | 1/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Lung | Slight focal inflammation | 1/10 | 0/10 | 0/10 | 1/10 | 2/10 | 0/10 |
| Thyroid gland | Exfoliation of follicle epithelial cell | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Adrenal gland | Central edema | 0/8 | 0/10 | 0/10 | 0/10 | 2/10 | 0/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, Y.; Wang, Y.; Chen, B.; Hu, Y.; Musha, Y.; He, X.; Tong, T.; Huang, K. A 90-Day Subchronic Toxicity Study of Consumption of GH-Transgenic Triploid Carp in Wistar Rats. Fishes 2022, 7, 10. https://doi.org/10.3390/fishes7010010
Guo J, Li Y, Wang Y, Chen B, Hu Y, Musha Y, He X, Tong T, Huang K. A 90-Day Subchronic Toxicity Study of Consumption of GH-Transgenic Triploid Carp in Wistar Rats. Fishes. 2022; 7(1):10. https://doi.org/10.3390/fishes7010010
Chicago/Turabian StyleGuo, Jingya, Yongming Li, Yaping Wang, Boyong Chen, Yingxin Hu, Yasheng Musha, Xiaoyun He, Tao Tong, and Kunlun Huang. 2022. "A 90-Day Subchronic Toxicity Study of Consumption of GH-Transgenic Triploid Carp in Wistar Rats" Fishes 7, no. 1: 10. https://doi.org/10.3390/fishes7010010
APA StyleGuo, J., Li, Y., Wang, Y., Chen, B., Hu, Y., Musha, Y., He, X., Tong, T., & Huang, K. (2022). A 90-Day Subchronic Toxicity Study of Consumption of GH-Transgenic Triploid Carp in Wistar Rats. Fishes, 7(1), 10. https://doi.org/10.3390/fishes7010010