Mapping and Assessing Commercial Fisheries Services in the Lithuanian Part of the Curonian Lagoon
Abstract
:1. Introduction
1.1. Study Area
1.2. Fish Communities in Curonian Lagoon
2. Materials and Methods
2.1. Data, CPUE Calculation, and Catch Value Estimation
2.2. Statistical Analysis
3. Results
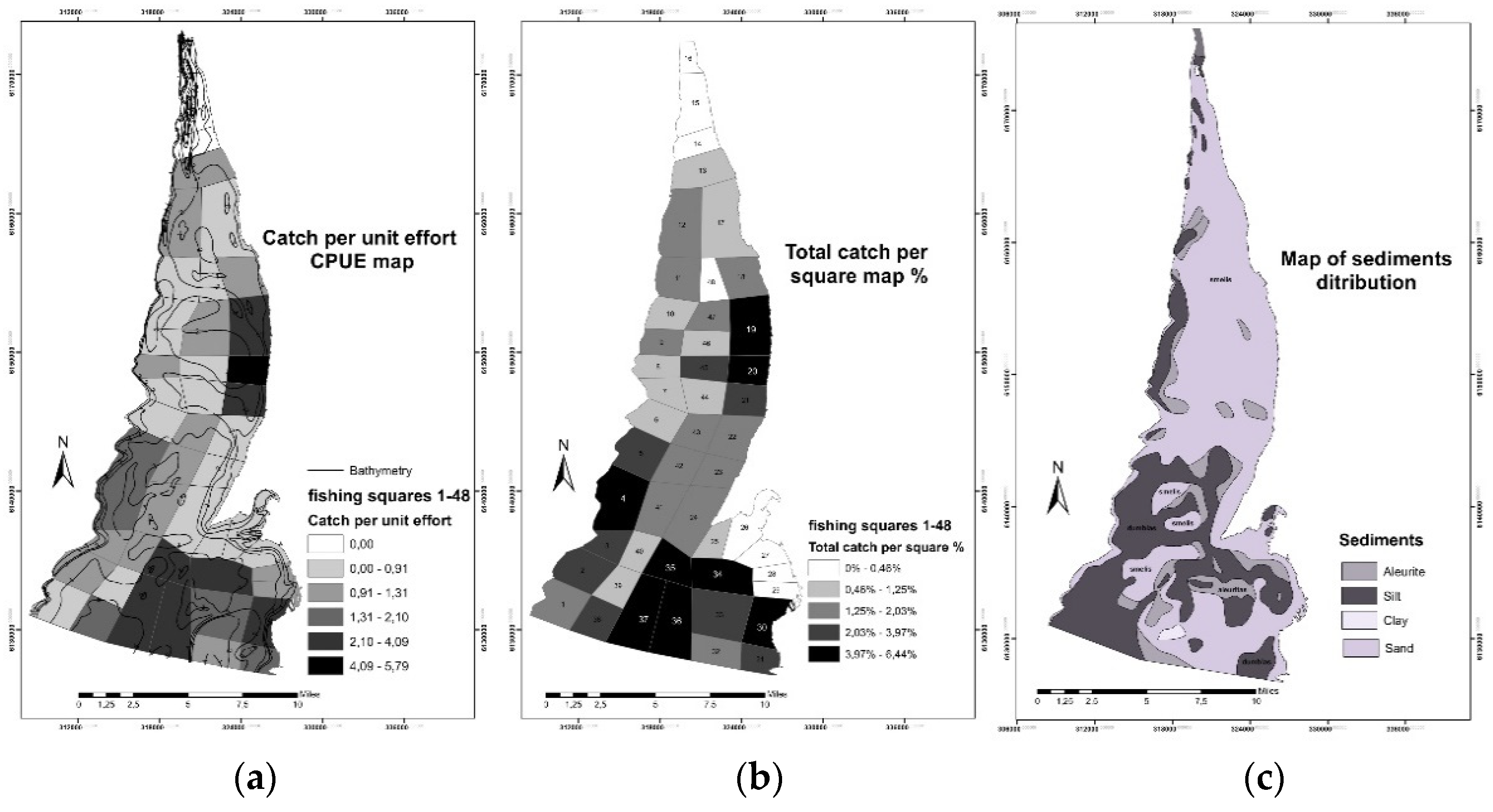
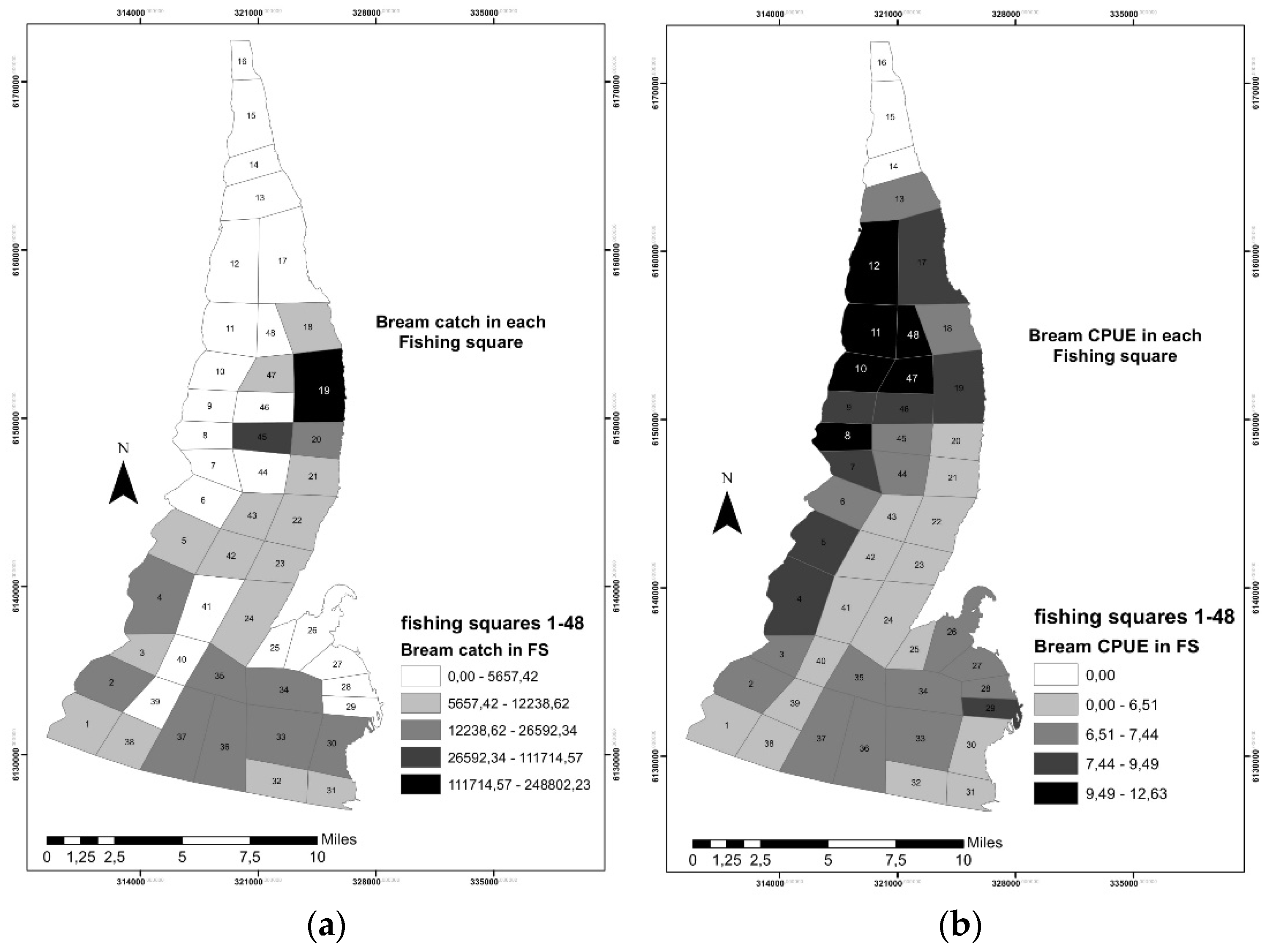
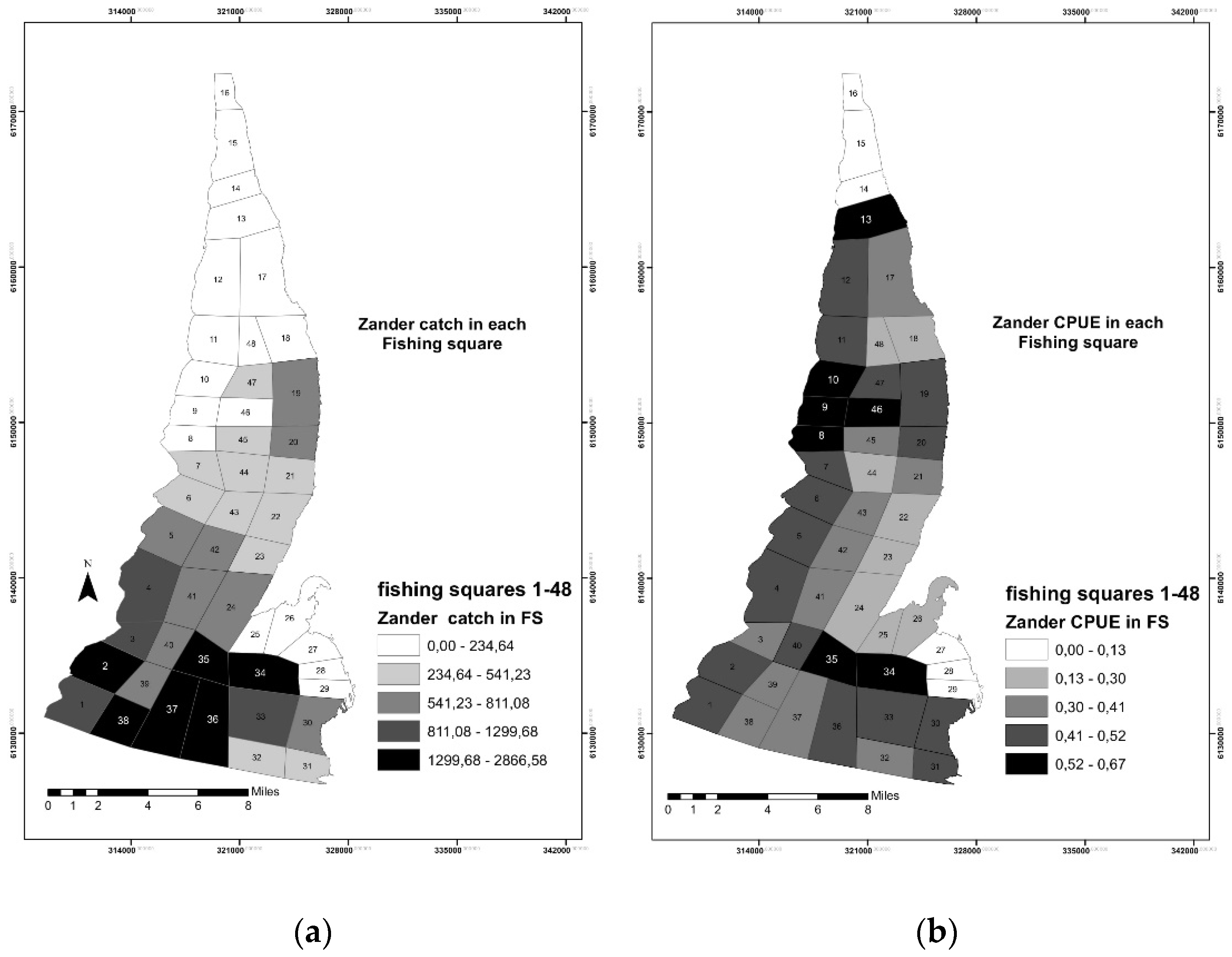
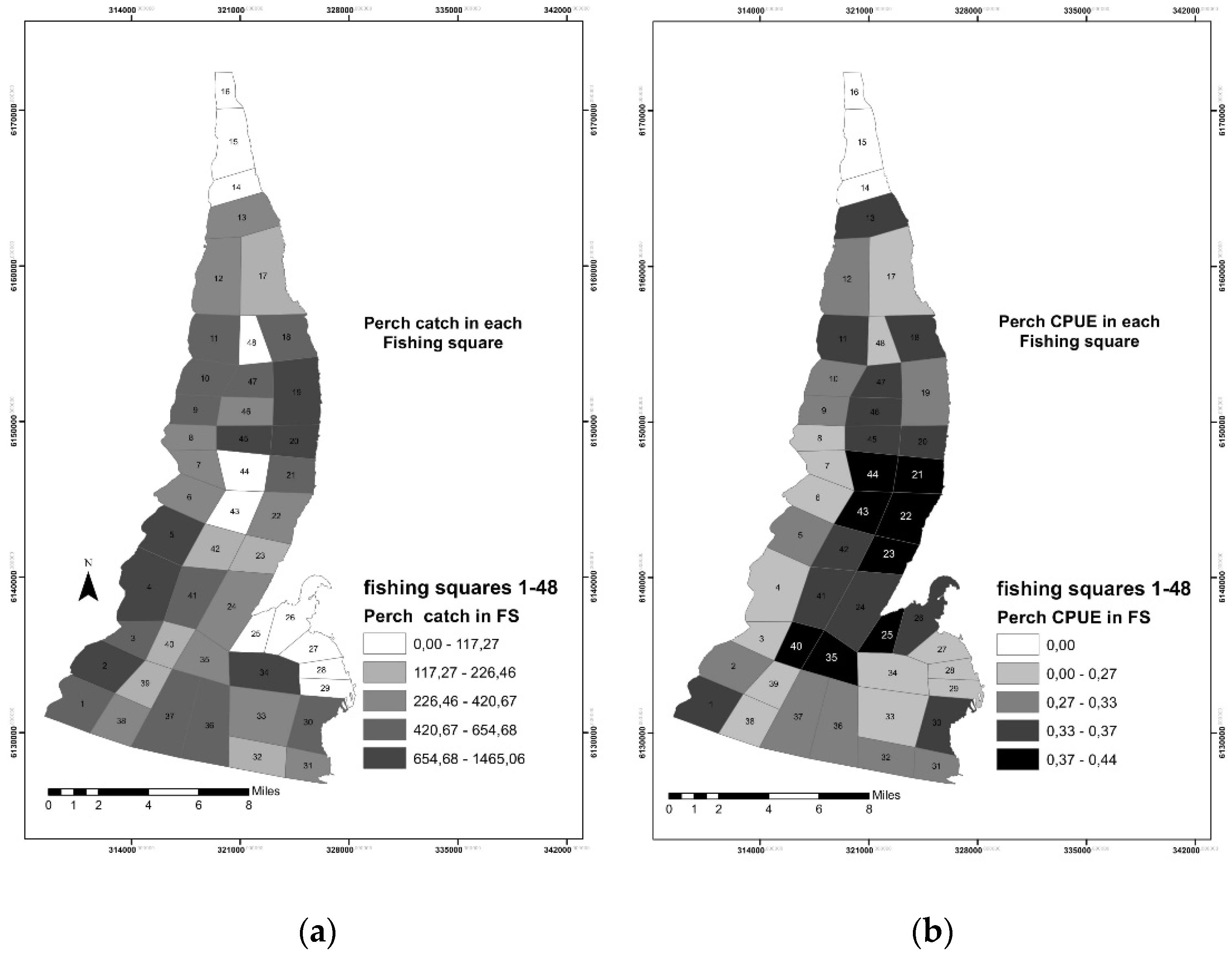
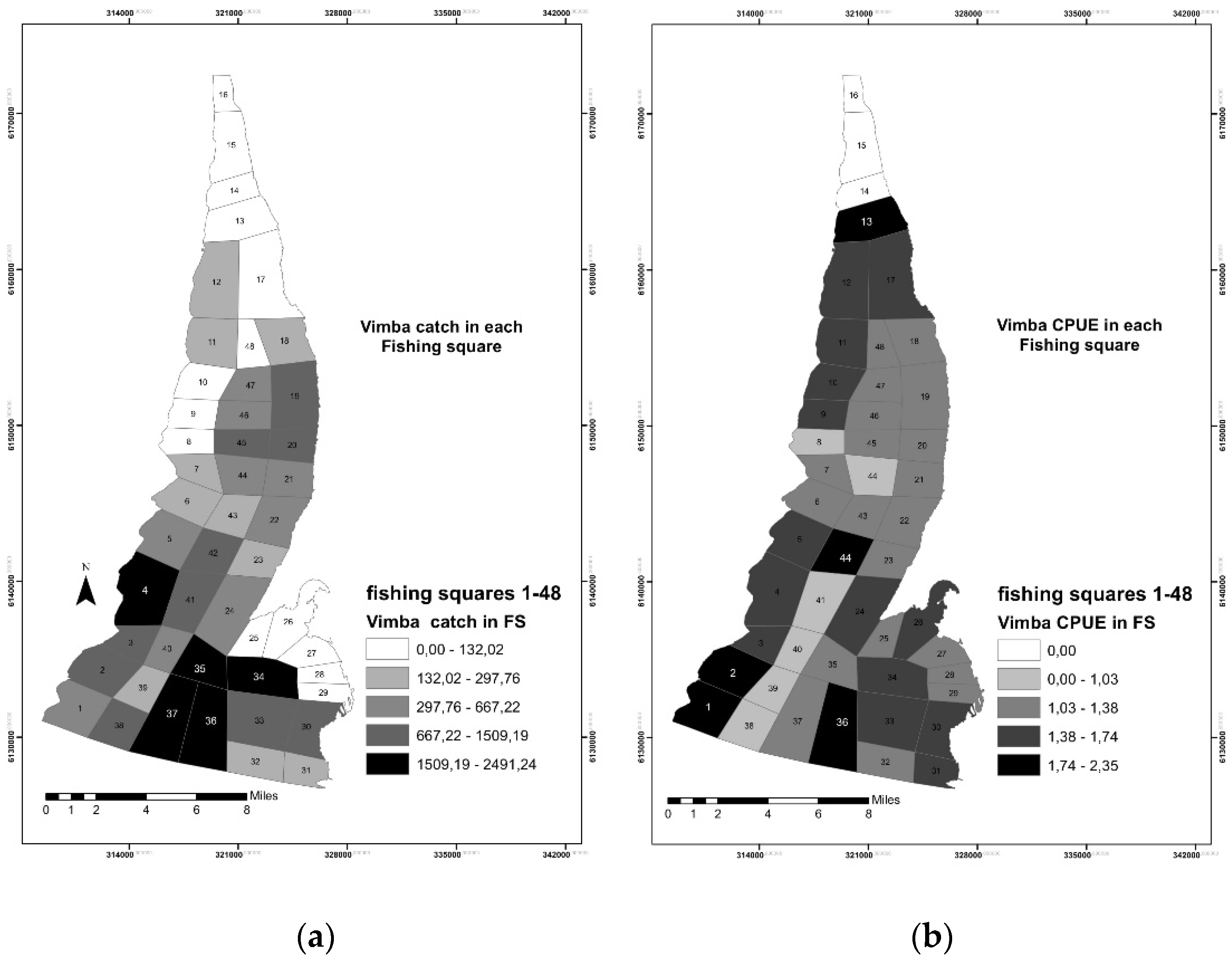
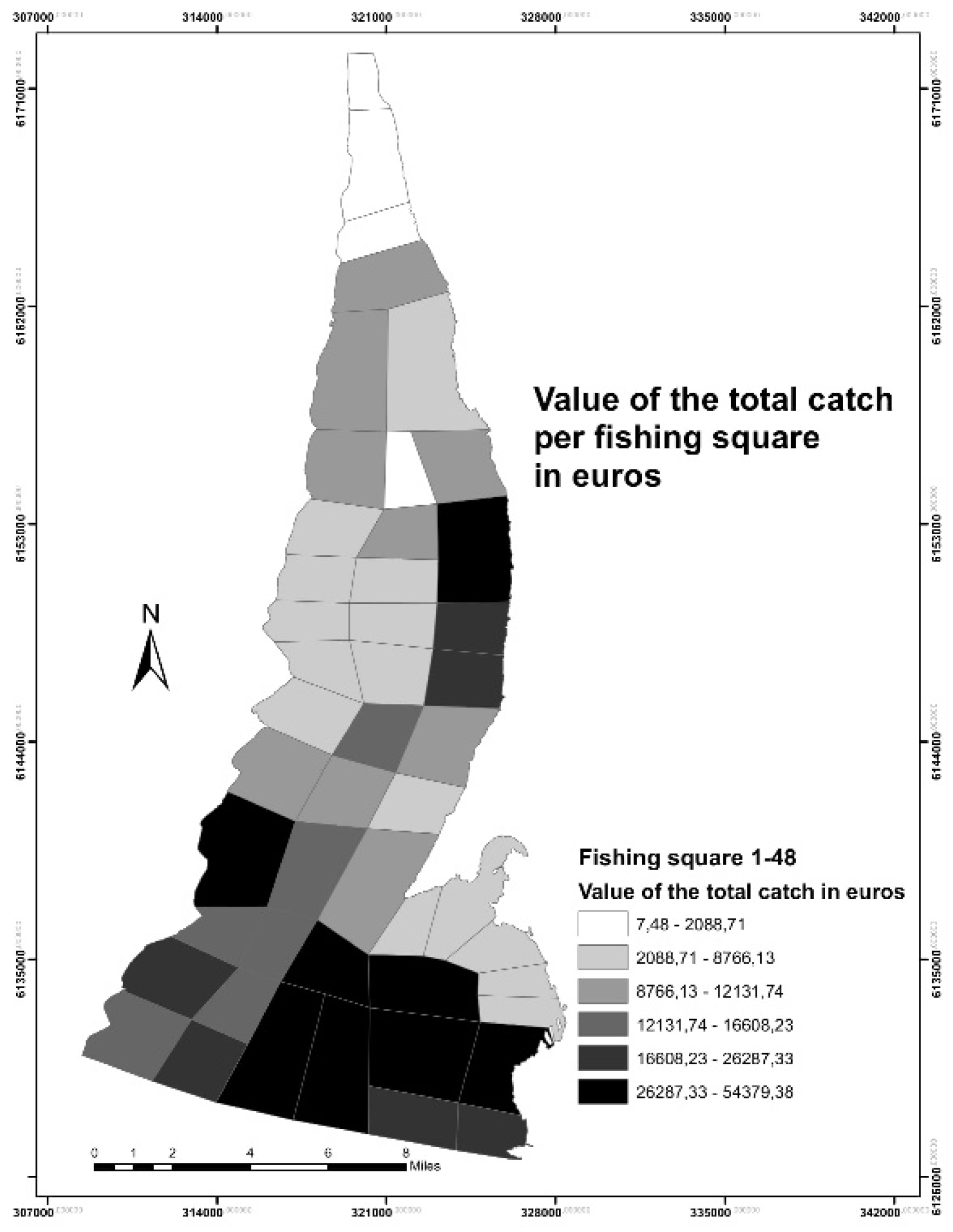
3.1. Fisheries Data Analysis and Mapping
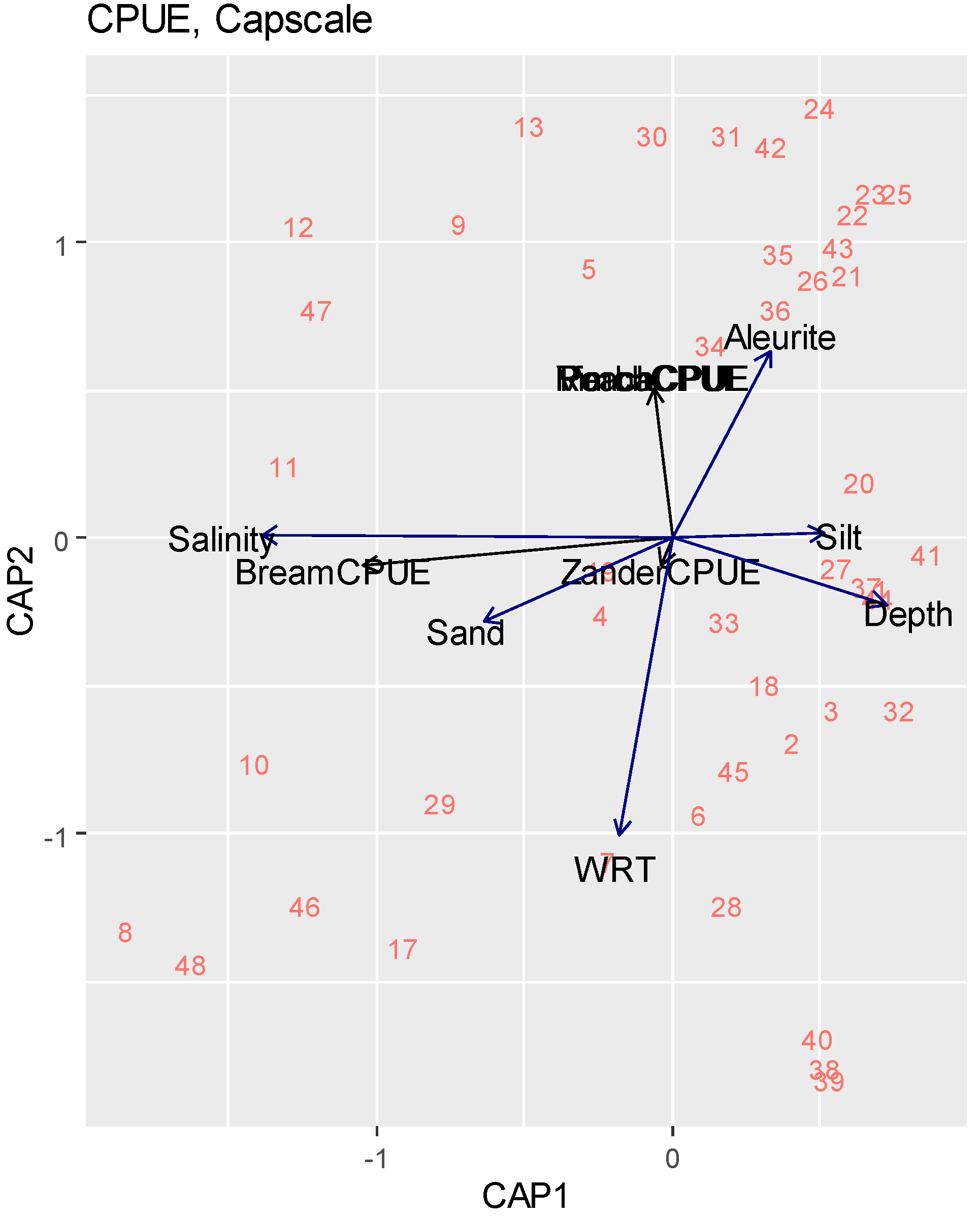
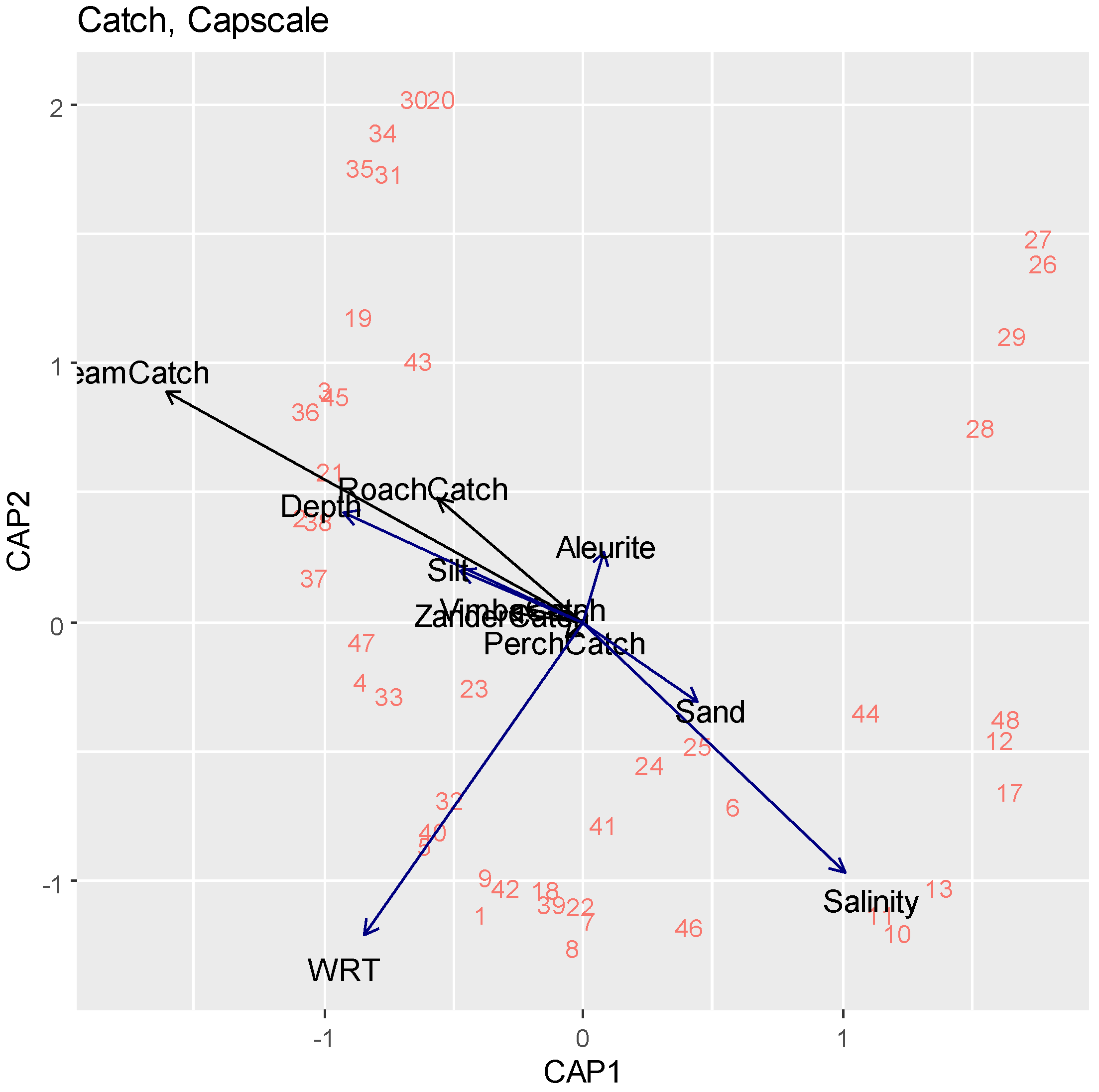
3.2. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franco, A.; Elliott, M.; Franzoi, P.; Torricelli, P. Life strategies of fishes in European estuaries: The functional guild approach. Marine Ecol. Progress Series 2008, 354, 219–228. Available online: https://www.int-res.com/abstracts/meps/v354/p219-228/ (accessed on 1 January 2022). [CrossRef] [Green Version]
- Wilson, S.K.; Sumaila, U.R.; Luisetti, T. Maintenance of fish diversity on disturbed coral reefs. Coral Reefs 2009, 28, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Pikitch, E.K.; Harmelin-Vivien, M.; Kulbicki, M. Ecosystem-Based Fishery Management. Science 2004, 305, 346–347. Available online: https://www.science.org/doi/10.1126/science.1098222 (accessed on 1 January 2022). [CrossRef]
- Carpenter, S.R.; Harmelin-Vivien, M.; Kulbicki, M. Ecological and economic analysis of lake eutrophication by nonpoint pollution. Aust. J. Ecol. 1998, 23, 68–79. [Google Scholar] [CrossRef]
- Post, D.M.; Kitchell, J.F. Trophic ontogeny and life history effects on interactions between age-0 fishes and zooplankton. Ergebnisse Limnol. 1997, 49, 1–12. [Google Scholar]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading trophic interactions and lake productivity. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Holmlund, C.M.; Hammer, M. Ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin, M. The links between biodiversity, ecosystem services and human well-being. Ecosyst. Ecol. New Synth. 2010, 1, 110–139. [Google Scholar]
- Crossman, N.D.; Summerfield, R.; Gring, A. A blueprint for mapping and modelling ecosystem services. Ecosyst. Serv. 2013, 4, 4–14. [Google Scholar] [CrossRef]
- Villamagna, A.M.; Angermeier, P.L.; Bennett, E.M. Capacity, pressure, demand, and flow: A conceptual framework for analyzing ecosystem service provision and delivery. Ecol. Complex. 2013, 15, 114–121. [Google Scholar] [CrossRef]
- Finlayson, M.; Cruz, R.D.; Davidson, N.; Alder, J.; Cork, S.; De Groot, R.S.; Lévêque, C.; Milton, G.R.; Peterson, G.; Pritchard, D.; et al. Millennium Ecosystem Assessment: Ecosystems and Human Well-Being: Wetlands and Water Synthesis; Island Press: Washington, DC, USA, 2005; Available online: https://www.millenniumassessment.org/documents/document.358.aspx.pdf (accessed on 1 January 2022).
- Gasiūnaitė, Z.R.; Harmelin-Vivien, M.; Kulbicki, M. The curonian lagoon. In Ecology of Baltic Coastal Waters; Springer: Berlin/Heidelberg, Germany, 2008; pp. 197–215. [Google Scholar]
- Petkuviene, J.; Summerfield, R.; Gring, A. Phosphorus cycling in a freshwater estuary impacted by cyanobacterial blooms. Estuar. Coasts 2016, 39, 1386–1402. [Google Scholar] [CrossRef]
- Razinkovas, A.; Sumaila, U.R.; Tremain, D. Preface: European lagoons—Need for further comparison across spatial and temporal scales. Hydrobiologia 2008, 611, 1–4. [Google Scholar] [CrossRef]
- Jakimavičius, D.; Kriaučiūnienė, J. The climate change impact on the water balance of the Curonian Lagoon. Water Resour. 2013, 40, 120–132. [Google Scholar] [CrossRef]
- Umgiesser, G.; Mornhe, R.; Whitfield, A. Seasonal renewal time variability in the Curonian Lagoon caused by atmospheric and hydrographical forcing. Ocean Sci. Discuss. 2015, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Christian, F.; Sumaila, U.R.; Luisetti, T. Hydraulic regime-based zonation scheme of the Curonian Lagoon. Hydrobiologia 2008, 611, 133–146. [Google Scholar] [CrossRef]
- Čerkasova, N.; Palmieri, M.G.; Luisetti, T. Curonian Lagoon drainage basin modelling and assessment of climate change impact. Oceanologia 2016, 58, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Gerulaitis, A.; Sumaila, U.R.; Luisetti, T. The monitoring of fish resources in the Curonian Lagoon and the Lower Nemunas, substantial of annual fishing limits and measures of regulation. In Fishery and Aquaculture; Baltic-ECO: Vilnius, Lithuania, 1994; pp. 92–96. [Google Scholar]
- Ložys, L. Seasonal migrations of pikeperch (Sander lucioperca L.) from the Curonian Lagoon to the Baltic Sea and advantages of the phenomenon. Acta Zool. Litu. 2003, 13, 188–194. [Google Scholar] [CrossRef]
- Gerulaitis, A.; Gaigalas, K.; Repečka, R.; Valušienė, V.; Rudzianskienė, G.; Overkaitė, T.; Skripkaitė, D. The investigations of fish stocks and catch prognoses in the Kursiu lagoon and Nemunas delta. In Fishery in Lithuania; The Lithuanian Society of Hydrobiologist: Vilnius, Lithuania, 1996; Volume II, pp. 165–182. [Google Scholar]
- Kesminas, V.; Domarkas, A. Perspectives of Fishery in Inland Waters; Žuvininkystė Lietuvoje: Klaipeda, Lithuania, 2008; Volume VIII, pp. 114–121. (In Lithuanian) [Google Scholar]
- Oksanen, J. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 January 2022).
- Whitfield, A.K. Ichthyofaunal assemblages in estuaries: A South African case study. Rev. Fish Biol. Fish. 1999, 9, 151–186. [Google Scholar] [CrossRef]
- Marshall, S.; Elliott, M. Environmental influences on the fish assemblage of the Humber estuary, UK. Estuar. Coast. Shelf Sci. 1998, 46, 175–184. [Google Scholar] [CrossRef]
- Hyndes, G.A.; Mornhe, R.; Whitfield, A. Does the composition of the demersal fish assemblages in temperate coastal waters change with depth and undergo consistent seasonal changes? Mar. Biol. 1999, 134, 335–352. [Google Scholar] [CrossRef]
- Wantiez, L.; Harmelin-Vivien, M.; Kulbicki, M. Spatial and temporal variation in a soft-bottom fish assemblage in St Vincent Bay, New Caledonia. Mar. Biol. 1996, 125, 801–812. [Google Scholar] [CrossRef]
- Dainys, J.; Mornhe, R.; Whitfield, A. Predicted Climate Change Effects on European Perch (Perca fluviatilis L.)—A Case Study from the Curonian Lagoon, South-Eastern Baltic; Estuarine, Coastal and Shelf Science; Academic Press—Elsevier Science: London, UK, 2019; Volume 221. [Google Scholar]
- Kupschus, S.; Tremain, D. Associations between fish assemblages and environmental factors in nearshore habitats of a subtropical estuary. J. Fish Biol. 2001, 58, 1383–1403. [Google Scholar] [CrossRef]
- Cochranke, K.; Smith, R.; Gerulaitis, A. Climate change implications for fisheries and aquaculture. FAO Fish. Aquac. 2009, 530, 212. [Google Scholar]
- Turner, R.K.; Palmieri, M.G.; Luisetti, T. Lessons from the construction of a climate change adaptation plan: A Broads Wetland case study. Integr. Environ. Assess. Manag. 2016, 12, 719–725. [Google Scholar] [CrossRef] [PubMed]

| df | AIC | F | Pr (>F) | |
|---|---|---|---|---|
| Salinity | 1 | 50.000 | 4.3562 | 0.005 ** |
| WRT | 1 | 49.804 | 2.1009 | 0.040 * |
| df | AIC | F | Pr (>F) | |
|---|---|---|---|---|
| Salinity | 1 | 96.411 | 3.9140 | 0.005 ** |
| WRT | 1 | 92.787 | 5.5911 | 0.005 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanauskas, E.; Skersonas, A.; Andrašūnas, V.; Elyaagoubi, S.; Razinkovas-Baziukas, A. Mapping and Assessing Commercial Fisheries Services in the Lithuanian Part of the Curonian Lagoon. Fishes 2022, 7, 19. https://doi.org/10.3390/fishes7010019
Ivanauskas E, Skersonas A, Andrašūnas V, Elyaagoubi S, Razinkovas-Baziukas A. Mapping and Assessing Commercial Fisheries Services in the Lithuanian Part of the Curonian Lagoon. Fishes. 2022; 7(1):19. https://doi.org/10.3390/fishes7010019
Chicago/Turabian StyleIvanauskas, Edgaras, Andrius Skersonas, Vaidotas Andrašūnas, Soukaina Elyaagoubi, and Artūras Razinkovas-Baziukas. 2022. "Mapping and Assessing Commercial Fisheries Services in the Lithuanian Part of the Curonian Lagoon" Fishes 7, no. 1: 19. https://doi.org/10.3390/fishes7010019







