Transcriptome Profiling Revealed Basis for Growth Heterosis in Hybrid Tilapia (Oreochromis niloticus ♀ × O. aureus ♂)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. RNA Extraction, Library Construction, and Transcriptome Sequencing
2.3. Data Filtering, Comparative Analysis, and Gene Functional Annotation
2.4. Quantification of Gene Expression Levels and Differential Expression Analysis
2.5. GO and KEGG Enrichment Analysis of DEGs
2.6. Validation of RNA-Seq Data by Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. Sequencing Data Statistics
3.2. Identification of Differentially Expressed Genes
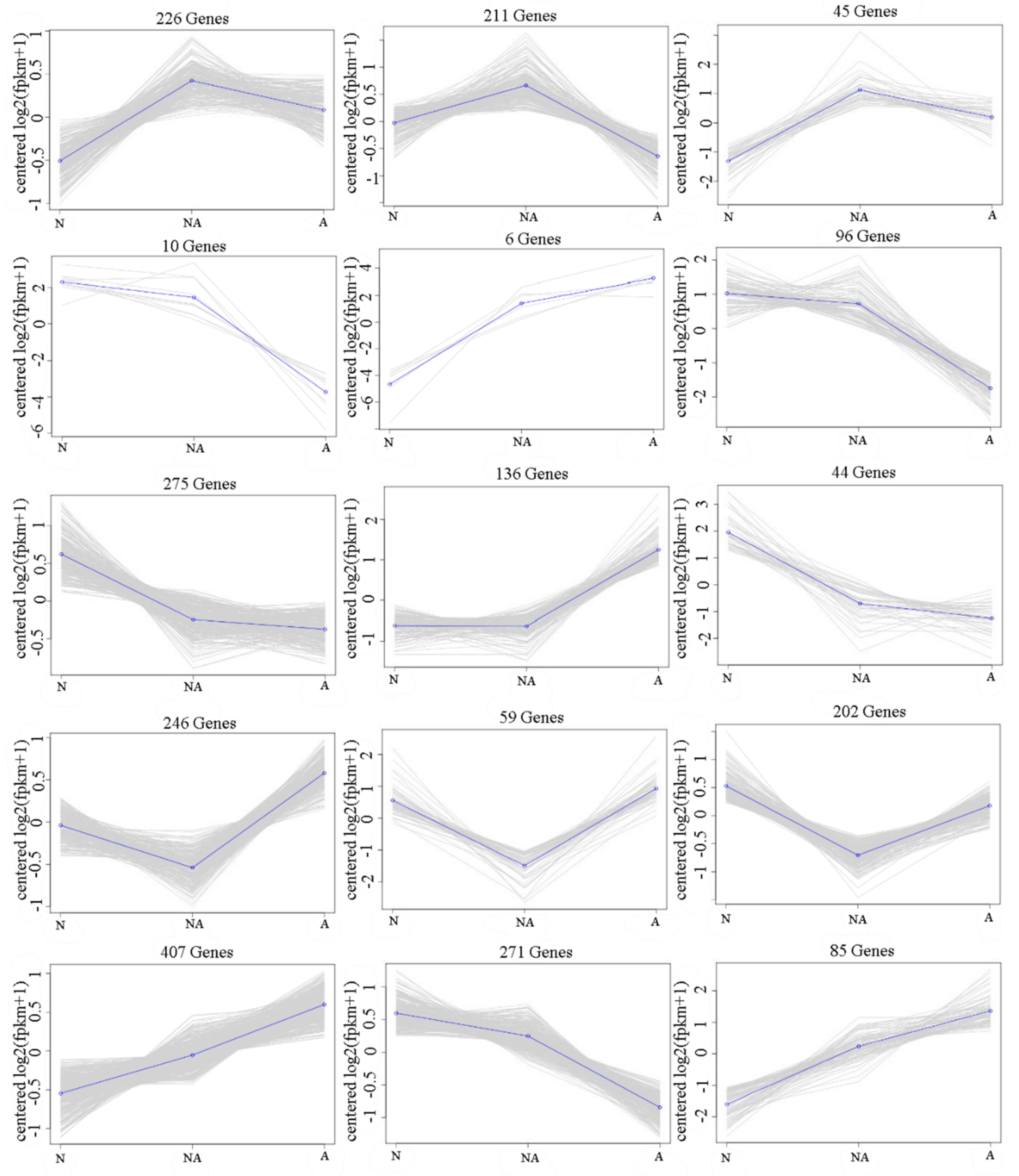
3.3. Trend Co-Expression Analysis of DEGs
3.4. GO and KEGG Enrichment Analysis of DEGs
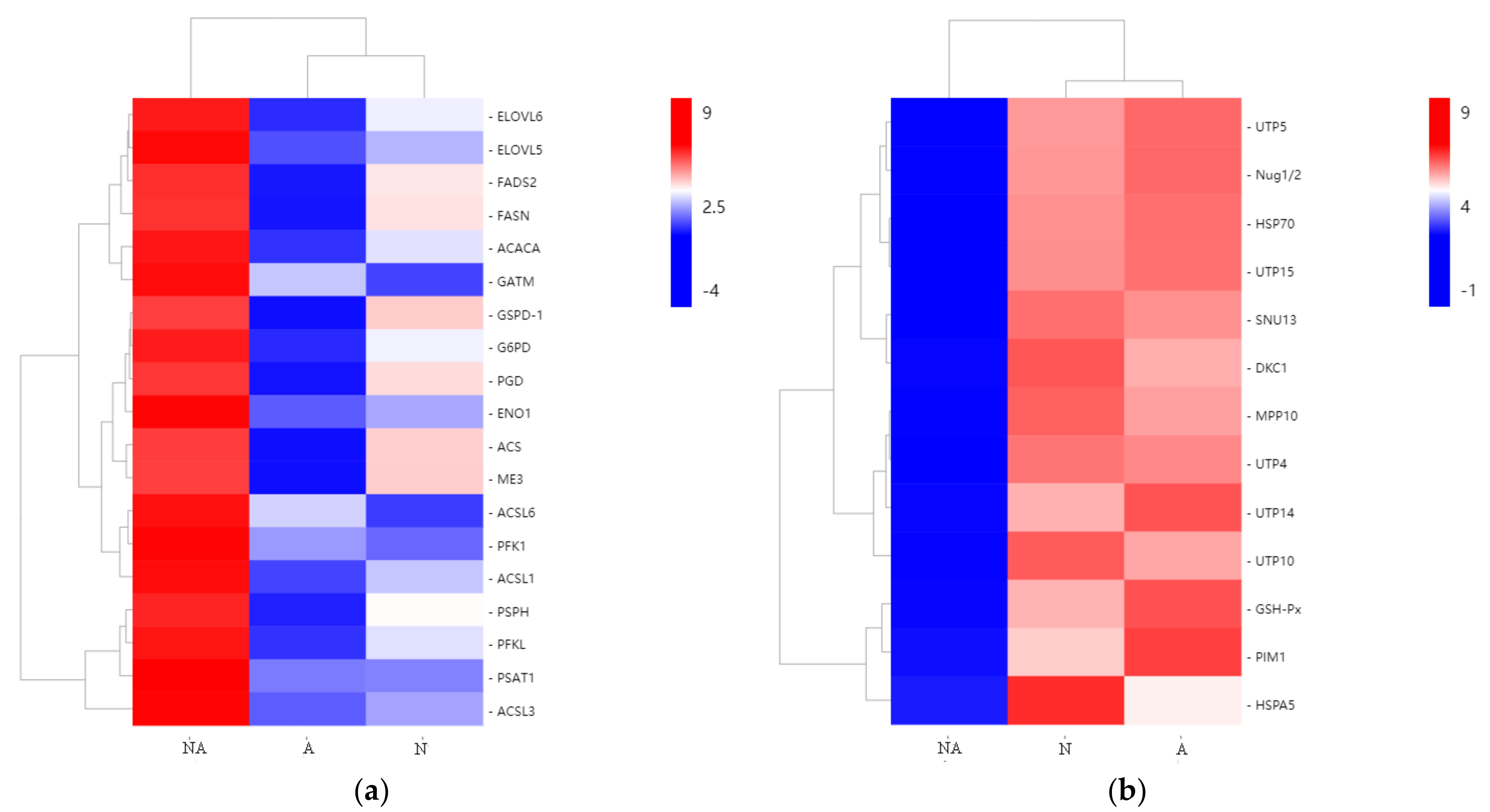
3.5. Analysis of DEGs Potentially Related to Hybrid Tilapia Heterosis
3.5.1. DEGs Related to Fatty Acid Metabolism
3.5.2. Carbon Metabolism-Related DEGs
3.5.3. Eukaryotic Biosynthesis and Defense-Related DEGs
3.6. Validation of RNA-Seq Data by qRT-PCR
4. Discussion
4.1. Analysis of Candidate DEGs Involved in Fatty Acid Metabolism
4.2. Analysis of Candidate DEGs Involved in Carbon Metabolism
4.3. Analysis of Candidate DEGs Involved in Ribosome Biosynthesis in Eukaryotes
4.4. Analysis of Candidate DEGs Involved in Basal Defense Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Li, Q. The DNA methylation level is associated with the superior growth of the hybrid crosses in the Pacific oyster Crassostrea gigas. Aquaculture 2022, 547, 737421. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.; Tao, M.; Qin, Q.; Zhang, C.; Luo, K.; Zhao, R.; Wang, J.; Ren, L.; Xiao, J.; et al. Establishment and application of distant hybridization technology in fish. Sci. China Life Sci. 2019, 62, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Yang, C.; Fan, S.; Huang, L.; Zhou, T.; Wang, Q.; Zhao, R.; Tang, C.; Tao, M.; et al. Comparative analyses of hypothalamus transcriptomes reveal fertility-, growth-, and immune-related genes and signal pathways in different ploidy cyprinid fish. Genomics 2021, 113, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Li, Q.; Xu, C.; Liu, S.; Kong, L.; Yu, H. Reciprocal hybrids derived from Crassostrea gigas and C. angulata exhibit high heterosis in growth, survival and thermos tolerance in northern China. Aquaculture 2021, 545, 737173. [Google Scholar] [CrossRef]
- Meng, L.; Li, Q.; Xu, C.; Liu, S.; Kong, L.; Yu, H. Hybridization improved stress resistance in the Pacific oyster: Evidence from physiological and immune responses. Aquaculture 2021, 545, 737227. [Google Scholar] [CrossRef]
- Xing, Q.; Yang, Z.; Zhu, X.; Liu, J.; Huang, X.; Hu, J.; Bao, Z. Interspecific hybridization between Patinopecten yessoensis (♀) and P. caurinus (♂) with heterosis in growth and temperature tolerance. Aquaculture 2022, 547, 737489. [Google Scholar] [CrossRef]
- Xiao, R.; Yuan, Y.; Zhu, F.; He, S.; Ge, Q.; Wang, X.; Taha, R.; Chen, K. Transcriptomics and proteomics-based analysis of heterosis on main economic traits of silkworm, Bombyx Mori. J. Proteomics 2020, 229, 103941. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Zhang, J.; Liang, X.; Zhang, X.; Wang, T.; Yin, S. Integrated analysis of transcriptomic, miRNA and proteomic changes of a novel hybrid yellow catfish uncovers key roles for miRNAs in heterosis. Mol. Cell. Proteomics 2019, 18, 1437–1453. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, Y.; Zhang, H.; Xiao, W. DNA methylation pattern is associated with elevated expression of DGAT2 in hybrid tilapia. Aquacult. Nutr. 2021, 27, 1750–1760. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, C.; Wang, D.; Li, X.F.; Xiao, L.; Zhang, X.; You, X.; Shi, Q.; Hu, G.; Fang, C.; et al. Transcriptome analysis reveals the molecular mechanisms underlying growth superiority in a novel grouper hybrid (Epinephelus fuscogutatus ♀ × E. lanceolatus ♂). BMC Genet. 2016, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Huang, Y.; Hu, G.; Zhang, X.; Ruan, Z.; Zhao, X.; Guo, C.; Tang, Z.; Li, X.; You, X.; et al. Comparative Transcriptomic study of muscle provides new insights into the growth superiority of a novel grouper hybrid. PLoS ONE 2016, 11, e168802. [Google Scholar] [CrossRef]
- Gao, F.; Lu, W.; Shi, Y.; Zhu, H.; Wang, Y.; Tu, H.; Gao, Y.; Zhou, L.; Gui, J.; Zhao, Z. Transcriptome profiling revealed the growth superiority of hybrid pufferfish derived from Takifugu obscurus ♀ × Takifugu rubripes ♂. Comp. Biochem. Physiol. Genom. Proteomics 2021, 40, 100912. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Watanabe, L.; Vianez, J.; Nunes, M.; Cardoso, J.; Lima, C.; Schneider, H.; Sampaio, I. Comparative analysis of the transcriptome of the Amazonian fish species Colossoma macropomum (tambaqui) and hybrid tambacu by next generation sequencing. PLoS ONE 2019, 14, e212755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Xu, Q.; Yan, J.; Yu, F.; Wang, F.; Xiao, J.; Luo, Y.; Zhong, H. Nonadditive and allele-specific expression of insulin-like growth factor 1 in Nile tilapia (Oreochromis niloticus, ♀) × blue tilapia (O. aureus, ♂) hybrids. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 232, 93–100. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, X.; Xu, Q.; Yan, J.; Han, Z.; Zheng, H.; Xiao, J.; Tang, Z.; Wang, F.; Luo, Y.; et al. Nonadditive and asymmetric allelic expression of growth hormone in hybrid tilapia. Front. Genet. 2019, 10, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lamer, J.T.; Gaughan, S.; Wachholtz, M.; Wang, C.; Lu, G. Transcriptomic comparison of invasive bigheaded carps (Hypophthalmichthys nobilis and Hypophthalmichthys molitrix) and their hybrids. Ecol. Evol. 2016, 6, 8452–8459. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Ji, H.; Xu, S.; Yang, Y.; Jia, C.; Zhu, F.; Meng, Q.; Sun, R.; Zhang, Z.; et al. Transcriptome profiles of F1 hybrids (Acanthopagrus schlegelii ♂ × Pagrus major ♀) and parents reveal hybrid effects on individual development. Aquac. Res. 2020, 51, 4011–4021. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, C.; Liu, J.; Chen, J.; Zou, S. Transcriptome analysis provides new insights into the growth superiority of a novel backcross variety, Megalobrama amblycephala ♀ × (M. amblycephala ♀ × Culter alburnus ♂). Aquaculture 2019, 512, 734317. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; He, Z.; Zheng, T.; Shao, J. De novo transcriptome analysis reveals insights into different mechanisms of growth and immunity in a Chinese soft-shelled turtle hybrid and the parental varieties. Gene 2017, 605, 54–62. [Google Scholar] [CrossRef]
- Gao, K.; Wang, Z.; Qiu, X.; Song, J.; Wang, H.; Zhao, C.; Wang, X.; Chang, Y. Transcriptome analysis of body wall reveals growth difference between the largest and smallest individuals in the pure and hybrid populations of Apostichopus japonicus. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100591. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Huang, Z.; Shen, Y.; Gan, Y.; Wang, Y.; Gong, S.; Lu, Y.; Luo, X.; You, W.; Ke, C. Transcriptome analysis reveals the molecular mechanisms of heterosis on thermal resistance in hybrid abalone. BMC Genom. 2021, 22, 650. [Google Scholar] [CrossRef]
- Bureau of Fisheries; Ministry of Agriculture and Rural Affairs; National Fisheries Technology Extension Center; China Society of Fisheries. 2020 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021; p. 25.
- Lago, A.D.A.; Rezende, T.T.; Dias, M.A.D.; Freitas, R.T.F.D.; Hilsdorf, A.W.S. The development of genetically improved red tilapia lines through the backcross breeding of two Oreochromis niloticus strains. Aquaculture 2017, 472, 17–22. [Google Scholar] [CrossRef]
- Bartie, K.L.; Taslima, K.; Bekaert, M.; Wehner, S.; Syaifudin, M.; Taggart, J.B.; de Verdal, H.; Rosario, W.; Muyalde, N.; Benzie, J.A.H.; et al. Species composition in the Molobicus hybrid tilapia strain. Aquaculture 2020, 526, 735433. [Google Scholar] [CrossRef]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Parrino, V.; De Marco, G.; Minutoli, R.; Lo Paro, G.; Giannetto, A.; Cappello, T.; De Plano, L.M.; Cecchini, S.; Fazio, F. Effects of pesticides on Chelon labrosus (Risso, 1827) evaluated by enzymatic activities along the north eastern Sicilian coastlines (Italy). Eur. Zool. J. 2021, 88, 540–548. [Google Scholar] [CrossRef]
- Schnable, P.S.; Springer, N.M. Progress toward understanding heterosis in crop plants. Annu. Rev. Plant Biol. 2013, 64, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, Q.; Liu, S.; Kong, L. Crossbreeding of three different shell color lines in the Pacific oyster reveals high heterosis for survival but low heterosis for growth. Aquaculture 2020, 529, 735621. [Google Scholar] [CrossRef]
- Gu, H.; Qi, X.; Jia, Y.; Zhang, Z.; Nie, C.; Li, X.; Li, J.; Jiang, Z.; Wang, Q.; Qu, L. Inheritance patterns of the transcriptome in hybrid chickens and their parents revealed by expression analysis. Sci. Rep. 2019, 9, 5750. [Google Scholar] [CrossRef]
- Yang, J.; Luo, S.; Li, J.; Zheng, Z.; Du, X.; Deng, Y. Transcriptome analysis of growth heterosis in pearl oyster Pinctada fucata martensii. FEBS Open Bio 2018, 8, 1794–1803. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; You, W.; Luo, X.; Ke, J.; Huang, M.; Guo, Y.; Ke, C. Integrated analysis of mRNA and miRNA changes in two Haliotis diversicolor genotypes and their hybrid. Front. Mar. Sci. 2021, 8, 667636. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Paiva, P.; Medina, F.E.; Viegas, M.; Ferreira, P.; Neves, R.; Sousa, J.; Ramos, M.J.; Fernandes, P.A. Animal fatty acid synthase: A chemical nanofactory. Chem. Rev. 2021, 121, 9502–9553. [Google Scholar] [CrossRef]
- Soo, H.J.; Sam, K.K.; Chong, J.; Lau, N.S.; Ting, S.Y.; Kuah, M.K.; Kwang, S.Y.; Ranjani, M.; Shu-Chien, A.C. Functional characterisation of fatty acyl desaturase, Fads2, and elongase, Elovl5, in the Boddart’s goggle-eyed goby Boleophthalmus boddarti (Gobiidae) suggests an incapacity for long-chain polyunsaturated fatty acid biosynthesis. J. Fish Biol. 2020, 97, 83–99. [Google Scholar] [CrossRef]
- Fothergill-Gilmore, L.A.; Michels, P.A. Evolution of glycolysis. Prog. Biophys. Mol. Biol. 1993, 59, 105–235. [Google Scholar] [CrossRef]
- Xiong, Y.; Lei, Q.Y.; Zhao, S.; Guan, K.L. Regulation of glycolysis and gluconeogenesis by acetylation of PKM and PEPCK. Cold Spring Harb. Symp. Quant. Biol. 2012, 76, 285–289. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Xie, X.; Teng, W.; Sun, X.; Liang, M.; Du, S.; Zhu, S.; Liu, X.; Nie, H.; Wang, Q. Transcriptomic analysis of the ark shell Scapharca kagoshimensis: De novo assembly and identification of genes and pathways involved growth. Aquac. Rep. 2020, 18, 100522. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, J.; Song, J.; Wang, H.; Gao, K.; Qiu, X.; Gou, M.; Li, X.; Hu, Z.; Wang, X.; et al. Comparative transcriptome analysis reveals growth-related genes in juvenile Chinese sea cucumber, Russian sea cucumber, and their hybrids. Mar. Biotechnol. 2018, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Abetov, D.A.; Kiyan, V.S.; Zhylkibayev, A.A.; Sarbassova, D.A.; Alybayev, S.D.; Spooner, E.; Song, M.S.; Bersimbaev, R.I.; Sarbassov, D.D. Formation of mammalian preribosomes proceeds from intermediate to composed state during ribosome maturation. J. Biol. Chem. 2019, 294, 10746–10757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J.; et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investig. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Cheng, T.C.; Wang, P.C.; Chen, S.C. Protective efficacy of four heat-shock proteins as recombinant vaccines against photobacteriosis in Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2021, 111, 179–188. [Google Scholar] [CrossRef]
- Groszmann, M.; Gonzalez-Bayon, R.; Lyons, R.L.; Greaves, I.K.; Kazan, K.; Peacock, W.J.; Dennis, E.S. Hormone-regulated defense and stress response networks contribute to heterosis in Arabidopsis F1 hybrids. Proc. Natl. Acad. Sci. USA 2015, 112, E6397–E6406. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Song, Q.; Shi, X.; Juenger, T.E.; Chen, Z.J. Natural variation in timing of stress-responsive gene expression predicts heterosis in intraspecific hybrids of Arabidopsis. Nat. Commun. 2015, 6, 8453. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Bayon, R.; Shen, Y.; Groszmann, M.; Zhu, A.; Wang, A.; Allu, A.D.; Dennis, E.S.; Peacock, W.J.; Greaves, I.K. Senescence and defense pathways contribute to heterosis. Plant Physiol. 2019, 180, 240–252. [Google Scholar] [CrossRef] [Green Version]





| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| FASN | AAGCGGTTGATGTCCTCGTAAG | TGGCTGTAAGGCAGTCCGTCTC |
| ELOVL6 | GCTGTGGTCACTCACCCTTGCT | CTGACTGTTTGAGCCCTTTCGT |
| ACACA | GGGAGTTCGGACAGCACCTAT | ATGACCCTGTTACCACCAAAGC |
| FADS2 | CACTTGTTTCCAATGATGCCG | CCAGAGGTCCCCAGAGGTTTT |
| ELOVL5 | CAGATCACGTTCCTGCACCTC | GGATGGCTGGAATGGCTGA |
| GATM | GCTGGGACGGACCTCTTTGT | GGGCGATCTGGGTTTGACA |
| SHMT | CCTTGACTATGCCCGCTTGA | GTGCGTCGTCGTGGTAACAAT |
| ME3 | GAGGATGTGGTGCGGGAACT | CAGCTTTGCTGGTCGGGTT |
| ACS | GCCTCGTCATCAAGCACCAT | GGCCTCATCCCACCAAACAT |
| ALT | ACCCCTCGTACCCGCTCTAT | AACGCAAACAATCCTCCTTCAA |
| ENO1 | TACCCAGTGGTGTCCATTGAGG | CGGAGCCAATCTGGTTGACTTT |
| NOG1 | CGCATTCTCGCCGAACAGA | CGCGTAGTGGCTATCGTCCTT |
| NOP56 | AAGCGGAGTCGGAGGAAGTAG | AGTCACAGGTGTTTCTGGGGTC |
| SNU13 | CTGAGGAAGGGAGCCAACGA | AGCGGCAGGTGGAGGATGAT |
| IGF-2 | ACGCAGAACAGCAGAATGAAGG | GCCGAGGCCATTTCCACTAC |
| GSH-Px | TGAGAAGTTTCTGGTGGGAAGG | TGCGTACTGTTCGAGCAGGTAT |
| β-actin | CCACACAGTGCCCATCTACGA | CCACGCTCTGTCAGGATCTTCA |
| Function | Gene | ID | NA-FPKM | N-FPKM | A-FPKM |
|---|---|---|---|---|---|
| Fatty acid metabolism | FASN | gene:ENSONIG00000007292 | 982.98 | 354.62 | 76.84 |
| ELOVL5 | gene:ENSONIG00000009696 | 3920 | 192.85 | 154.27 | |
| ELOVL6 | gene:ENSONIG00000001109 | 535.45 | 249.52 | 134.74 | |
| ACSL6 | gene:ENSONIG00000017218 | 29.69 | 13.55 | 17.75 | |
| ACSL1 | gene:ENSONIG00000017586 | 265 | 7.70 | 4.93 | |
| ACSL3 | gene:ENSONIG00000014644 | 0.77 | 0.13 | 0.09 | |
| FADS2 | gene:ENSONIG00000015532 | 410.86 | 216.16 | 89.65 | |
| Carbon metabolism | ENO1 ME3 | gene:ENSONIG00000002418 gene:ENSONIG00000000106 | 89.38 257.54 | 31.23 82.64 | 24.72 8.00 |
| ACS | gene:ENSONIG00000018471 | 205.65 | 86.71 | 15.60 | |
| PFK1 | gene:ENSONIG00000011877 | 23.28 | 10.96 | 12.04 | |
| PSPH | gene:ENSONIG00000008118 | 10.72 | 4.49 | 1.79 | |
| PSAT1 | gene:ENSONIG00000016998 | 6.34 | 1.26 | 1.22 | |
| GATM | gene:ENSONIG00000000198 | 412.59 | 29.92 | 66.46 | |
| ACACA | gene:ENSONIG00000001922 | 265.98 | 89.27 | 42.66 | |
| PGD | gene:ENSONIG00000002336 | 47.47 | 30.54 | 14.74 | |
| GSPD-1 | gene:ENSONIG00000017037 | 44.85 | 34.61 | 20.25 | |
| G6PD | gene:ENSONIG00000000416 | 44.67 | 28.09 | 19.16 | |
| PFKL | gene:ENSONIG00000009075 | 5.72 | 3.32 | 2.32 | |
| Defense-related genes | HSPA5 | gene:ENSONIG00000002826 | 188.08 | 678.45 | 3833 |
| GSH-Px | gene:ENSONIG00000017717 | 84.06 | 218.45 | 2979 | |
| PIM1 | gene:ENSONIG00000000258 | 52.95 | 130.25 | 200.23 | |
| HSP70 | gene:ENSONIG00000005214 | 2.33 | 7.61 | 8.46 | |
| Ribosome biogenesis in eukaryotes | SNU13 | gene:ENSONIG00000019849 | 12.13 | 34.88 | 32.03 |
| DKC1 | gene:ENSONIG00000013889 | 10.35 | 29.10 | 23.45 | |
| UTP5 | gene:ENSONIG00000019540 | 3.80 | 12.45 | 14.62 | |
| Nug1/2 | gene:ENSONIG00000019335 | 2.57 | 13.09 | 16.23 | |
| UTP15 | gene:ENSONIG00000014323 | 2.51 | 7.15 | 7.78 | |
| MPP10 | gene:ENSONIG00000002999 | 1.29 | 5.41 | 4.33 | |
| UTP4 | gene:ENSONIG00000004191 | 1.27 | 5.00 | 4.66 | |
| UTP14 | gene:ENSONIG00000020563 | 1.14 | 3.82 | 5.42 | |
| UTP10 | gene:ENSONIG00000019095 | 0.66 | 2.04 | 1.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zou, Z.; Li, D.; Xiao, W.; Yu, J.; Chen, B.; Xue, L.; Yang, H. Transcriptome Profiling Revealed Basis for Growth Heterosis in Hybrid Tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Fishes 2022, 7, 43. https://doi.org/10.3390/fishes7010043
Zhu J, Zou Z, Li D, Xiao W, Yu J, Chen B, Xue L, Yang H. Transcriptome Profiling Revealed Basis for Growth Heterosis in Hybrid Tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Fishes. 2022; 7(1):43. https://doi.org/10.3390/fishes7010043
Chicago/Turabian StyleZhu, Jinglin, Zhiying Zou, Dayu Li, Wei Xiao, Jie Yu, Binglin Chen, Liangyi Xue, and Hong Yang. 2022. "Transcriptome Profiling Revealed Basis for Growth Heterosis in Hybrid Tilapia (Oreochromis niloticus ♀ × O. aureus ♂)" Fishes 7, no. 1: 43. https://doi.org/10.3390/fishes7010043
APA StyleZhu, J., Zou, Z., Li, D., Xiao, W., Yu, J., Chen, B., Xue, L., & Yang, H. (2022). Transcriptome Profiling Revealed Basis for Growth Heterosis in Hybrid Tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Fishes, 7(1), 43. https://doi.org/10.3390/fishes7010043







