Abstract
The antimicrobial and immunostimulant proprieties of aromatic plant extracts have been widely described, but their effects on serum blood biochemistry in fish have not. For this study, we assessed the changes in serum blood biochemical parameters in rainbow trout fed with a fish diet supplemented with a basil supercritical extract (F1-BEO). Our hypothesis was that treatment and time would be associated with changes in 10 serum blood biochemical parameters. F1-BEO was added to a commercial feed (0.5, 1, 2, 3% w/w). The fish were fed for 30 days, and the blood samples were collected at 2 time points (15 and 30 days). A two-way ANOVA showed a significant effect of treatment, time, and interaction treatment × time on creatinine, urea, total protein, albumin, magnesium, and phosphorus (p < 0.05), a significant effect of both time and interaction treatment × time on cholesterol (CHOL), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT) levels, and a significant effect of time on triglycerides (p < 0.05). While changes in several of the parameters were observed, the levels remained within the normal range for rainbow trout. Notably, after 30 days there was a significant decrease in CHOL in fish treated with 0.5% and 1% w/w F1-BEO (p < 0.05). The statistically non-significant increase in GOT and GPT in the fish fed with F1-BEO up to 3% w/w indicated a positive effect of basil on liver health. Our findings suggest a potential use for basil extracts (for example., F1-BEO) in fish feed to reduce antibiotic use and improve fish welfare.
1. Introduction
The use of antimicrobials is crucial for food-producing animals and public health protection; however, their irrational and irresponsible use is a leading cause of antimicrobial resistance [1,2,3]. When antibacterial treatment is the only option after the failure of preventive actions [4], the administration of antimicrobials should be prudent and rational to maintain their efficacy against major pathogens [4]. The uncontrolled use of antimicrobials in aquaculture has led to wide-reaching consequences: the emergence of antibiotic resistant bacteria in aquatic environments, greater antibiotic resistance in fish pathogens, the transfer of these resistance determinants to other bacteria in aquatic ecosystems, to animal and to human pathogens, and alteration of the bacterial flora in sediments and the water column [5,6,7]. International, European, and national agencies recommend strategies to reduce the use of antibiotics in food-producing livestock to minimize the spread of antibiotic resistance [8]. The challenge is to identify new sustainable antimicrobials. A potential source for structurally diverse and complex antimicrobials is plant-derived products [9].
Phytotherapy refers to the use of plants to prevent or treat diseases in humans and animals [10]. Around the turn of the twentieth century, it began to compete with modern medicine, particularly antibiotic compounds [11]. For example, essential oils and polyphenol enhanced extracts (PEEs) have become increasingly utilized in the aquaculture industry to improve industrial and environmental sustainability [12]. Some essential oils extracted from aromatic plants are known to generate biological activity [13], through which they exhibit strong antibacterial and antioxidant effects [13,14]. Their use in medicated feed could help reduce antimicrobials use and environmental pollution and improve animal welfare and food safety [15,16].
Volatile organic compounds (VOCs) and other functional molecules have biocidal properties against fish pathogens [17], as well as the ability to strengthen the immune system in response to disease [18,19,20,21]. VOCs can be extracted from essential oils by solvent extraction and supercritical fluid extraction (CO2-SFE) [22]. CO2-SFE is a green technology that has evolved as a cost-effective, ecologically friendly, and scalable technique for the synthesis of essential oils and VOC-enriched extracts. SFE has a lower solvent recovery, heat degradation of molecules, and extraction time than other standard methods [23]. Side fractions characterized by VOCs combined with polyphenolic compounds and lipophilic compounds are formed during the extraction of VOCs from aromatic plant matrices [24]. These side fractions are regarded as waste products and incur costs for manufacturers because they fail to meet the quality standards of traditional essential oils.
Basil (Ocimum spp.), a member of the Lamiaceae family, is a fragrant perennial shrub that thrives in a variety of climates around the world [25]. One of the world’s most popular aromatic herbs is sweet basil (Ocimum basilicum) [26,27], which contains natural antioxidants such as flavonoids and phenolic acids that may contribute to fish health [28]. Essential oils and O. basilicum extracts have proven effective antioxidant [29], antimicrobial [30,31,32], insecticidal [33,34], nematocidal [27] and fungistatic [35] agents in aquaculture. However, studies investigating the effects of basil extract on blood biochemistry parameters in fish are still scant to date.
Monitoring blood biochemical parameters is a non-lethal means of determining the health status of fish [36,37,38]. Hematobiochemical analysis is useful in diagnostics for predicting the health status of farmed fish species [37,39], and blood biochemistry parameters are basic physiological markers in fish [38]. Previous studies have investigated changes in blood chemistry in response to disease [40], contaminants [41,42], parasitic infestation [43], environmental stressors [44], inappropriate stocking density [45,46], and farm practices [47]. For the present study we assessed the changes in serum blood parameters in rainbow trout (Oncorhynchus mykiss) fed with a fish diet supplemented with a waste product derived from the supercritical fluid extraction of basil (F1-BEO) enriched in polyphenols and VOCs. Our hypothesis was that there would be a measurable time-treatment effect on serum blood parameters. To achieve this, we added the F1-BEO extracted by CO2-SFE to a commercial fish diet (proportions of 0.5% w/w, 1% w/w, 2% w/w, 3% w/w) for 30 days under controlled conditions.
2. Materials and Methods
2.1. Chemical Profile of Supercritical Fluid Basil Extract (F1-BEO)
The basil supercritical fluid extract (F1-BEO) was purchased from Exenia Group s.r.l. (Pinerolo, Turin, Italy). F1-BEO was extracted using a supercritical fluid extractor (SCF-100; Separeco s.r.l, Pinerolo, Italy) from dried, clean sweet basil leaves (size, 0.3 to 0.5 cm; residual humidity < 10%). The first fraction (F1-BEO; yield 3.7% w/w), considered a waste product, contained mainly lipophilic compounds mixed with VOCs, the second fraction (F2-BEO; yield 4.8% w/w) was almost totally composed of VOCs. In order to enhance the waste product, the first fraction was chosen for the purposes of this study. The Exenia Group s.r.l. also provided the chemical profiling of F1-BEO as reported hereinafter [29]. Spectrophotometric evaluation showed that the F1-BEO contained bioactive compounds with a total polyphenol content and total flavan-3-ol content of 32.97 ± 1.63 mmol gallic acid equivalent (GAE) per 100 g of fresh weight and 21.21 ± 1.04 mmol A2-type proanthocyanidin content equivalent (A2-PACE) per 100 g of fresh weight, respectively. Several polyphenolic compounds were identified by HPLC-ESI-MS/MS in the F1-BEO: flavones (scolymoside, isomyricetin, myricetin diglucoside, cynaroside, myricetin, luteolin), flavonols (nicotiflorin, isoquercitrin, astragalin, kaempferol, quercetin, rutin), flavanols (aro-madendrin, arthromerin B, taxifolin), and eight polyphenolic acids (one of which is a derivative of hydroxycinnamic acid [chicoric acid] and seven in the salvianolic acid family). The F1-BEO also contained several VOCs (GC-MS; mg per 100 g of fresh weight): 1,8-cineole (9.33), linalool (25.29), estragol (18.79), eugenol (4.49), methylcinnamylate (8.71), methyleugenol (6.58), b-caryophyllene (7.47), α-bergamotene (19.34). The F1-BEO fraction was composed of about 10% of fats; palmitic acid, linoleic acid, and oleic acid accounted for 77% of the total content of fatty acids (GC-MS and GC-FID analysis).
2.2. Diet Formulation and Rainbow Trout Nutrition
The diet was prepared at the experimental facility of the Department of Agricultural, Forest and Food Sciences (Carmagnola, Turin, Italy). Supplementation with the basil extract was made with commercial feed (Alterna Eel, Skretting Italia, Verona, Italy; ingredients: fish meal, fish oil, wheat gluten, poultry blood meal, soybean protein concentrate, swine hemoglobin, whey powder; proximate composition: protein 48%, fat 11%, ash 8%, fiber 1%, vitamin A, vitamin D3, zinc sulphate, potassium, manganese, copper, iron sulphate, anhydrous calcium iodate). The waste fraction derived from the supercritical fluid extraction of basil was added to the commercial feed in proportions of 0.5% w/w, 1% w/w, 2% w/w, and 3% F1-BEO w/w. The control diet contained only commercial feed without F1-BEO. The mixture was mixed to obtain material for pellet preparation. The pellets were obtained using a 4.0 mm die meat grinder and dried at 30 °C for 48 h. The five diets (A: control; B: 0.5%; C: 1%; D: 2%; E: 3% F1-BEO) were stored in dark bags at 4 °C until use.
For the purpose of this study, 430 sex-reversed female rainbow trout exhibiting a sterile filiform gonad were purchased from a private fish farm in northwest Italy. Thirty individuals were randomly selected for anatomopathological, parasitological, bacteriological, and virologic examination following methods previously reported [48] to ensure that the fish were in optimal health. The fish were conditioned for 20 days before the study began in the same tanks and environmental conditions as those used during the study. The fish were fed by hand to apparent visual satiation seven days a week. After an acclimatization period, 400 fish were lightly anesthetized with tricaine methane sulfonate (MS-222; 70 mg L−1; Sigma-Aldrich, Milan, Italy), individually weighed using a technical balance (KERN KB 2400-2N, 0.01 g accuracy, Kern & Sohn GmbH, Balingen, Germany) and randomly equally distributed in the tanks. Fish weight was also monitored during the experiment since it could influence blood parameters [38].
The fish were kept for 30 days in 20 square fiberglass tanks (capacity, 400 L) supplied with artesian well water (constant temperature 13 ± 1 °C) in an open system (flow-through); each tank had a water inflow of 8 L min−1. Dissolved oxygen was measured every day (range, 8.4–9.5 mg L−1; water pH 7.5 ± 0.6). The fish were exposed to a natural photoperiod (12 h light/12 h dark). The five experimental diets (A, B, C, D, E) were randomly assigned to the 20 tanks (four replicate tanks per diet). The fish were fed by hand seven days a week. The daily feed quantity was set at 1% of tank biomass. The tank biomass was kept constant at 20 kg m−3 by lowering the water level in each tank and based on the fish biomass removed for analysis. Mortality was checked every day. For the purposes of this study, 40 fish from each group (10 fish per tank; four replicates per diet) were sampled at 15 days (T1) and at 30 days (T2). At each time point (T1 and T2), the fish were captured using a landing net and euthanized using an overdose (170 mg kg−1) of MS-222. Blood samples were collected in the morning (8–11 a.m.) by caudal vessels puncture with a 5 mL syringe and transferred in 9 mL Vacuette® tubes containing serum clot activator in a 16 × 100 red cap-black ring (Greiner Bio-One GmbH, Kremsmünster, Austria) and transported refrigerated (4 °C) to the laboratory within a few hours. Each fish was then necropsied and subjected to bacteriological, virological, and parasitological examination [48]. Liver and gut samples were also collected from each fish and immediately fixed in 10% neutral buffered formalin after capture. The samples were then processed by standard paraffin wax techniques [49]. This was performed because diseases and alterations are known to influence blood fish parameters [50].
2.3. Sample Preparation and Analysis
For blood chemistry analysis, serum was obtained by centrifugation (15 min, 2000× g at 10 °C), controlled visually to rule out hemolysis which could influence the results, and stored at −80 °C until biochemical analysis (1 week later). Biochemical parameters included: total proteins (PROT), albumin (ALB), cholesterol (CHOL), triglycerides (TRIGL), creatinine (CREAT), urea (UREA), magnesium (MG), phosphorus (PHOS), glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT). Serum concentrations were analyzed with an automated system photometer (I-Lab Aries Chemical Analyzer, Instrumentation Laboratory S.p.A., Milan, Italy) using the following reagents (Instrumentation Laboratory S.p.A., Milan, Italy): IL Test TM Phosphorus, IL Test TM Magnesium, IL Test TM Albumin, IL Test TM ALT/GPT, IL Test TM AST/GOT, IL Test TM Urea, IL Test TM Triglycerides, IL Test TM Cholesterol, IL Test TM Creatinine Enzymatic, IL Test TM Total Protein. Quality control was performed with 2 levels of control serum samples (SeraChem® Control Level 1 [Cat. No 0018162412] and SeraChem® Control Level 2 [Cat. No. 001816251], Instrumentation Laboratory S.p.A., Milan, Italy) before each assay, and calibration was performed with ReferrIL G (Instrumentation Laboratory S.p.A., Milan, Italy).
2.4. Ethical Statement
The study protocol was designed according to the guidelines of European Union Council 2010/63/EU for the use and care of experimental animals, ARRIVE guidelines, and the principle of the 3Rs was applied. The study protocol was approved by the Institutional Review Board of the Italian Ministry of Health (authorization no. 196/2020-PR).
2.5. Statistical Analysis
Normality and homoscedasticity of data were assessed using the Shapiro-Wilk and the Levene test, respectively. As the data were normally distributed, a one-way ANOVA was used to compare fish total weight at the 2 time points (T1 and T2) between treatment groups (A control; B 0.5%; C 1%; D 2%; E 3% w/w F1-BEO). The Tukey’s test was used as a post-hoc test; two-way ANOVA, with time (T1 and T2), treatment group (A: control; B: 0.5%; C: 1%; D: 2%; E: 3% w/w F1-BEO), and time × treatment interaction as independent variables was used to test statistically significant differences in serum biochemical parameters between the 5 treatment groups at the 2 time points. Dunnett’s multiple comparison test was used to compare the groups treated with F1-BEO (B: 0.5%; C: 1%; D: 2%; E: 3% w/w) against the control group (A diet without F1-BEO). Trends in changes in serum blood parameters for the five groups (A, B, C, D, E) and the 2 time points (T1 and T2) times were checked by principal component analysis (PCA). Statistical significance was set at p < 0.05. Statistical analysis was performed using R software (RStudio, Inc., Boston, MA, USA, version 3.5.2).
3. Results
There were no significant differences in the weight of fish between the 5 experimental groups at 15 days (T1) and at 30 days (T2) (one-way ANOVA; p > 0.05). The mean fish weight ranged from 249.15 g (E) to 252.23 g (A) at T0, from 279.89 g (B) to 282.20 g (A) at T1, and from 310.75 g (E) to 313.15 g (A) at T2 (Table 1).

Table 1.
Mean and standard deviation (±SD) of fish total body weight (g) at 0 (T0), 15 (T1), and 30 days in the five treatment groups (A: control; B: 0.5%; C: 1%; D: 2%; E: 3% w/w F1-BEO). N = 40 rainbow trout (Oncorhynchus mykiss) for each treatment.
No mortality was observed at the end of the experiment. All of the fish were healthy, as confirmed by the absence of internal and external lesions at necropsy and histological analysis. Parasitological, bacteriological, and virological examination tested negative at both T1 and T2 in all treatments.
Table 2 presents the results of two-way ANOVA for treatment, time, and interaction treatment × time on serum biochemical blood parameters. Analysis showed a significant effect (p < 0.05) of treatment, time, and interaction treatment × time on CREAT, PROT, ALB, MG, PHOS, and UREA levels. There was a significant effect (p < 0.05) of both time and interaction treatment × time on CHOL, GOT, and GPT levels, and a significant effect of time on TRIGL (p < 0.05) (Table 2).

Table 2.
Results of two-way ANOVA of time (T1—15 days; T2—30 days), treatment (A: control; B: 0.5%; C: 1%; D: 2%; E: 3% w/w F1-BEO) and interaction (time × treatment) on serum blood paraments in rainbow trout (Oncorhynchus mykiss). Degrees of freedom (dfn = numerator, dfd = denominator) and F statistics (F) are provided. Asterisks denote significant differences: *** p < 0.001; ** p < 0.01; * p < 0.05. ALB denotes albumin; CHOL cholesterol; CREA creatinine; GOT glutamic oxaloacetic transaminase; GPT glutamic pyruvic transaminase; MG magnesium; PHOS phosphorus; PROT total proteins; TRIGL triglycerides; UREA urea.
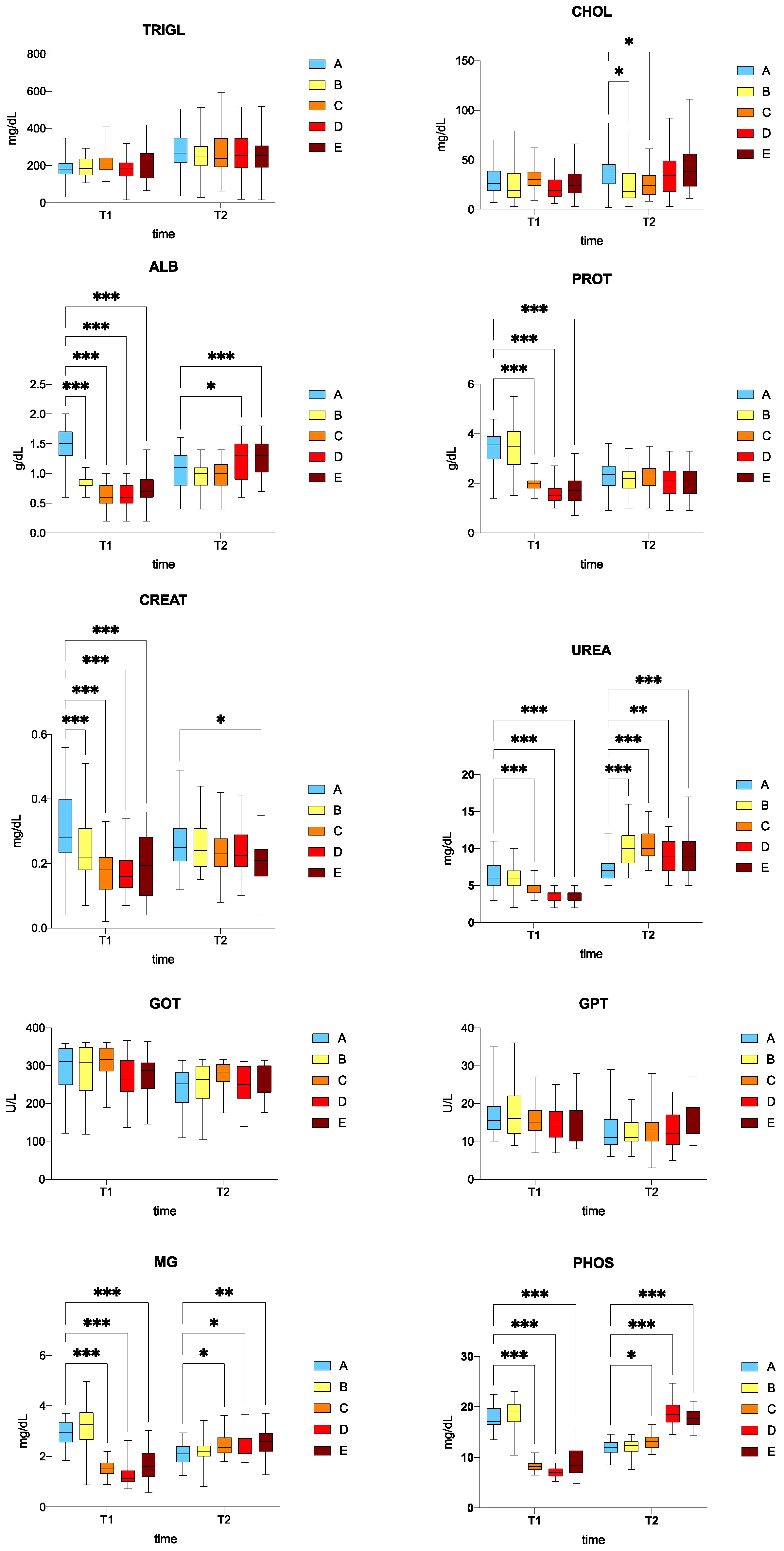
Serum biochemical parameters are reported in Figure 1.
Figure 1.
Boxplots of blood biochemical parameters in the serum of rainbow trout (Oncorhynchus mykiss). Asterisks indicate significant differences according to Dunnett’s multiple comparison test (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001). A: control, B: 0.5%, C: 1%, D: 2%, E: 3% w/w F1-BEO; T1—15 days; T2—30 days. ALB denotes albumin; CHOL cholesterol; CREA creatinine; GOT glutamic oxaloacetic transaminase; GPT glutamic pyruvic transaminase; MG magnesium; PHOS phosphorus; PROT total proteins; TRIGL triglycerides; UREA urea.
The triglyceride (TRIGL) levels in the treated fish (B, C, D, E) were comparable to those of the control group (A) at both T1 and T2. There was no significant difference in cholesterol (CHOL) between the treated (B, C, D, E) and the control group (A) at T1. There was a significant difference in CHOL levels (downward trend) between the fish fed with 0.5% (B) and 1% (C) w/w F1-BEO compared to the control group (A) (p < 0.05). At T1, albumin (ALB) levels were significantly decreased (p < 0.05) in the fish fed with 0.5% (B), 1% (C), 2% (D), and 3% (E) w/w F1-BEO compared to the control group (A), whereas a significant increase in ALB levels was recorded for the fish fed with 2% (D) and 3% (E) w/w F1-BEO compared to the control group (A) at T2 (p < 0.05). At T1, a significant downward trend in total protein content (PROT) was noted for the fish fed with 1% (C), 2% (D), 3% (E) w/w F1-BEO compared to the control group (A) (p < 0.05), while the PROT level in the fish fed with 0.5% w/w F1-BEO was comparable to that of the control group. A similar, albeit not statistically significant, trend was observed at T2 (p > 0.05). The creatinine (CREAT) level at T1 was significantly lower (p < 0.05) in the fish fed with 0.5% (B), 1% (C), 2% (D), and 3% (E) w/w F1-BEO compared to the control group (A). A downward trend in CREAT was recorded at T2, which was only statistically significant for the fish fed with F1-BEO 3% w/w (diet E) compared to the control group (diet A) (p < 0.05). There was a significant decrease in urea (UREA) levels (p < 0.05) in the fish fed with 1% (C), 2% (D),and 3% w/w (E) F1-BEO compared to the control group (A) at T2, whereas a significantly higher concentration was observed in the fish fed with 0.5% (B), 1% (C), 2% (D), and 3% (E) w/w F1-BEO compared to the control group (A) at T2 (p < 0.05). There were no significant differences in glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase between the fish fed with F1-BEO (B–E) and the control group (A) at both T1 and T2. Finally, there was a significant decrease in magnesium (MG) and phosphorus (PHOS) levels (p < 0.05) in the fish fed with 1% (C), 2% (D), and 3% (E) w/w F1-BEO compared to the control group (A) at T1, which was a significant increased at T2 in the fish fed with 1% (C), 2% (D), and 3% (E) w/w F1-BEO compared to the control group (A) (p < 0.05).
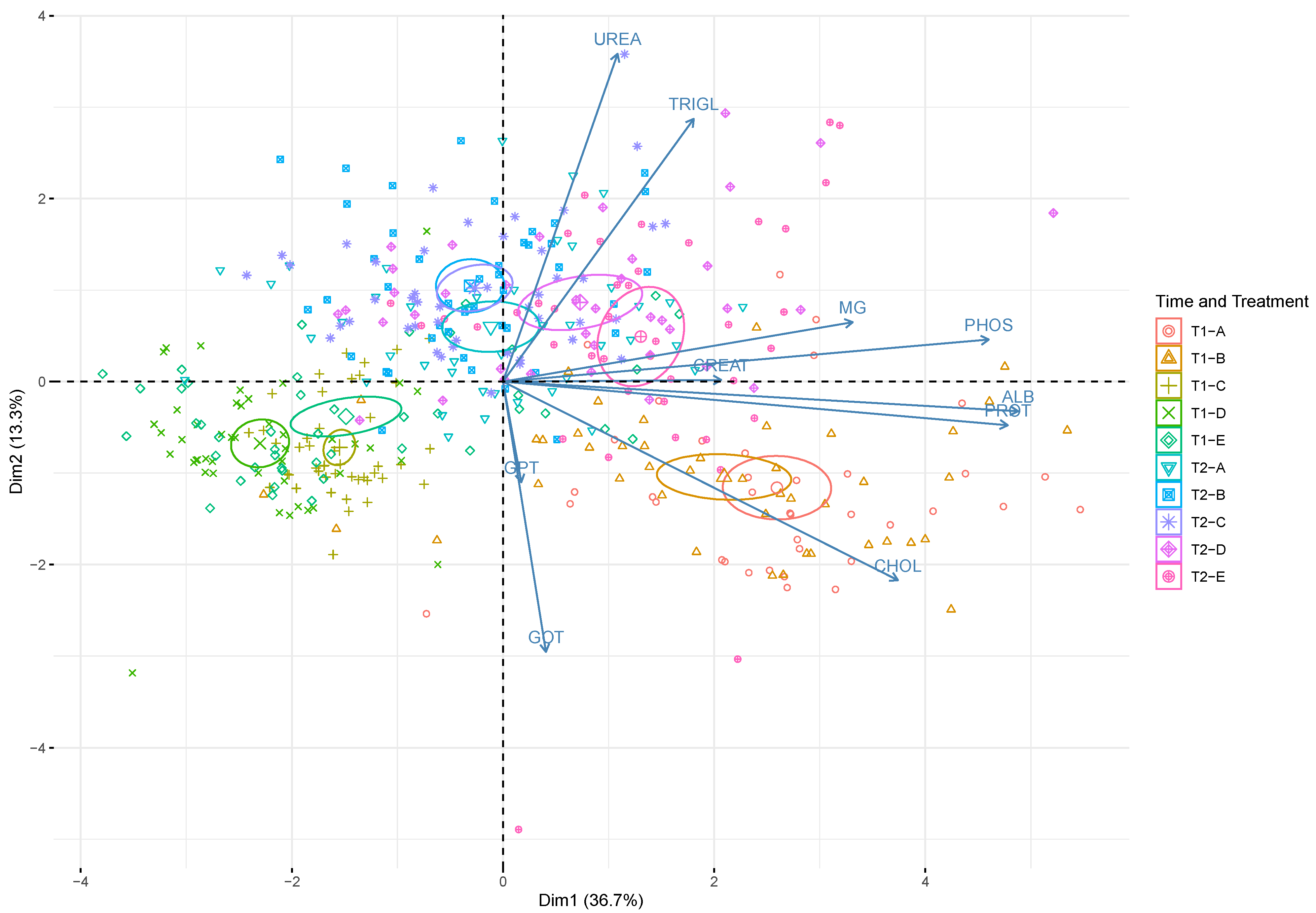
Principal component analysis (PCA) (Figure 2) graphically confirms the results from two-way ANOVA. It clearly demonstrates the effect of both time and treatment on serum blood biochemistry. PCA showed that the first (PC1) and the second (PC2) components accounted for meaningful amounts of total variance (50%): PC1 explained 36.7% of total variance and was positively correlated with PROT, ALB, CHOL, MG, and PHOS, whereas PC2 explained 13.7% of total variance and was positively correlated with TRIGL and UREA and negatively correlated with GOT and GPT. The 2 time points (T1, T2) are arranged according to serum biochemical parameter levels. There was a clear separation between T1 (upper half of the plot) and T2 (lower half of the plot). Blood samples (T2; diets D and E) on the upper right side of the plot are located in relation to the higher TRIGL and UREA concentrations. The blood samples in the lower right part of the plot (T1; diets A, B) are located in relation to the higher levels of CHOL, PROT, ALB, and CREAT.
Figure 2.
Principal component analysis performed on serum blood biochemical parameters in rainbow trout (Oncorhynchus mykiss). The scores of each time point (T1—15 days; T2—30 days) and treatment (A: control; B: 0.5%; C: 1%; D: 2%; E: 3% w/w F1-BEO) are denoted by a color and a symbol (largest symbol corresponds to average value). Confidence ellipses (95%) plot values at each sampling time point/treatment. ALB denotes albumin; CHOL cholesterol; CREA creatinine; GOT glutamic oxaloacetic transaminase; GPT glutamic pyruvic transaminase; MG magnesium; PHOS phosphorus; PROT total proteins; TRIGL triglycerides; UREA urea.
4. Discussion
The serum blood biochemistry parameters in rainbow trout proved highly sensitive to the F1-BEO extract added to a commercial fish diet. Our hypothesis that treatment (increasing percentage of F1-BEO) and time would influence serum blood biochemical parameters in rainbow trout was confirmed by two-way ANOVA and PCA analyses. Time, treatment, and their interaction had an effect on most of the serum biochemical parameters we analyzed. Differently, we recorded no significant difference in weight between the fish fed with F1-BEO extract and the controls at T2. Of note, however, is that our study was not designed to evaluate the growth performance of fish fed with the F1-BEO extract, which would have taken months of observation to determine. The fish weight was monitored only to see whether this variable might influence serum blood parameters. Since no significant difference in weight between the groups was found, weight was not entered in the statistical analyses. A previous study that monitored fish weight for at least one year suggested that changes could have a significant effect on blood parameters in rainbow trout [38].
The factor “treatment” did not seem to have an effect on triglyceride and cholesterol levels, whereas there was a significant decrease in cholesterol levels in the fish fed with 0.5% (B) and 1% (C) w/w F1-BEO at T2. The CHOL and TRIGL levels we recorded were lower than the normal range reported for rainbow trout (mean 247.38 mg dL−1 and 347.51 mg dL−1, respectively) [51]. Cholesterol is a component of cell membranes; it is chiefly synthesized by the liver and involved in steroid hormone synthesis. Its blood levels can be influenced by hepatic activity, nutrition, sex, and sexual development [52]. Triglycerides are the major lipids in adipose tissue and an essential form of fat storage in the body. Since triglyceride levels in the blood reflect dietary fat intake, they should be evaluated whenever a new diet is initiated [53]. F1-BEO may be effective at lowering cholesterol because it contains flavones and a flavonoid known to lower cholesterol and triglycerides [54,55]. Moreover, Brum et al. [56] found that clove basil essential oil (Ocimum gratissimum) supplemented to feed reduced serum cholesterol and triglyceride levels in the Nile tilapia (Oreochromis niloticus). None of these previous studies explained the biochemical mechanism underlying this effect, however, probably because of the multiple mechanisms involved in lipid elimination and because of the plant extract’s potential lipid-lowering effects. Previous studies also reported that supplementation with other essential oils (for example, bergamot peel oil) in O. niloticus feeds reduced cholesterol and triglyceride levels [57].
Creatinine is the end product of energy in muscle tissue [58]. It is formed from creatine, and its blood concentration changes with the level of muscle activity [58]. Since fish eliminate creatinine through the kidney, creatinine blood levels can be used to measure kidney filtration efficiency [59]. Creatinine, together with urea, provides an accurate estimation of kidney filtration. Our results showed a general decrease in creatinine, with lower mean levels in fish fed with 3% w/w F1-BEO at both T1 and T2. Farag et al. [21] found that serum creatinine levels declined in O. niloticus treated with parsley (Petroselinum crispum) essential oil at 2 dose levels (1–2 mL essential oil/kg basal diet) compared to the control group. The same findings were shared by Dawood et al. [60] who recorded lower creatinine levels in O. niloticus fed for 15 and 30 days with menthol essential oil compared to the controls and by Shourbela et al. [61] who reported reduced creatinine levels in O. niloticus fed with a diet supplemented with oregano essential oil.
Urea is a nitrogenous end product of metabolism that provides an estimation of how well fish kidneys are working. Generally, the urea levels were increased at both T1 and T2 in the fish fed with F1-BEO and the control group. Higher levels may result from increased impaired excretion or increased synthesis or decreased urinary clearance by the kidney or decreased degradation of nitrogenous compounds [59]. However, since few factors other than kidney function are known to affect creatinine concentration (which decreased between T0 and T2), we excluded kidney dysfunction. Urea levels of up to 20 mg/dL are considered normal in some fish species [59]. Farag et al. [21] recorded a mean urea level of 18.75 ± 0.25 mg/dL in O. niloticus fed with a commercial diet supplemented with 2 mL/kg of parsley essential oil, which was much higher than those we observed.
The main components of blood plasma are proteins. They play an important role in providing the body with building material [20] and perform a wide range of functions. As such, they are among the main parameters in evaluating the physiological state of an animal. Active participants in metabolic processes, proteins provide an energy substrate [62]. Protein synthesis occurs primarily in the liver. Serum proteins turn blood from a complex solution of many diverse substances into a specialized tissue in which metabolism takes place [63]. Total protein is a measure of protein metabolism; low concentrations may be found in liver disorders [64] and nephritic syndromes [50]. The protein content of blood may change with season, stage of maturity, spawning, type of food consumed, and feeding habit [37]. Generally, we observed a decreasing trend in total protein content, especially during the first 15 days (T1), suggesting an alteration in plasma volume. However, stress may alter the total protein level [64]. At T2, the total protein levels were similar in the treated and the control group, indicating that supplementation with F1-BEO up to 2% w/w had no effect on the osmotic equilibrium of plasma. These findings are shared by Brum et al. [56] in O. niloticus fed with a diet supplemented with clove basil essential oils (0.5%, 1.0%, 1.5% w/w). Generally, the total protein content was in line with that reported for the fish fed with a commercial diet and kept in controlled conditions (range at T2, 2.4–4.5 g dL−1).
The level of serum albumin is also of diagnostic importance in laboratory animals, as it relates to general nutritional status, vascular system integrity, and liver function [65,66]. Albumin plays a key role in the formation and maintenance of osmotic blood pressure and in the transport of numerous substances [63]. Albumin serves as a reserve of amino acids for protein synthesis. In addition, due to the large surface of the micelles and their high negative charge, proteins of this fraction adsorb and transport substances (for example, bilirubin, bile acid salts, hormones, toxins), exerting a regulatory effect on metabolic processes [67]. In the present study, serum albumin levels were significantly decreased at T1 in the fish fed with F1-BEO extract, whereas the levels were significantly increased in the fish fed with 2% and 3% w/w F1-BEO. Hypoalbuminemia in fish may result from exposure to pollutants and other stress [68]. This decreasing trend was similar to that reported above for total protein content, suggesting that the fish had been exposed to stress during the first 15 days of the study. However, the levels were increased at T2. Similar results are shared by Abdel-Tawwab et al. [32] in Clarias gariepinus fed with a diet supplemented with the leaf extract of the clove basil (Ocimum gratissimum) (proportion of 5, 10, 15 g/kg diet) and by Dawood et al. [60] in O. niloticus fed with a diet supplemented with 0.25% menthol essential oil. The albumin levels were generally in line with those Fazio et al. [69], Vigiani et al. [70] and Manera and Britti [51] reported for Italian trout farms (mean concentration 1.83 g dL−1, 1.38 g dL−1, and 0.84 g dL−1, respectively).
We noted no significant difference in GPT and GOT between the treatment and the control groups at T1 and T2. While GOT and GPT activity mainly occurs in the cytoplasmic compartment, increased enzyme activity indicates lysis of hepatic origin. In other words, the non-significant increase in GOT and GPT in the fish fed with F1-BEO up to 3% w/w suggests the absence of negative effects of basil on the health status of the liver cells. Since F1-BEO has a high antioxidant capacity, it may enhance liver health by preserving the hepatic tissue membranes.
Magnesium is found in all body cells; it is involved in cellular energy production, muscle contraction, mineralization, and bone development [71]. Furthermore, phosphorus is essential for energy production, nerve and muscle function, and bone growth [72]. It plays an important buffer role in maintaining the body’s acid-base balance. The same trend for these 2 elements was reported for total protein and albumin levels, where the concentrations were significantly decreased at T1 and then increased at T2 in the fish fed with F1-BEO compared to the control group. However, previous studies reported fluctuations in fish fed with a commercial diet and kept under controlled conditions [38]. The range of magnesium and phosphorus levels are in line with those reported for the fish fed with a commercial diet and kept in controlled condition (range at T2, 1.43–3.37 mg dL−1 and 14.20–21.30 for magnesium and phosphorus, respectively).
5. Conclusions
The study findings show that the F1-BEO extract exerts effects on serum blood biochemical parameters in rainbow trout. The levels were consistently within the normal range for this fish species. Furthermore, the changes in certain parameters demonstrate that F1-BEO has potential lipid-lowering effects, as reported by previous studies with other essential oils. The non-significant increase in GOT and GPT indicates that F1-BEO extract did not affect liver health, suggesting its potentially use in fish feed. Further studies are needed to evaluate its effects on growth performance and immune parameters. Assessment of the antibiotic sensitivity of our F1-BEO to common pathogens that threaten aquaculture industry is another challenge that needs to be addressed as a sustainable alternative to current antibiotics. The use of essential oils and plant extracts in medicated feed in livestock could provide a useful strategy to reduce antimicrobials use and environmental pollution and to improve animal wellbeing and food safety.
Author Contributions
Conceptualization, P.P. and M.P.; Data curation, P.P.; Funding acquisition, P.P.; Investigation, P.P., S.B., C.V., G.P., L.D., M.R., R.B., G.R., A.C.E., A.D., D.B. and M.P.; Methodology, P.P., S.B., C.V., G.P., L.D., M.R., R.B., G.R., A.C.E., D.B. and M.P.; Project administration, P.P. and A.D.; Supervision, M.P.; Writing—original draft, P.P.; Writing—review & editing, S.B., C.V., M.R., G.R., A.C.E., A.D., D.B. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Ministry of Health, Ricerca Finalizzata, grant number GR-2013-02355796.
Institutional Review Board Statement
The study protocol was designed according to the guidelines of European Union Council 2010/63/EU for the use and care of experimental animals, ARRIVE guidelines, and the principle of the 3Rs was applied. The study protocol was approved by the Institutional Review Board of the Italian Ministry of Health (authorization no. 196/2020-PR).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Alfonso Botto (Exenia Group s.r.l., Pinerolo, Turin, Italy), Cinzia Margherita Bertea, Giuseppe Mannino and Marco Micera (Department of Life Sciences and Systems Biology, University of Turin, Turin, Italy) for providing the chemical profile of the F1-BEO extract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burnham, C.A.D.; Leeds, J.; Nordmann, P.; O’Grady, J.; Patel, J. Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 2017, 15, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar]
- Kaprou, G.D.; Bergšpica, I.; Alexa, E.A.; Alvarez-Ordóñez, A.; Prieto, M. Rapid methods for antimicrobial resistance diagnostics. Antibiotics 2021, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Watts, J.E.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Sicuro, B.; Pastorino, P.; Barbero, R.; Barisone, S.; Dellerba, D.; Menconi, V.; Righetti, M.; De Vita, V.; Prearo, M. Prevalence and antibiotic sensitivity of bacteria isolated from imported ornamental fish in Italy: A translocation of resistant strains? Prev. Vet. Med. 2020, 175, 104880. [Google Scholar] [CrossRef]
- Hockenhull, J.; Turner, A.E.; Reyher, K.K.; Barrett, D.C.; Jones, L.; Hinchliffe, S.; Buller, H.J. Antimicrobial use in food-producing animals: A rapid evidence assessment of stakeholder practices and beliefs. Vet. Rec. 2017, 181, 510. [Google Scholar] [CrossRef]
- Jeyavani, J.; Sibiya, A.; Sivakamavalli, J.; Divya, M.; Preetham, E.; Vaseeharan, B.; Faggio, C. Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: A review. Aquacult. Int. 2022, 1–16. [Google Scholar] [CrossRef]
- Falzon, C.C.; Balabanova, A. Phytotherapy: An introduction to herbal medicine. Prim. Care 2017, 44, 217–227. [Google Scholar] [CrossRef]
- Raman, R.P. Applicability, feasibility and efficacy of phytotherapy in aquatic animal health management. Am. J. Plant Sci. 2017, 8, 257. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Thaya, R. Medicinal plant derivatives as immunostimulants: An alternative to chemotherapeutics and antibiotics in aquaculture. Aquac. Int. 2014, 22, 1079–1091. [Google Scholar] [CrossRef]
- Souza, C.D.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential oils as stress-reducing agents for fish aquaculture: A review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, C.; Pasquetti, M.; Giovannetti, G.; Visioni, S.; Re, G.; Giorgi, M.; Gambino, G.; Peano, A. In vitro and in vivo evaluation of a new phytotherapic blend to treat acute externa otitis in dogs. J. Vet. Pharmacol. Ther. 2021, 44, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Citarasu, T. Herbal biomedicines: A new opportunity for aquaculture industry. Aquac. Int. 2010, 18, 403–414. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Firmino, J.P.; Galindo-Villegas, J.; Reyes-López, F.E.; Gisbert, E. Phytogenic bioactive compounds shape fish mucosal immunity. Front. Immunol. 2021, 12, 695973. [Google Scholar] [CrossRef]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; Gültepe, N.; Türker, A. Evaluation of the effects of essential oil extracted from sweet orange peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture 2015, 437, 282–286. [Google Scholar] [CrossRef]
- Martin, S.A.; Król, E. Nutrigenomics and immune function in fish: New insights from omics technologies. Dev. Comp. Immunol. 2017, 75, 86–98. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Haghjou, M.; Nematollahi, A.; Goudarzian, F. Effects of rosemary essential oil on growth performance and hematological parameters of young great sturgeon (Huso huso). Aquaculture 2020, 521, 734909. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Abd El-Aziz, R.M.; Zaglool, A.W.; Moselhy, A.A.; Abou-Zeid, S.M. Effect of parsley essential oil on digestive enzymes, intestinal morphometry, blood chemistry and stress-related genes in liver of Nile tilapia fish exposed to Bifenthrin. Aquaculture 2022, 546, 737322. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E. Comparison of Extraction Methods for the Rapid Determination of Essential Oil Content and Composition of Basil. J. Am. Soc. Hortic. Sci. 1990, 115, 458–462. [Google Scholar] [CrossRef]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and Classical Methods for the Extraction of Mitragyna speciosa Leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Devani, B.M.; Jani, B.L.; Balani, P.C.; Akbari, S.H. Optimization of Supercritical CO2 Extraction Process for Oleoresin from Rotten Onion Waste. Food Bioprod. Process. 2020, 119, 287–295. [Google Scholar] [CrossRef]
- Simon, J.E.; Quinn, J.; Murray, R.G. Basil: A source of essential oils. Adv. Crop Sci. 1990, 1, 484–489. [Google Scholar]
- Abdou, M.A.H.; Abdalla, M.Y.A.; Hegazy, A.A.; Marzok, Z.S. Physiological studies on clove basil plant. J. Plant Prod. Sci. 2011, 2, 1451–1469. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Magara, G.; Prearo, M.; Vercelli, C.; Barbero, R.; Micera, M.; Botto, A.; Caimi, C.; Caldaroni, B.; Bertea, C.M.; Mannino, G.; et al. Modulation of antioxidant defense in farmed rainbow trout (Oncorhynchus mykiss) fed with a diet supplemented by the waste derived from the supercritical fluid extraction of basil (Ocimum basilicum). Antioxidants 2022, 11, 415. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Antimicrobial properties of basil and its possible application in food packaging. J. Agric. Food Chem. 2003, 51, 3197–3207. [Google Scholar] [CrossRef]
- Brum, A.; Pereira, S.A.; Owatari, M.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Effect of dietary essential oils of clove basil and ginger on Nile tilapia (Oreochromis niloticus) following challenge with Streptococcus agalactiae. Aquaculture 2017, 468, 235–243. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; El-Ashram, A.M.; Tahoun, A.; Abdel-Razek, N.; Awad, S.M. Effects of dietary sweet basil (Ocimum basilicum) oil on the performance, antioxidants and immunity welfare, and resistance of Indian shrimp (Penaeus indicus) against Vibrio parahaemolyticus infection. Aquac. Nutr. 2021, 27, 1244–1254. [Google Scholar] [CrossRef]
- Ling Chang, C.; Kyu Cho, I.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, J.; Tak, J.H. Synergistic mechanism of insecticidal activity in basil and mandarin essential oils against the tobacco cutworm. J. Pest Sci. 2021, 94, 1119–1131. [Google Scholar] [CrossRef]
- Wyenandt, C.A.; Simon, J.E.; Pyne, R.M.; Homa, K.; McGrath, M.T.; Zhang, S.; Raid, R.N.; Ma, L.J.; Wick, R.; Guo, L.; et al. Basil downy mildew (Peronospora belbahrii): Discoveries and challenges relative to its control. Phytopathology 2015, 105, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Casella, S.; Arfuso, F.; Marafioti, S.; Piccione, G.; Fazio, F. Effect of storage time on haematological parameters in mullet, Mugil cephalus. Cell Biochem. Funct. 2013, 31, 412–416. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Pastorino, P.; Bergagna, S.; Dezzutto, D.; Barbero, R.; Righetti, M.; Pagliasso, G.; Gasco, L.; Gennero, S.; Pizzul, E.; Dondo, A.; et al. Long-term assessment of baseline blood biochemistry parameters in rainbow trout (Oncorhynchus mykiss) maintained under controlled conditions. Animals 2020, 10, 1466. [Google Scholar] [CrossRef]
- Nabi, N.; Ahmed, I.; Wani, G.B. Hematological and serum biochemical reference intervals of rainbow trout, Oncorhynchus mykiss cultured in Himalayan aquaculture: Morphology, morphometrics and quantification of peripheral blood cells. Saudi J. Biol. Sci. 2022, 29, 2942–2957. [Google Scholar] [CrossRef]
- Rehulka, J. Haematological analyses in rainbow trout Oncorhynchus mykiss affected by viral haemorrhagic septicaemia (VHS). Dis. Aquat. Org. 2003, 56, 185–193. [Google Scholar] [CrossRef]
- Taheri Mirghaed, A.; Ghelichpour, M.; Mirzargar, S.S.; Joshaghani, H.; Ebrahimzadeh Mousavi, H. Toxic effects of indoxacarb on gill and kidney histopathology and biochemical indicators in common carp (Cyprinus carpio). Aquac. Res. 2018, 49, 1616–1627. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, Y.B.; Choi, J.H. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- González, M.P.; Muñoz, J.L.; Valerio, V.; Vargas-Chacoff, L. Effects of the ectoparasite Caligus rogercresseyi on Salmo salar blood parameters under farm conditions. Aquaculture 2016, 457, 29–34. [Google Scholar] [CrossRef]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Uiuiu, P.; Lațiu, C.; Păpuc, T.; Craioveanu, C.; Ihuț, A.; Sava, A.; Răducu, C.; Șonea, C.; Constantinescu, R.; Cocan, D.; et al. Multi-approach assessment for stress evaluation in rainbow trout females, Oncorhynchus mykiss (Walbaum, 1792) from three different farms during the summer season. Animals 2021, 11, 1810. [Google Scholar] [CrossRef]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; John Wiley & Sons: Ames, IA, USA, 2010; p. 519. [Google Scholar]
- Pastorino, P.; Prearo, M.; Pizzul, E.; Bertoli, M.; Francese, D.R.; Menconi, V.; Mugetti, D.; Bozzetta, E.; Varello, K. Hepatic Steatosis in a Bullhead (Cottus gobio) Population from a High-Mountain Lake (Carnic Alps): Adaptation to an Extreme Ecosystem? Water 2019, 11, 2570. [Google Scholar] [CrossRef]
- Clauss, T.M.; Dove, A.D.; Arnold, J.E. Hematologic disorders of fish. Vet. Clin. N. Am. 2008, 11, 445–462. [Google Scholar] [CrossRef]
- Manera, M.; Britti, D. Assessment of blood chemistry normal ranges in rainbow trout. J. Fish Biol. 2006, 69, 1427–1434. [Google Scholar] [CrossRef]
- Banaee, M.; Nemadoost Haghi, B.; Tahery, S.; Shahafve, S.; Vaziriyan, M. Effects of sub-lethal toxicity of paraquat on blood biochemical parameters of common carp, Cyprinus carpio (Linnaeus, 1758). Iran. J. Toxicol. 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Van der Boon, J.; Van Den Thillart, G.E.; Addink, A.D. The effects of cortisol administration on intermediary metabolism in teleost fish. Comp. Biochem. Phys. A 1991, 100, 47–53. [Google Scholar] [CrossRef]
- Kurowska, E.M.; Manthey, J.A. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J. Agric. Food Chem. 2004, 52, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, C.C.; Oyoo-Okoth, E.; Mugo-Bundi, J.; Orina, P.S.; Chemoiwa, E.J.; Aloo, P.A. Effects of dietary administration of stinging nettle (Urtica dioica) on the growth performance, biochemical, hematological and immunological parameters in juvenile and adult Victoria Labeo (Labeo victorianus) challenged with Aeromonas hydrophila. Fish Shellfish. Immunol. 2015, 44, 533–541. [Google Scholar] [CrossRef]
- Brum, A.; Cardoso, L.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Histological changes in Nile tilapia fed essential oils of clove basil and ginger after challenge with Streptococcus agalactiae. Aquaculture 2018, 490, 98–107. [Google Scholar] [CrossRef]
- Kesbiç, O.S. Effects of juniper berry oil on growth performance and blood parameters in common carp (Cyprinus carpio). Aquac. Res. 2019, 50, 342–349. [Google Scholar] [CrossRef]
- Campbell, T.W. Clinical Chemistry of Fish and Amphibian. In Veterinary Hematology and Clinical Chemistry; Thrall, M.A., Weiser, G., Allison, R.W., Campbell, T.W., Eds.; Wiley-Blackwel: Ames, IA, USA, 2012; pp. 607–614. [Google Scholar]
- Ajeniyi, S.A.; Solomon, R.J. Urea and creatinine of Clarias gariepinus in three different commercial ponds. Nat. Sci. 2014, 12, 124–138. [Google Scholar]
- Dawood, M.A.; Zommara, M.; Eweedah, N.M.; Helal, A.I. The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in Nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 2020, 515, 734571. [Google Scholar] [CrossRef]
- Shourbela, R.M.; El-Hawarry, W.N.; Elfadadny, M.R.; Dawood, M.A. Oregano essential oil enhanced the growth performance, immunity, and antioxidative status of Nile tilapia (Oreochromis niloticus) reared under intensive systems. Aquaculture 2021, 542, 736868. [Google Scholar] [CrossRef]
- Lapirova, T.B.; Flerova, E.A. Comparative analysis of some immunophysiological blood parameters of pike Esox lucius (L.) and walleye Stizostedion lucioperca (L.). Vestn. Astrakh. Gos. Tekh. Univ. Ser. Ryb. Khoz. 2013, 1, 140–146. [Google Scholar]
- Chernyavskikh, S.D.; Borodaeva, Z.A.; Borisovskiy, I.P.; Ostapenko, S.I.; Galtseva, O.A. Blood protein spectrum in representatives of the fish superclass. Eurasian J. Biosci. 2019, 13, 979–981. [Google Scholar]
- John, P.J. Alteration of certain blood parameters of freshwater teleost Mystus vittatus after chronic exposure to Metasystox and Sevin. Fish Physiol. Biochem. 2007, 33, 15–20. [Google Scholar] [CrossRef]
- Gopal, V.; Parvathy, S.; Balasubramanian, P.R. Effect of heavy metals on the blood protein biochemistry of the fish Cyprinus carpio and its use as a bio-indicator of pollution stress. Environ. Monit. Assess. 1997, 48, 117–124. [Google Scholar] [CrossRef]
- Januar, H.I.; Fajarningsih, N.D.; Zilda, D.S.; Bramandito, A.; Wright, A.D. Concentration of fish serum albumin (FSA) in the aqueous extract of Indonesian Perciformes fishes’ muscle tissue. Nat. Prod. Res. 2015, 29, 2230–2232. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Aydin, S.; Kutlu, B. Alterations of growth performance and blood chemistry in Nile tilapia (Oreochromis nuoticus) affected b copper sulfate in long-term exposure. Turk. J. Fish Aquat. Sc. 2015, 15, 481–488. [Google Scholar]
- Chen, C.Y.; Wooster, G.A.; Bowser, P.R. Comparative blood chemistry and histopathology of tilapia infected with Vibrio vulnificus or Streptococcus iniae or exposed to carbon tetrachloride, gentamicin, or copper sulfate. Aquaculture 2004, 239, 421–443. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Piccione, G.; Kesbiç, O.S.; Acar, Ü. Comparative study of some hematological and biochemical parameters of Italian and Turkish farmed rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Turk. J. Fish. Aquat. Sc. 2016, 16, 715–721. [Google Scholar]
- Vigiani, V.; Lupi, P.; Mecatti, M. Some haematochemical parameters of intensively farmed rainbow trout (Oncorhynchus mykiss). Ital. J. Anim. Sci. 2005, 4, 574–576. [Google Scholar] [CrossRef][Green Version]
- Zaprudnova, R.A. Change in magnesium concentration in erythrocytes in fish under stress. Regul. Mech. 2018, 9, 391–395. [Google Scholar] [CrossRef]
- Ye, C.X.; Wan, F.; Sun, Z.Z.; Cheng, C.H.; Ling, R.Z.; Fan, L.F.; Wang, A.L. Effect of phosphorus supplementation on cell viability, anti-oxidative capacity and comparative proteomic profiles of puffer fish (Takifugu obscurus) under low temperature stress. Aquaculture 2016, 452, 200–208. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).