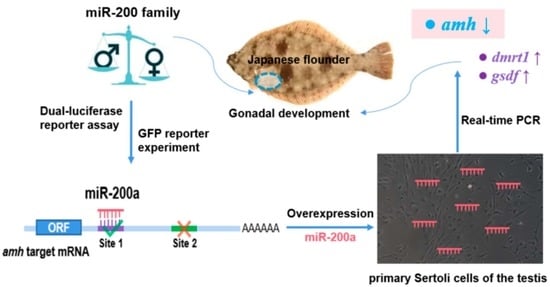
The miR-200 Family Targeting amh Affects the Gonadal Development of Japanese Flounder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Real-Time PCR
2.3. Prediction of Target Genes of miR-200 Family by Bioinformatics
2.4. Construction of Wild and Mutant Recombinant Plasmid
2.5. Cell Transfection and Luciferase Assay
2.6. Isolation and miRNA Overexpression of Primary Sertoli Cells from the Testis
3. Results
3.1. Differential Expression of the miR-200 Family in Male and Female Gonads
3.2. Prediction of Target Sites between the miR-200 Family and amh
3.3. Target Identification between the miR-200 Family and amh
3.4. Sex-Related Gene Expression Differences in Sertoli Cells and Germ Cells
3.5. Expression Changes of amh, dmrt1 and gsdf after Overexpression of miR-200a
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.; Norbury, C.; Gilbert, R.C. The long and short of microRNA. Cell 2013, 153, 516–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, J.; Xiong, S.; Li, Z.; Wu, J.; Zhou, L.; Gui, J.F.; Mei, J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci. Rep. 2015, 5, 15906. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.L.; Sun, J.C.; Lu, Y.; Liu, Y.K.; Cao, H.Y.; Zhang, H.Y.; Calin, G.A. MiR-200 family and cancer: From a meta-analysis view. Mol. Asp. Med. 2019, 70, 57–71. [Google Scholar] [CrossRef]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008, 22, 894–907. [Google Scholar] [CrossRef] [Green Version]
- Hasuwa, H.; Ueda, J.; Ikawa, M.; Okabe, M. MiR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 2013, 341, 71–73. [Google Scholar] [CrossRef]
- Jing, J.; Wu, J.; Wei, L.; Xiong, S.; Ma, W.; Jin, Z.; Wang, W.; Gui, J.F.; Jie, M. Sex-biased miRNAs in gonad and their potential roles for testis development in yellow catfish. PLoS ONE 2014, 9, e107946. [Google Scholar] [CrossRef]
- Xiong, S.; Ma, W.; Jing, J.; Zhang, J.; Dan, C.; Gui, J.F.; Mei, J. An miR-200 cluster on chromosome 23 regulates sperm motility in zebrafish. Endocrinology 2018, 159, 1982–1991. [Google Scholar] [CrossRef] [Green Version]
- De Siqueira-Silva, D.H.; da Silva Rodrigues, M.; Nobrega, R.H. Testis structure, spermatogonial niche and Sertoli cell efficiency in Neotropical fish. Gen. Comp. Endocrinol. 2019, 273, 218–226. [Google Scholar] [CrossRef]
- Yang, X.Y.; Jia, Z.W. The role of EGF-like factor signaling pathway in granulosa cells in regulation of oocyte maturation and development. Heredita 2019, 41, 137–145. [Google Scholar]
- Yan, Y.L.; Batzel, P.; Titus, T.; Sydes, J.; Desvignes, T.; BreMiller, R.; Draper, B.; Postlethwait, J.H. A hormone that lost its receptor: Anti-Mullerian hormone (AMH) in zebrafish gonad development and sex determination. Genetics 2019, 213, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mari, A.; Yan, Y.L.; Bremiller, R.A.; Wilson, C.; Canestro, C.; Postlethwait, J.H. Characterization and expression pattern of zebrafish anti-Mullerian hormone (Amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns 2005, 5, 655–667. [Google Scholar] [CrossRef]
- Yan, X.; Xiong, X.; Chen, Y.G. Feedback regulation of TGF-beta signaling. Acta Biochim. Biophys. Sin. 2018, 50, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Marca, A.; Ferraretti, A.P.; Palermo, R.; Ubaldi, F.M. The use of ovarian reserve markers in IVF clinical practice: A national consensus. Gynecol. Endocrinol. 2016, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Mei, J.; Li, Z.; Zhang, X.; Zhou, L.; Gui, J.F. Distinct and cooperative roles of amh and dmrt1 in self-renewal and differentiation of male germ cells in zebrafish. Genetics 2017, 207, 1007–1022. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A tandem duplicate of anti-Mullerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile Tilapia, Oreochromis niloticus. PLoS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef] [Green Version]
- Shuhei, N.; Ikuko, W.; Toshiya, N.; Jean-Yves, P.; Atsushi, T.; Yoshihito, T.; Nathalie, D.C.; Minoru, T. Hyperproliferation of mitotically active germ cells due to defective anti-Müllerian hormone signaling mediates sex reversal in medaka. Development 2012, 139, 2283–2287. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, Q.H.; Xiao, Y.S.; Xu, S.H.; Wang, X.Y.; Yang, J.K.; Song, Z.C.; You, F.; Li, J. Effects of environmental stress (sex steroids and heat) during sex differentiation in Japanese flounder (Paralichthys olivaceus): Insight from germ cell proliferation and gsdf-amh-cyp19a1a expression. Aquaculture 2020, 515, 734536. [Google Scholar] [CrossRef]
- Eiichi, Y. Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture 1999, 173, 235–246. [Google Scholar]
- Gu, Y.; Zhang, L.; Chen, X. Differential expression analysis of Paralichthys olivaceus microRNAs in adult ovary and testis by deep sequencing. Gen. Comp. Endocrinol. 2014, 204, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.; Liu, H.J.; Si, F.; Wang, Y.F.; Jiang, X.F. Histological observation of gonadal differentiation in cultured Japanese flounder Paralichthys olivaceus. J. Dalian Fish. Univ. 2008, 23, 451–454. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, S.; Zou, Y.; Wu, Z.; Wang, L.; Liu, Y.; You, F. Amh dominant expression in Sertoli cells during the testicular differentiation and development stages in the olive flounder Paralichthys olivaceus. Gene 2020, 755, 144906. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.M.; Donahoe, P.K. Mullerian Inhibiting Substance: A gonadal hormone with multiple functions. Endocr. Rev. 1993, 14, 152–164. [Google Scholar]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, G.; Li, M.; Zhu, F.; Liu, Q.; Naruse, K.; Herpin, A.; Nagahama, Y.; Li, J.; Hong, Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 2016, 6, 19738. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, H.; Ijiri, S.; Kobayashi, T.; Izumi, H.; Kuramochi, Y.; Wang, D.-S.; Mizuno, S.; Nagahama, Y. Gonadal soma-derived factor (gsdf), a TGF-beta superfamily gene, induces testis differentiation in the teleost fish Oreochromis niloticus. Mol. Cell. Endocrinol. 2015, 415, 87–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond, C.S.; Murphy, M.W.; O’Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Primer | Sequence (5′→3′) |
|---|---|
| miR-200a-F | UAACACUGUCUGGUAACGAUGU |
| miR-200b-F | UAAUACUGCCUGGUAAUGAUGA |
| miR-141-F | UAACACUGUCUGGUAACGAUG |
| miR-429-F | UAAUACUGUCUGGUAAUGCCGU |
| mQ Primer | AGTGCAGGGTCCGAGGTATT |
| amh-F | CAGATAGGAGTGCGGAACA |
| amh-R | AAGAGCCTCGGCAGTTGT |
| dmrt1-F | CAGCCTCCTTCTCACCC |
| dmrt1-R | CCTCGCACTCAGCCTTG |
| 3′-amh GLO-F | GGACTAGTAACCCACCGCAAGCAATC |
| 3′-amh GLO-R | CATGCATGCATGCCCTCCTGGCACAAGAAAGC |
| 3′-amh EGFP-F | CTAGTTGTTTAAACGAGCTCGAGGGGATTTGGGTTCTGAAGG |
| 3′-amh EGFP-R | GCCTGCAGGTCGACTCTAGACTAGCCCTCCTGCTCTTCCCTA |
| β-catin-F | GGAAATCGTGCGTGACATTAAG |
| β-catin-R | CCTCTGGACAACGGAACCTCT |
| 5s-F | CCATACCACCCTGAACAC |
| 5s-R | CGGTCTCCCATCCAAGTA |
| amh-mt-F | TACATGTCTCCGATACACATATTTATTCAGGGAGGTCTTT |
| amh-mt-R | GTGTATCGGAGACATGTATATGACACTTACTTCATCTAACAAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, K.; Zhang, F.; Wu, J.; Zhang, J. The miR-200 Family Targeting amh Affects the Gonadal Development of Japanese Flounder. Fishes 2022, 7, 129. https://doi.org/10.3390/fishes7030129
Zhang H, Li K, Zhang F, Wu J, Zhang J. The miR-200 Family Targeting amh Affects the Gonadal Development of Japanese Flounder. Fishes. 2022; 7(3):129. https://doi.org/10.3390/fishes7030129
Chicago/Turabian StyleZhang, Haoran, Kun Li, Fayang Zhang, Jikui Wu, and Junling Zhang. 2022. "The miR-200 Family Targeting amh Affects the Gonadal Development of Japanese Flounder" Fishes 7, no. 3: 129. https://doi.org/10.3390/fishes7030129
APA StyleZhang, H., Li, K., Zhang, F., Wu, J., & Zhang, J. (2022). The miR-200 Family Targeting amh Affects the Gonadal Development of Japanese Flounder. Fishes, 7(3), 129. https://doi.org/10.3390/fishes7030129