Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis
Abstract
:1. Introduction
2. Nutrients and Bioactive Compounds
2.1. Proteins, Bioactive Peptides, Amino Acids, Taurine, and Anserine
2.2. Lipids and Omega–3 Polyunsaturated Fatty Acids
2.3. Vitamins, Pro-Vitamins and Carotenoids
2.4. Phytosterols and Squalene
2.5. Minerals and Trace Elements
2.6. Chitin, Chitosan and Chito-Oligosaccharides
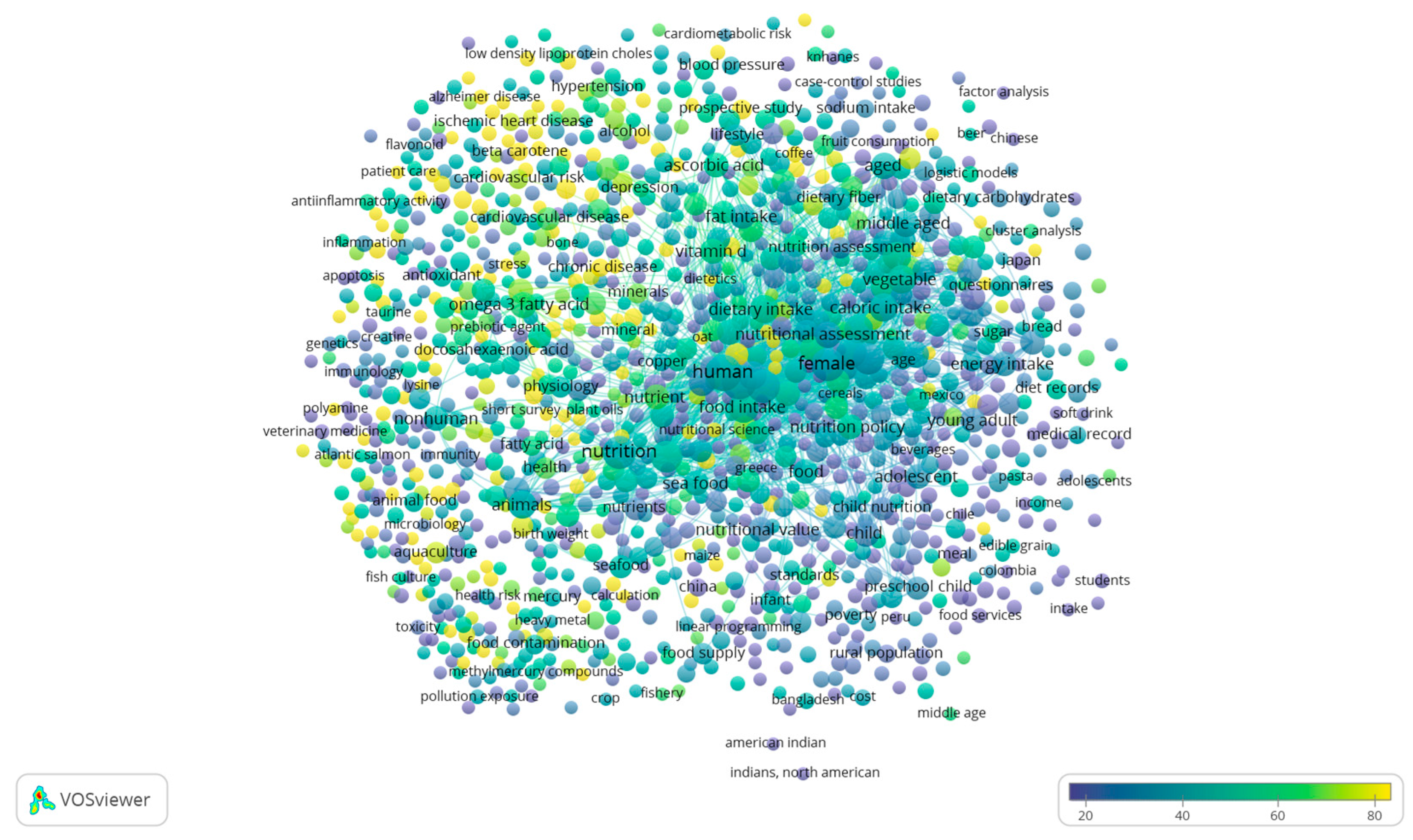
3. Literature Quantitative Research Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, H.; Langkilde, A.M.; Undeland, I.; Lindqvist, H.; Langkilde, A.M.; Undeland, I.; Rådendal, T.; Sandberg, A.S. Herring (Clupea harengus) supplemented diet influences risk factors for CVD in overweight subjects. Eur. J. Clin. Nutr. 2007, 61, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringleb, P.A.; Bousser, M.-G.; Ford, G.; Bath, P.; Brainin, M.; Caso, V.; Cervera, Á.; Chamorro, A.; Cordonnier, C.; Csiba, L.; et al. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 2008, 25, 457–507. [Google Scholar]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Zampelas, A.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Chrysohoou, C.; Skoumas, Y.; Stefanadis, C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: The ATTICA study. J. Am. Coll. Cardiol. 2005, 46, 120–124. [Google Scholar] [CrossRef] [Green Version]
- CREA Centro di Ricerca Alimenti e Nutrizione. Linee Guida Per Una Sana Alimentazione; Centro di Ricerca Alimenti e Nutrizione: Roma, Italy, 2018; ISBN 978-88-96597-01-9. Available online: https://www.crea.gov.it/web/alimenti-e-nutrizione/-/linee-guida-per-una-sana-alimentazione-2018 (accessed on 10 May 2022).
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. In Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Government Printing Office: Washington, DC, USA, December 2020. Available online: DietaryGuidelines.gov (accessed on 10 May 2022).
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in human health. Foods 2020, 9, 370. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Mohanty, B.; Mahanty, A.; Ganguly, S.; Sankar, T.V.; Chakraborty, K.; Rangasamy, A.; Paul, B.; Sarma, D.; Mathew, S.; Asha, K.K.; et al. Amino acid compositions of 27 food fishes and their importance in clinical nutrition. J. Amino Acids 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Pyz-Łukasik, R.; Chałabis-Mazurek, A.; Gondek, M. Basic and functional nutrients in the muscles of fish: A review. Int. J. Food Prop. 2020, 23, 1941–1950. [Google Scholar] [CrossRef]
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: A randomized controlled trial. Diabetes Care 2007, 30, 2816–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geahchan, S.; Baharlouei, P.; Rahman, M.A. Marine Collagen: A Promising Biomateria l for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Harnedy-Rothwell, P.A.; McLaughlin, C.M.; O’Keeffe, M.B.; Le Gouic, A.V.; Allsopp, P.J.; McSorley, E.M.; Sharkey, S.; Whooley, J.; McGovern, B.; O’Harte, F.P.; et al. Identification and characterisation of peptides from a boarfish (Capros aper) protein hydrolysate displaying in vitro dipeptidyl peptidase-IV (DPP-IV) inhibitory and insulinotropic activity. Food Res. Int. 2020, 131, 108989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Tu, M.; Chen, H.; Xu, Z.; Wang, Z.; Liu, H.; Zhao, G.; Zhu, B.; Du, M. Identification and inhibitory activity against α-thrombin of a novel anticoagulant peptide derived from oyster (Crassostrea gigas) protein. Food Func. 2018, 9, 6391–6400. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef]
- Novoa, B.; Romero, A.; Álvarez, L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral activity of myticin C peptide from mussel: An ancient defense against herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [Green Version]
- Tejpal, C.S.; Vijayagopal, P.; Elavarasan, K.; Prabu, D.L.; Lekshmi, R.G.K.; Asha, K.K.; Anandan, R.; Chatterjee, N.S.; Mathew, S. Antioxidant, functional properties and amino acid composition of pepsin-derived protein hydrolysates from whole tilapia waste as influenced by pre-processing ice storage. J. Food Sci. Technol. 2017, 54, 4257–4267. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Gormley, T.R.; Neumann, T.; Fagan, J.D. Taurine content of raw and processed fish fillets/portions. Eur. Food Res. Technol. 2007, 225, 837–842. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, J.; Lin, Y. Taurine content in chinese food and daily taurine intake of chinese men. In Taurine 3; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1998; Volume 442, pp. 501–505. [Google Scholar] [CrossRef]
- Dragnes, B.T.; Larsen, R.; Ernstsen, M.H.; Mæhre, H.; Elvevoll, E.O. Impact of processing on the taurine content in processed seafood and their corresponding unprocessed raw materials. Int. J. Food Sci. Nutr. 2009, 60, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tørris, C.; Småstuen, M.C.; Molin, M. Nutrients in fish and possible associations with cardiovascular disease risk factors in metabolic syndrome. Nutrients 2018, 10, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Rizzolo, D.A.; Miro, A.; Gomis, R. Prevention of type 2 diabetes through sardines consumption: An Integrative review. Food Rev. Inter. 2021, 38, 1–19. [Google Scholar] [CrossRef]
- KP, A.D.; Martin, A. Recent insights into the molecular regulators and mechanisms of taurine to modulate lipid metabolism: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–13. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Kromhout, D.; Bosschieter, E.B.; Coulander de Lezenne, C. The inverse relation between fish consumption and 20-year mortality from coronary hearth disease. N. Engl. J. Med. 1985, 312, 1205–1209. [Google Scholar] [CrossRef]
- Phillipson, B.E.; Rothrock, D.W.; Connor, W.E.; Harris, W.S.; Illingworth, D.R. Reduction of plasma lipids, lipoproteins and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N. Engl. J. Med. 1985, 312, 1210–1216. [Google Scholar] [CrossRef]
- Herold, P.M.; Kinsella, J.E. Fish oil consumption and decreased risk of cardiovascular disease: A comparison of findings from animal and human feeding trials. Am. J. Clin. Nutr. 1986, 43, 566–598. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. N-3 Fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [Green Version]
- Khawaja, O.A.; Gaziano, J.M.; Djousse, L. N-3 fatty acids for prevention of cardiovascular disease. Curr. Atheroscler. Rep. 2014, 16, 450. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Morris, M.-C.; Bennett, D.A.; Berr, C.; Amouyel, P.; Dartigues, J.-F.; Tzourio, C.; Chasman, D.I.; Grodstein, F. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am. J. Epidemiol. 2018, 187, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Crivello, F.; Mazoyer, B.; Debette, S.; Tzourio, C.; Samieri, C. Fish intake and mri burden of cerebrovascular disease in older adults. Neurology 2021, 97, E2213–E2222. [Google Scholar] [CrossRef]
- Di Lena, G.; Nevigato, T.; Rampacci, M.; Casini, I.; Caproni, R.; Orban, E. Proximate composition and lipid profile of red mullet (Mullus barbatus) from two sites of the Tyrrhenian and Adriatic seas (Italy): A seasonal differentiation. J. Food Compos. Anal. 2016, 45, 121–129. [Google Scholar] [CrossRef]
- Prato, E.; Biandolino, F. Total lipid content and fatty acid composition of commercially important fish species from the Mediterranean, Mar Grande Sea. Food Chem. 2012, 131, 1233–1239. [Google Scholar] [CrossRef]
- SINU; 2014 SINU, Società Italiana di Nutrizione Umana. Livelli di Assunzione di Riferimento di Nutrienti ed Energia per la Popolazione Italiana. (IV Revisione); SICS (Società Italiana di Comunicazione Scientifica e Sanitaria): Milano, Italy, 2014. [Google Scholar]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Mattila, P.; Piironen, V.; Uusi-Rauva, E.; Koivistoinen, P. Cholecalciferol and 25-Hydroxycholecalciferol contents in fish and fish products. J. Food Compos. Anal. 1995, 8, 232–243. [Google Scholar] [CrossRef]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in Seafood and Aquaculture Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Fujisawa, A.; Hara, A.; Dunlap, W.C. An unusual vitamin E constituent (Alpha-tocomonoenol) provides enhanced antioxidant protection in marine organisms adapted to cold-water environments. Proc. Natl. Acad. Sci. USA 2001, 98, 13144–13148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zingg, J.M. Modulation of signal transduction by vitamin E. Mol. Asp. Med. 2007, 28, 481–506. [Google Scholar] [CrossRef] [PubMed]
- Theriault, A.; Jun-Tzu, C.; Qi, W. Abdul Gapor and Khosrow Adeli. Tocotrienol: A Review of Its Therapeutic Potential. Clin. Biochem. 1999, 32, 309–319. [Google Scholar] [CrossRef]
- Sen, C.K.L.; Khanna, S.L.; Roy, S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol. Asp. Med. 2007, 28, 692–728. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Parinandi, N.L.; Kotha, S.R.; Roy, S.; Rink, C.; Bibus, D.; Sen, C.K. Nanomolar vitamin E α-tocotrienol inhibits glutamate induced activation of phospholipase A2 and causes neuroprotection. J. Neurochem. 2010, 112, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Volume 5: Nutrition and Health; Springer: Basel, Switzerland, 2009. [Google Scholar]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef]
- De Jong, A.; Plat, J.; Bast, A.; Godschalk, R.W.L.; Basu., S.; Mensink., R.P. Effects of plant sterol and stanol ester consumption on lipid metabolism antioxidant status, and markers of oxidative stress, endothelial function, and low-grade inflammation in paitents on current statin treatment. Eur. J. Clin. Nutr. 2008, 62, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Talati, R.; Sobieraj, D.M.; Makanji, S.S.; Phung, O.J.; Coleman, C.I. The comparative efficacy of plant sterols and stanols on serum lipids: A systematic review and meta-analysis. J. Am. Diet. Assoc. 2010, 110, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Couvreur, P. Squalene: A natural triterpene for use in disease management and therapy. Adv. Drug Deliv. Rev. 2009, 61, 1412–1426. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Franco, F.; Martinho, F.; Carvalho, L.; Pereira, M.E.; Coelho, J.P.; Pardal, M.A. Essential mineral content variations in commercial marine species induced by ecological and taxonomical attributes. J. Food Compos. Anal. 2021, 103, 104118. [Google Scholar] [CrossRef]
- Zhang, Z.; Cogswell, M.E.; Gillespie, C.; Fang, J.; Loustalot, F.; Dai, S.; Carriquiry, A.L.; Kuklina, E.V.; Hong, Y.; Merritt, R.; et al. Association between usual sodium and potassium intake and blood pressure and hypertension among U.S. adults: NHANES 2005–2010. PLoS ONE 2013, 8, e75289. [Google Scholar] [CrossRef] [PubMed]
- Di Lena, G.; Casini, I.; Caproni, R.; Fusari, A.; Orban, E. Total mercury levels in commercial fish species from Italian fishery and aquaculture. Food Addit. Contam. Part B Surveill. 2017, 10, 118–127. [Google Scholar] [CrossRef]
- Sprague, M.; Chau, T.C.; Givens, D.I. Iodine Content of Wild and Farmed Seafood and Its Estimated Contribution to UK Dietary Iodine Intake. Nutrients 2022, 14, 195. [Google Scholar] [CrossRef]
- Guan, G.; Azad, A.K.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [Green Version]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a valuable biomolecule from seafood industry waste in the design of green food packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef]
- Aikawa, H. On the Summer Plankton in the Waters of the Western Aleutian Islands in 1928. Bull. Jap. Soc. Sci. Fish. 1932, 1, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Zulqurnain, S.S.; Sultana, T.; Mahboob, S. Fatty acid profile variations after exposure to textile industry effluents in Indian Major Carps [Variações do perfil de ácidos graxos após exposição a efluentes da indústria têxtil nas principais carpas indianas]. Braz. J. Biol. 2022, 84, e254252. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Wintersteen, K.A. The Fishmeal Revolution: The Industrialization of the Humboldt Current Ecosystem; University of California Press: Berkeley, CA, USA, 2021; pp. 1–225. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake responses to reduced nutrient loading-An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Moi, D.A.; Romero, G.Q.; Jeppesen, E.; Kratina, P.; Alves, D.C.; Antiqueira, P.A.P.; Teixeira de Mello, F.; Figueiredo, B.R.S.; Bonecker, C.C.; Pires, A.P.F.; et al. Regime shifts in a shallow lake over 12 years: Consequences for taxonomic and functional diversities, and ecosystem multifunctionality. J. Anim. Ecol. 2022, 91, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Shepon, A.; Makov, T.; Hamilton, H.A.; Müller, D.B.; Gephart, J.A.; Henriksson, P.J.; Troell, M.; Golden, C.D. Sustainable optimization of global aquatic omega-3 supply chain could substantially narrow the nutrient gap. Resour. Conserv. Recycl. 2022, 181, 106260. [Google Scholar] [CrossRef]
- Taylor, V.F.; Karagas, M.R. Exposure to arsenolipids and inorganic arsenic from marine-sourced dietary supplements. Chemosphere 2022, 296, 133930. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.-H.; Ye, C.; Chen, H.-S.; Xu, J.; Dong, X.-H.; Liu, X.-S.; Li, D. Effects of aquaculture on the shallow lake aquatic ecological environment of Lake Datong, China. Environ. Sci. Eur. 2022, 34, 19. [Google Scholar] [CrossRef]
- Besnard, L.; Duchatelet, L.; Bird, C.S.; Le Croizier, G.; Michel, L.; Pinte, N.; Lepoint, G.; Schaal, G.; Vieira, R.P.; Gonçalves, J.M.S.; et al. Diet consistency but large-scale isotopic variations in a deep-sea shark: The case of the velvet belly lantern shark, Etmopterus spinax, in the northeastern Atlantic region and Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2022, 182, 103708. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. Text mining and visualization using VOSviewer. ISSI Newsl. 2011, 7, 50–54. [Google Scholar]
- Waltman, L.; Van Eck, N.J.; Noyons, E.C. A unified approach to mapping and clustering of bibliometric networks. J. Inform. 2011, 4, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. Visualizing bibliometric networks. In Measuring Scholarly Impact: Methods and Practice; Ding, Y., Rousseau, R., Wolfram, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 285–320. [Google Scholar]
- Shalders, T.C.; Champion, C.; Coleman, M.A.; Benkendorff, K. The nutritional and sensory quality of seafood in a changing climate. Mar. Environ. Res. 2022, 176, 105590. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jayachandran, M.; Bai, W.; Xu, B. A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem. 2022, 369, 130874. [Google Scholar] [CrossRef] [PubMed]
- Koehn, J.Z.; Allison, E.H.; Villeda, K.; Chen, Z.; Nixon, M.; Crigler, E.; Zhao, L.; Chow, M.; Vaitla, B.; Thilsted, S.H.; et al. Fishing for health: Do the world’s national policies for fisheries and aquaculture align with those for nutrition? Fish Fish. 2022, 23, 125–142. [Google Scholar] [CrossRef]
- Meng, C.; Wang, K.; Xu, G. Metals in Ten Commercial Demersal Fish from the East China Sea: Contribution to Aquatic Products Nutrition and Toxic Risk Assessment. Biol Trace Elem Res 2022, 1–9. [Google Scholar] [CrossRef]
- Simmance, F.A.; Cohen, P.J.; Huchery, C.; Sutcliffe, S.; Suri, S.K.; Tezzo, X.; Thilsted, S.H.; Oosterveer, P.; McDougall, C.; Ahern, M.; et al. Nudging fisheries and aquaculture research towards food systems. Fish Fish. 2021, 23, 34–53. [Google Scholar] [CrossRef]
- Luten, J.B. Marine Functional Food; Wageningen Academic Publishers: Wageningen, The Netherlands, 2009; pp. 1–174. [Google Scholar]
- Lee, C.-S.; Lim, C.; Gatlin, D.M.; Webster, C.D. Dietary nutrients, additives, and fish health. Diet. Nutr. Addit. Fish Health. 2015, 1–355. [Google Scholar]
- Newton, P. Seaweeds: Biodiversity Environmental Chemistry and Ecological Impacts; Nova Science Pub.: Hauppauge, NY, USA, 2017; pp. 1–190. [Google Scholar]
- Tlusty, M.F.; Tyedmers, P.; Bailey, M.; Ziegler, F.; Henriksson, P.J.; Béné, C.; Bush, S.; Newton, R.; Asche, F.; Little, D.C.; et al. Reframing the sustainable seafood narrative. Glob. Environ. Chang. 2019, 59, 101991. [Google Scholar] [CrossRef]
- Tiwari, A.; Pritam, S.; Mishra, K.; Khan, M.; Upmanyu, N.; Ghosh, D. Nutraceuticals from marine bionetworks. Curr. Nutr. Food Sci. 2019, 15, 338–344. [Google Scholar] [CrossRef]
- Lucarini, M.; Zuorro, A.; Di Lena, G.; Lavecchia, R.; Durazzo, A.; Benedetti, B.; Lombardi-Boccia, G. Sustainable Management of Secondary Raw Materials from the Marine Food-Chain: A Case-Study Perspective. Sustainability 2020, 12, 8997. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Meenatchi, R.; Pachillu, K.; Bansal, S.; Brindangnanam, P.; Arockiaraj, J.; Kiran, G.S.; Selvin, J. Identification and characterization of the novel bioactive compounds from microalgae and cyanobacteria for pharmaceutical and nutraceutical applications. J. Basic Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Tan, X.; Zhang, Y.; Li, F.; Luo, P.; Liu, H. Molecular Targets and Related Biologic Activities of Fucoidan: A Review. Mar. Drugs 2020, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Gunasegavan, R.D.; Mustar, S.; Lee, J.C.; Mohd Noh, M.F. Isolation of Industrial Important Bioactive Compounds from Microalgae. Molecules 2021, 26, 943. [Google Scholar] [CrossRef]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.P.; Chew, K.W.; Ling, T.C. Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere 2022, 291 Pt 2, 132932. [Google Scholar] [CrossRef]
- Arora, K.; Kumar, P.; Bose, D.; Li, X.; Kulshrestha, S. Potential applications of algae in biochemical and bioenergy sector. 3 Biotech. 2021, 11, 296. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durazzo, A.; Di Lena, G.; Gabrielli, P.; Santini, A.; Lombardi-Boccia, G.; Lucarini, M. Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis. Fishes 2022, 7, 132. https://doi.org/10.3390/fishes7030132
Durazzo A, Di Lena G, Gabrielli P, Santini A, Lombardi-Boccia G, Lucarini M. Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis. Fishes. 2022; 7(3):132. https://doi.org/10.3390/fishes7030132
Chicago/Turabian StyleDurazzo, Alessandra, Gabriella Di Lena, Paolo Gabrielli, Antonello Santini, Ginevra Lombardi-Boccia, and Massimo Lucarini. 2022. "Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis" Fishes 7, no. 3: 132. https://doi.org/10.3390/fishes7030132
APA StyleDurazzo, A., Di Lena, G., Gabrielli, P., Santini, A., Lombardi-Boccia, G., & Lucarini, M. (2022). Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis. Fishes, 7(3), 132. https://doi.org/10.3390/fishes7030132








