Diel Vertical Habitat Use Observations of a Scalloped Hammerhead and a Bigeye Thresher in the Northern Gulf of Mexico
Abstract
:1. Introduction
2. Materials and Methods
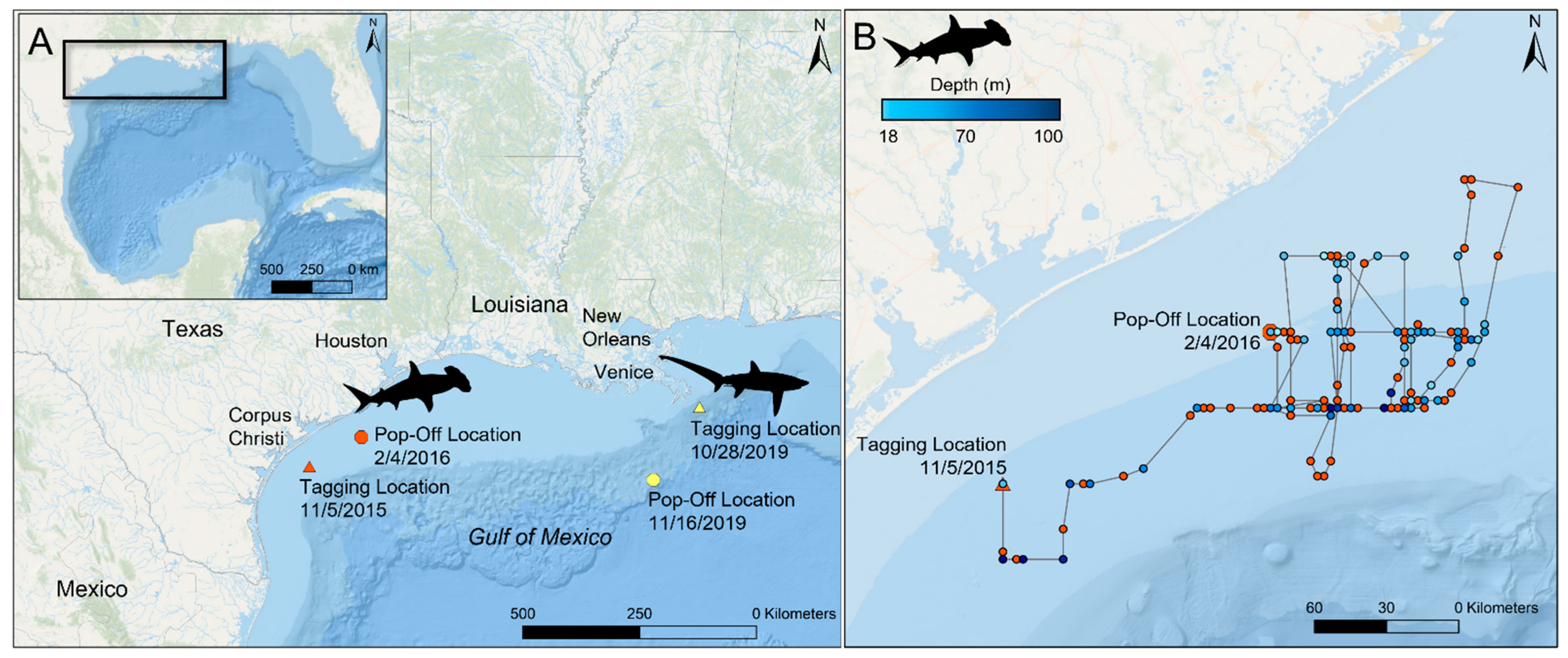
2.1. Scalloped Hammerhead Shark
2.2. Bigeye Thresher Shark
2.3. Data Analysis
3. Results and Discussion
3.1. Scalloped Hammerhead Shark
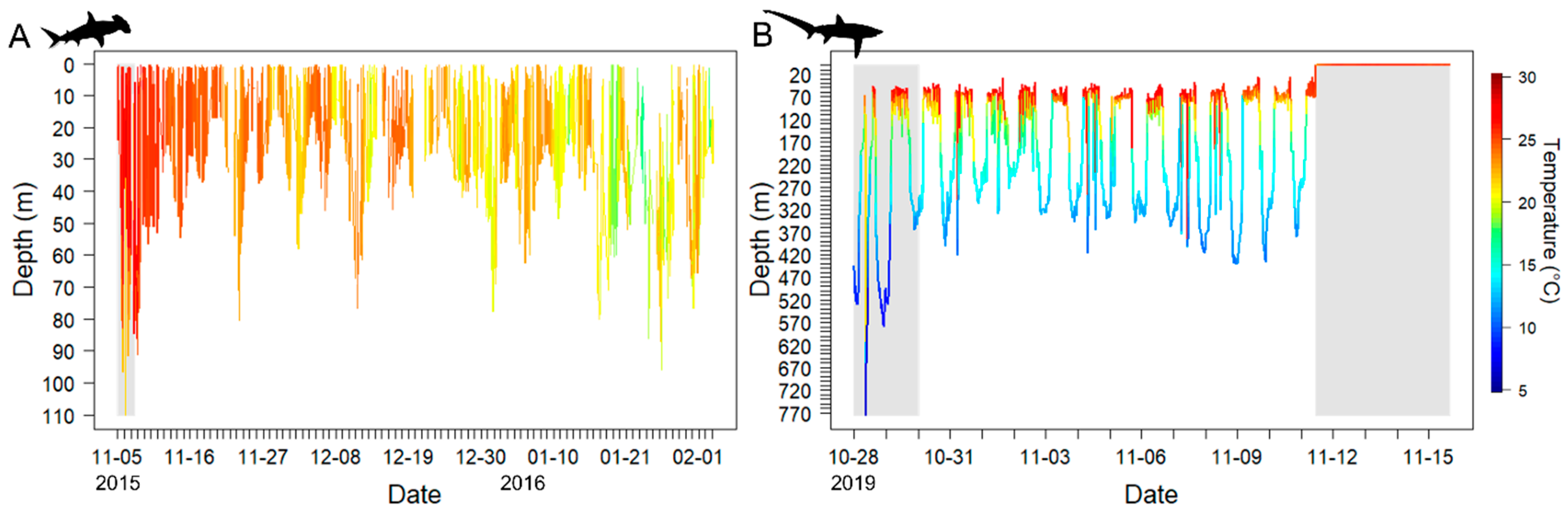
3.1.1. PSAT Tag Summary
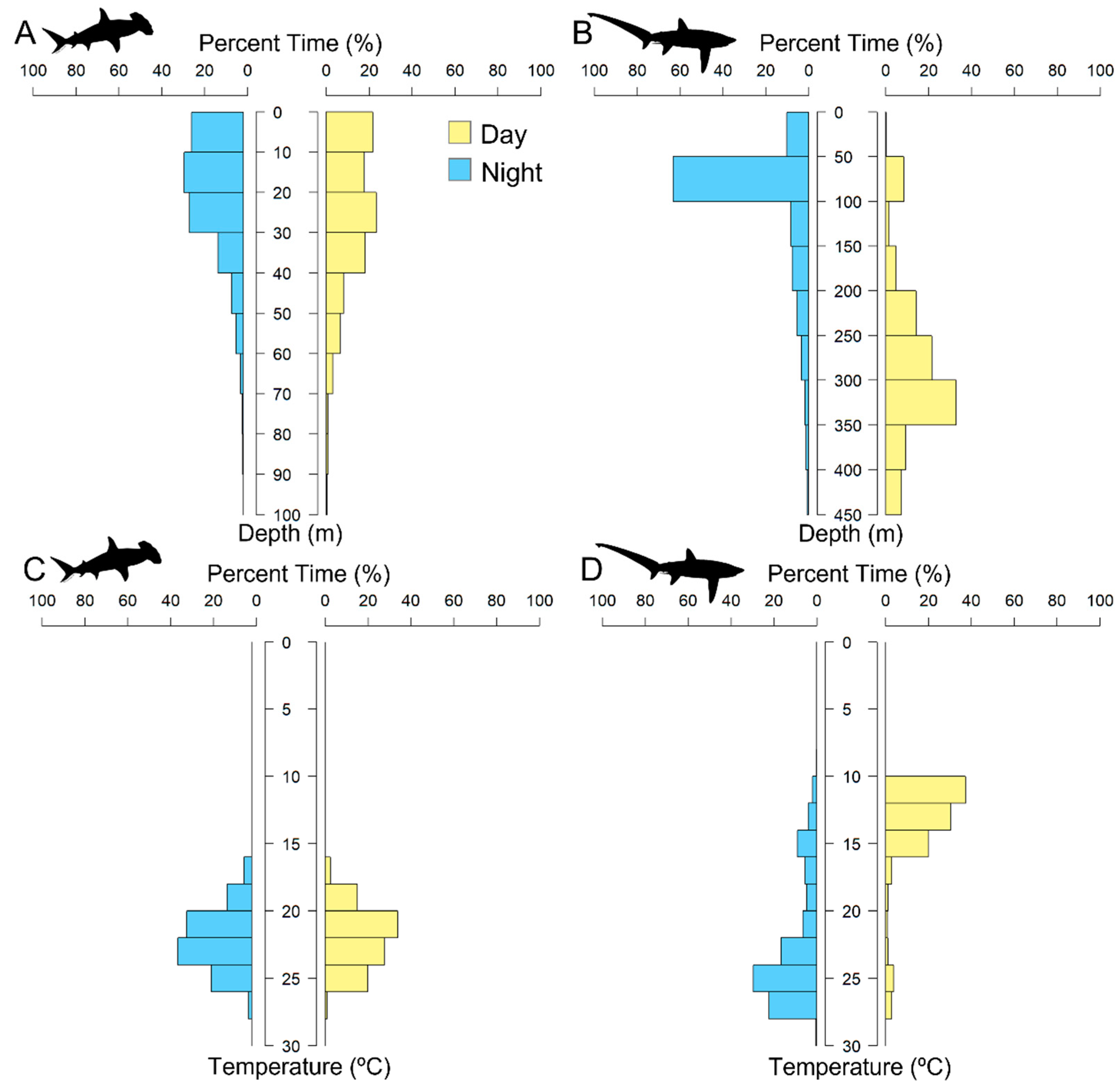
3.1.2. Scalloped Hammerhead Shark Discussion
3.2. Bigeye Thresher Shark
3.2.1. High-Rate PSAT X-Tag Summary
3.2.2. Bigeye Thresher Shark Discussion
3.3. Potential for Fishery Interactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, J.K.; Myers, R.A. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol. Lett. 2004, 7, 135–145. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Klimley, A.P. The biology and conservation status of the large hammerhead shark complex: The great, scalloped, and smooth hammerheads. Rev. Fish Biol. Fish. 2018, 28, 777–794. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.; de Young, B. Cascading effects of overfishing marine systems. Trends Ecol. Evolut. 2005, 20, 579–581. [Google Scholar] [CrossRef]
- Myers, R.A.; Baum, J.K.; Shepherd, T.D.; Powers, S.P.; Peterson, C.H. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 2007, 315, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Honig, M.; Ryan, P.; Underhill, L.; Compagno, L.J. Pelagic shark bycatch in the tuna-and swordfish-directed longline fishery off southern Africa. Afr. J. Mar. Sci. 2009, 31, 215–225. [Google Scholar] [CrossRef]
- Mucientes, G.R.; Queiroz, N.; Sousa, L.L.; Tarroso, P.; Sims, D.W. Sexual segregation of pelagic sharks and the potential threat from fisheries. Biol. Lett. 2009, 5, 156–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, A.; Simpfendorfer, C.; White, W.; Johnson, G.; McAuley, R.; Heupel, M. Crossing lines: A multidisciplinary framework for assessing connectivity of hammerhead sharks across jurisdictional boundaries. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Wells, R.J.D.; TinHan, T.C.; Dance, M.A.; Drymon, J.M.; Falterman, B.; Ajemian, M.J.; Stunz, G.W.; Mohan, J.A.; Hoffmayer, E.R.; Driggers III, W.B. Movement, Behavior and Habitat Use of a Marine Apex Predator, the Scalloped Hammerhead. Front. Mar. Sci. 2018, 5, 321. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, I.; Watanabe, Y.Y.; Papastamatiou, Y.P.; Sato, K.; Meyer, C.G. Yo-yo vertical movements suggest a foraging strategy for tiger sharks Galeocerdo cuvier. Mar. Ecol. Prog. Ser. 2011, 424, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Andrzejaczek, S.; Gleiss, A.C.; Pattiaratchi, C.B.; Meekan, M.G. Patterns and drivers of vertical movements of the large fishes of the epipelagic. Rev. Fish Biol. Fish. 2019, 29, 335–354. [Google Scholar] [CrossRef]
- Powers, S.P.; Fodrie, F.J.; Scyphers, S.B.; Drymon, J.M.; Shipp, R.L.; Stunz, G.W. Gulf-wide decreases in the size of large coastal sharks documented by generations of fishermen. Mar. Coast. Fish. 2013, 5, 93–102. [Google Scholar] [CrossRef] [Green Version]
- White, C.F.; Lyons, K.; Jorgensen, S.J.; O'Sullivan, J.; Winkler, C.; Weng, K.C.; Lowe, C.G. Quantifying habitat selection and variability in habitat suitability for juvenile white sharks. PLoS ONE. 2019, 14, e0214642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compagno, L.J. FAO species catalogue. Vol. 4. Sharks of the World. An annoted and illustrated catalogue of sharks species known to date. Part. 2. Carcharhiniformes. FAO Fish. Synop. 1984, 4, 125. [Google Scholar]
- Compagno, L.J. Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date; Food & Agriculture Org: Rome, Italy, 2001; Volume 2. [Google Scholar]
- Sepulveda, C.; Wang, M.; Aalbers, S. Post-release survivorship and movements of bigeye thresher sharks, Alopias superciliosus, following capture on deep-set buoy gear. Fish. Res. 2019, 219, 105312. [Google Scholar] [CrossRef]
- Aalbers, S.A.; Wang, M.; Villafana, C.; Sepulveda, C.A. Bigeye thresher shark Alopias superciliosus movements and post-release survivorship following capture on linked buoy gear. Fish. Res. 2021, 236, 105857. [Google Scholar] [CrossRef]
- Rigby, C.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.; Jabado, R.; Liu, K.; Marshall, A.; Pacoureau, N. Bigeye Thresher Shark (Alopias superciliousus) 2019. Available online: iucnredlist.org (accessed on 23 June 2022).
- Rigby, C.; Dulvy, N.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.; Herman, K.; Jabado, R.; Liu, K. Scalloped Hammerhead Shark (Sphyrna lewini) 2019. Available online: iucnredlist.org (accessed on 23 June 2022).
- Carlson, J.K.; Goldman, K.J.; Lowe, C.G. Metabolism, energetic demand, and endothermy. In Biology of Sharks and Their Relatives; CRC Press: Boca Raton, FL, USA, 2004; Volume 10, pp. 203–224. [Google Scholar]
- Afonso, A.S.; Hazin, F.H.; Carvalho, F.; Pacheco, J.C.; Hazin, H.; Kerstetter, D.W.; Murie, D.; Burgess, G.H. Fishing gear modifications to reduce elasmobranch mortality in pelagic and bottom longline fisheries off Northeast Brazil. Fish. Res. 2011, 108, 336–343. [Google Scholar] [CrossRef]
- Gulak, S.; de Ron Santiago, A.; Carlson, J. Hooking mortality of scalloped hammerhead Sphyrna lewini and great hammerhead Sphyrna mokarran sharks caught on bottom longlines. Afr. J. Mar. Sci. 2015, 37, 267–273. [Google Scholar] [CrossRef]
- Cortes, E.; Brown, C.A.; Beerhircher, L. Relative abundance of pelagic sharks in the western North Atlantic Ocean, including the Gulf of Mexico and Caribbean Sea. Gulf Caribb. Res. 2007, 19, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Camhi, M.D.; Valenti, S.V.; Fordham, S.V.; Fowler, S.L.; Gibson, C. The Conservation Status of Pelagic Sharks and Rays: Report of the IUCN Shark Specialist Group Pelagic Shark Red List Workshop; IUCN Species Survival Commission Shark Specialist Group: Newbury, UK, 2009; 78p. [Google Scholar]
- Piercy, A.N.; Carlson, J.K.; Sulikowski, J.A.; Burgess, G.H. Age and growth of the scalloped hammerhead shark, Sphyrna lewini, in the north-west Atlantic Ocean and Gulf of Mexico. Mar. Freshwater. Res. 2007, 58, 34–40. [Google Scholar] [CrossRef]
- Vaske Júnior, T.; Vooren, C.M.; Lessa, R.P. Feeding strategy of the night shark (Carcharhinus signatus) and scalloped hammerhead shark (Sphyrna lewini) near seamounts off northeastern Brazil. Braz. J. Oceanogr. 2009, 57, 97–104. [Google Scholar] [CrossRef]
- Bush, A. Diet and diel feeding periodicity of juvenile scalloped hammerhead sharks, Sphyrna lewini, in Kāne'ohe Bay, Ō'ahu, Hawai'i. Environ. Biol. Fishes. 2003, 67, 1–11. [Google Scholar] [CrossRef]
- Rojas, Y.E.T.; Osuna, F.P.; Herrera, A.H.; Magaña, F.G.; García, S.A.; Villalobos Ortíz, H.; Sampson, L. Feeding grounds of juvenile scalloped hammerhead sharks (Sphyrna lewini) in the south-eastern Gulf of California. Hydrobiologia. 2014, 726, 81–94. [Google Scholar] [CrossRef]
- Klimley, A.P.; Nelson, D.R. Diel movement patterns of the scalloped hammerhead shark (Sphyrna lewini) in relation to El Bajo Espiritu Santo: A refuging central-position social system. Behav. Ecol. Sociobiol. 1984, 15, 45–54. [Google Scholar] [CrossRef]
- Klimley, P.A.; Butler, S.; Nelson, D.R.; Stull, A.T. Diel movements of scalloped hammerhead sharks, Sphyrna lewini Griffith and Smith, to and from a seamount in the Gulf of California. J. Fish. Biol. 1988, 33, 751–761. [Google Scholar] [CrossRef]
- Klimley, P.A. Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar. Biol. 1993, 117, 1–22. [Google Scholar] [CrossRef]
- Bessudo, S.; Soler, G.A.; Klimley, P.A.; Ketchum, J.; Arauz, R.; Hearn, A.; Guzmán, A.; Calmettes, B. Vertical and horizontal movements of the scalloped hammerhead shark (Sphyrna lewini) around Malpelo and Cocos Islands (Tropical Eastern Pacific) using satellite telemetry. Boletín Investig. Mar. Costeras-INVEMAR 2011, 40, 91–106. [Google Scholar] [CrossRef]
- Jorgensen, S.J.; Klimley, P.A.; Muhlia-Melo, A. Scalloped hammerhead shark Sphyrna lewini, utilizes deep-water, hypoxic zone in the Gulf of California. J. Fish. Biol. 2009, 74, 1682–1687. [Google Scholar] [CrossRef]
- Hoffmayer, E.R.; Franks, J.S.; Driggers, W.B.; Howey, P.W. Diel vertical movements of a scalloped hammerhead, Sphyrna lewini, in the northern Gulf of Mexico. Bull. Mar. Sci. 2013, 89, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Spaet, J.L.; Lam, C.H.; Braun, C.D.; Berumen, M.L. Extensive use of mesopelagic waters by a Scalloped hammerhead shark (Sphyrna lewini) in the Red Sea. Anim. Biotelem 2017, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Snodgrass, D.J.; Orbesen, E.S.; Walter, J.F.; Hoolihan, J.P.; Brown, C.A. Potential impacts of oil production platforms and their function as fish aggregating devices on the biology of highly migratory fish species. Rev. Fish Biol. Fish. 2020, 30, 405–422. [Google Scholar] [CrossRef]
- Preti, A.; Kohin, S.; Dewar, H.; Ramon, D. Feeding habits of the bigeye thresher shark (Alopias superciliosus) sampled from the California-based drift gillnet fishery. CalCOFI Rep 2008, 49, 202–211. [Google Scholar]
- Coelho, R.; Fernandez-Carvalho, J.; Santos, M.N. Habitat use and diel vertical migration of bigeye thresher shark: Overlap with pelagic longline fishing gear. Mar. Environ. Res. 2015, 112, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Cortés, E.; Arocha, F.; Beerkircher, L.; Carvalho, F.; Domingo, A.; Heupel, M.; Holtzhausen, H.; Santos, M.N.; Ribera, M.; Simpfendorfer, C. Ecological risk assessment of pelagic sharks caught in Atlantic pelagic longline fisheries. Aquat. Liv. Resour. 2010, 23, 25–34. [Google Scholar] [CrossRef]
- Liu, K.-M.; Chiang, P.-J.; Chen, C.-T. Age and growth estimates of the bigeye thresher shark, Alopias superciliosus, in northeastern Taiwan waters. Fish. Bull. 1998, 96, 482–491. [Google Scholar]
- Weng, K.C.; Block, B.A. Diel vertical migration of the bigeye thresher shark (Alopias superciliosus), a species possessing orbital retia mirabilia. Fishery Bulletin 2004, 102, 221–229. [Google Scholar]
- Musyl, M.K.; Brill, R.; Curran, D.S.; Fragoso, N.M.; McNaughton, L.; Nielsen, A.; Kikkawa, B.S.; Moyes, C.D. Postrelease survival, vertical and horizontal movements, and thermal habitats of five species of pelagic sharks in the central Pacific Ocean. Fishery Bulletin 2011, 109, 341. [Google Scholar]
- Carlson, J.K.; Gulak, S. Habitat use and movement patterns of oceanic whitetip, bigeye thresher and dusky sharks based on archival satellite tags. Collect. Vol. Sci. Pap. ICCAT 2012, 68, 1922–1932. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Thieurmel, B.; Elmarhraoui, A.; Thieurmel, M.B. Package “Suncalc”. Available online: https://cran.r-project.org/web/packages/suncalc/suncalc.pdf (accessed on 1 December 2019).
- Hoolihan, J.P.; Luo, J.; Abascal, F.J.; Campana, S.E.; De Metrio, G.; Dewar, H.; Domeier, M.L.; Howey, L.A.; Lutcavage, M.E.; Musyl, M.K. Evaluating post-release behaviour modification in large pelagic fish deployed with pop-up satellite archival tags. ICES J. Mar. Sci. 2011, 68, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Arostegui, M.; Gaube, P.; Berumen, M.L.; DiGiulian, A.; Jones, B.; Røstad, A.; Braun, C. Vertical movements of a pelagic thresher shark (Alopias pelagicus): Insights into the species’ physiological limitations and trophic ecology in the Red Sea. End. Spec. Res. 2020, 43, 387–394. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rex, P.T.; Maloney, K.; Johnston, M.; Verbeck, D.; Allen, N.; Holland, K. Observations of a species-record deep-dive by a central Pacific female scalloped hammerhead shark (Sphyrna lewini). J. Fish. Biol. 2022; early view. [Google Scholar] [CrossRef]
- Royer, M.; Maloney, K.; Meyer, C.; Cardona, E.; Payne, N.; Whittingham, K.; Silva, G.; Holland, K. Scalloped hammerhead sharks swim on their side with diel shifts in roll magnitude and periodicity. Anim. Biotelem 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Royer, M.A. Thermoregulation Strategies of Deep Diving Ectothermic Sharks; University of Hawai'i at Manoa: Honolulu, HI, USA, 2020. [Google Scholar]
- Nakano, H.; Matsunaga, H.; Okamoto, H.; Okazaki, M. Acoustic tracking of bigeye thresher shark Alopias superciliosus in the eastern Pacific Ocean. Mar. Ecol. Prog. Ser. 2003, 265, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Stoehr, A.A.; Donley, J.M.; Aalbers, S.A.; Syme, D.A.; Sepulveda, C.; Bernal, D. Thermal effects on red muscle contractile performance in deep-diving, large-bodied fishes. Fish. Physiol. Biochem. 2020, 46, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Orbesen, E.S.; Snodgrass, D.; Shideler, G.S.; Brown, C.A.; Walter, J.F. Diurnal patterns in Gulf of Mexico epipelagic predator interactions with pelagic longline gear: Implications for target species catch rates and bycatch mitigation. Bull. Mar. Sci. 2017, 93, 573–589. [Google Scholar] [CrossRef]
- Calich, H.; Estevanez, M.; Hammerschlag, N. Overlap between highly suitable habitats and longline gear management areas reveals vulnerable and protected regions for highly migratory sharks. Mar. Ecol. Prog. Ser. 2018, 602, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.S.; Cudney, J.L.; Blankinship, D.R. Characteristics and trends in the nighttime and daytime United States Atlantic recreational swordfish fishery based on fishery-dependent data. Bull. Mar. Sci. 2017, 93, 539–555. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, T.; Meese, E.N.; Drymon, J.M.; Stunz, G.W.; Falterman, B.; Menjivar, E.; Wells, R.J.D. Diel Vertical Habitat Use Observations of a Scalloped Hammerhead and a Bigeye Thresher in the Northern Gulf of Mexico. Fishes 2022, 7, 148. https://doi.org/10.3390/fishes7040148
Anderson T, Meese EN, Drymon JM, Stunz GW, Falterman B, Menjivar E, Wells RJD. Diel Vertical Habitat Use Observations of a Scalloped Hammerhead and a Bigeye Thresher in the Northern Gulf of Mexico. Fishes. 2022; 7(4):148. https://doi.org/10.3390/fishes7040148
Chicago/Turabian StyleAnderson, Taylor, Emily N. Meese, James Marcus Drymon, Gregory W. Stunz, Brett Falterman, Elias Menjivar, and R. J. David Wells. 2022. "Diel Vertical Habitat Use Observations of a Scalloped Hammerhead and a Bigeye Thresher in the Northern Gulf of Mexico" Fishes 7, no. 4: 148. https://doi.org/10.3390/fishes7040148
APA StyleAnderson, T., Meese, E. N., Drymon, J. M., Stunz, G. W., Falterman, B., Menjivar, E., & Wells, R. J. D. (2022). Diel Vertical Habitat Use Observations of a Scalloped Hammerhead and a Bigeye Thresher in the Northern Gulf of Mexico. Fishes, 7(4), 148. https://doi.org/10.3390/fishes7040148







