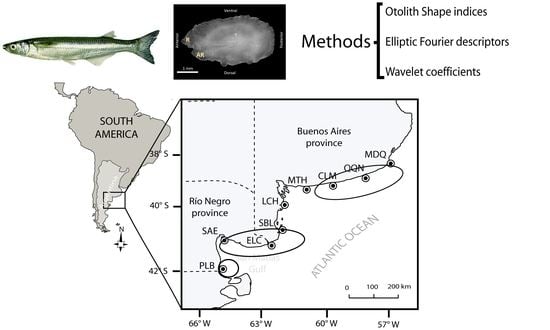
Unravelling Stock Spatial Structure of Silverside Odontesthes argentinensis (Valenciennes, 1835) from the North Argentinian Coast by Otoliths Shape Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Shape Analysis
2.4. Morphometric Analysis
2.5. Multivariate Analysis
3. Results
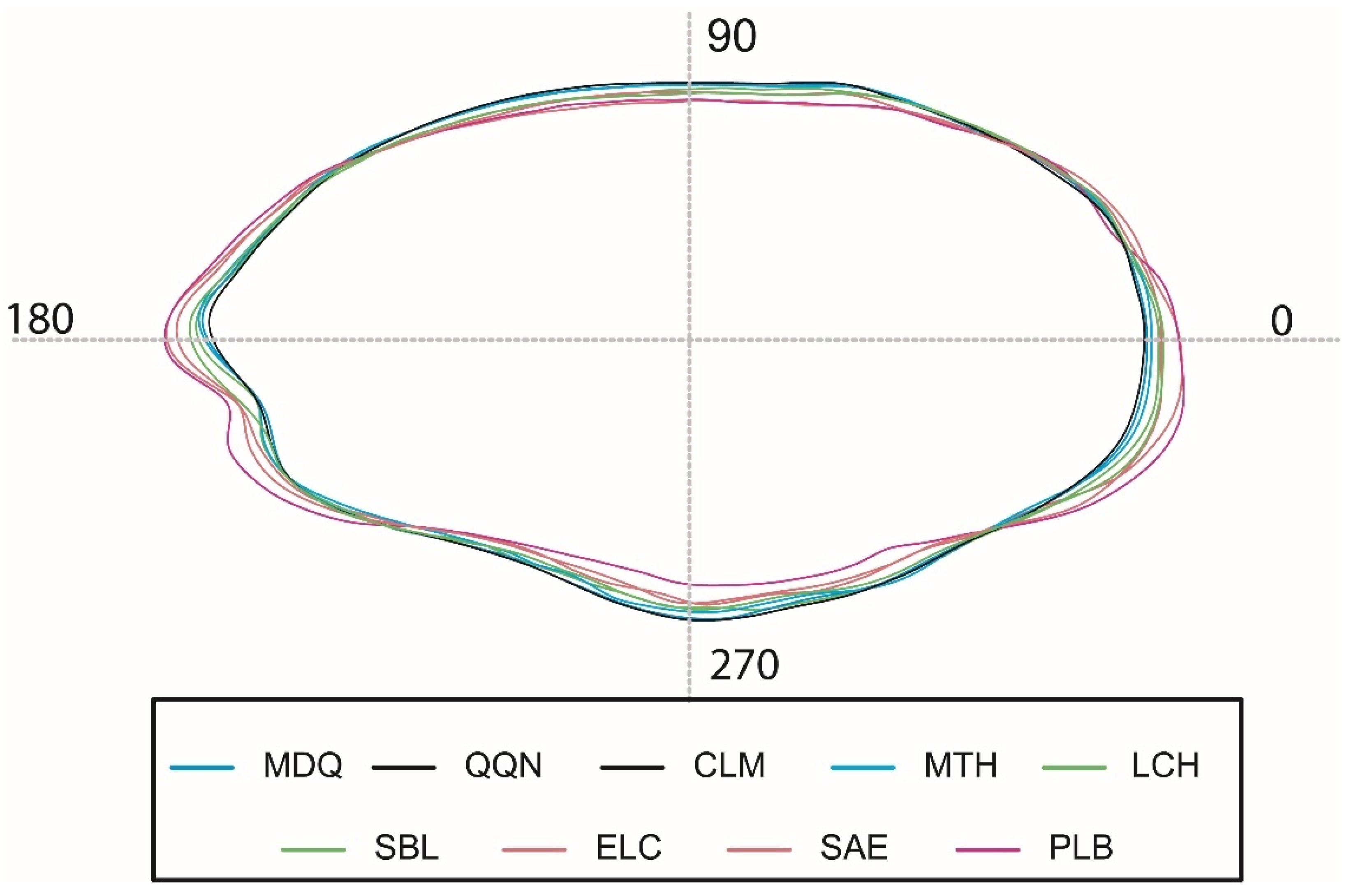
3.1. Mean Shape Features
3.2. Shape Indices
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carr, M.H.; Robinson, S.P.; Wahle, C.; Davis, G.; Kroll, S.; Murray, S.; Schumacker, E.J.; Williams, M. The Central Importance of Ecological Spatial Connectivity to Effective Coastal Marine Protected Areas and to Meeting the Challenges of Climate Change in the Marine Environment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 6–29. [Google Scholar] [CrossRef] [Green Version]
- Dizon, A.E.; Lockyer, C.; Perrin, W.F.; Demaster, D.P.; Sisson, J. Rethinking the Stock Concept: A Phylogeographic Approach. Conser. Biol. 1992, 6, 24–36. [Google Scholar] [CrossRef]
- Begg, G.A.; Waldman, J.R. An Holistic Approach to Fish Stock Identification. Fish. Res. 1999, 43, 35–44. [Google Scholar] [CrossRef]
- Stephenson, R.L. Stock Complexity in Fisheries Management: A Perspective of Emerging Issues Related to Population Sub-Units. Fish. Res. 1999, 43, 247–249. [Google Scholar] [CrossRef]
- Kerr, L.A.; Hintzen, N.T.; Cadrin, S.X.; Clausen, L.W.; Dickey-Collas, M.; Goethel, D.R.; Hatfield, E.M.C.; Kritzer, J.P.; Nash, R.D.M. Lessons Learned from Practical Approaches to Reconcile Mismatches between Biological Population Structure and Stock Units of Marine Fish. ICES J. Mar. Sci. 2017, 74, 1708–1722. [Google Scholar] [CrossRef] [Green Version]
- Côté, C.L.; Gagnaire, P.A.; Bourret, V.; Verreault, G.; Castonguay, M.; Bernatchez, L. Population Genetics of the American Eel (Anguilla rostrata): FST = 0 and North Atlantic Oscillation Effects on Demographic Fluctuations of a Panmictic Species. Mol. Ecol. 2013, 22, 1763–1776. [Google Scholar] [CrossRef] [PubMed]
- Abaunza, P.; Murta, A.G.; Campbell, N.; Cimmaruta, R.; Comesaña, A.S.; Dahle, G.; García Santamaría, M.T.; Gordo, L.S.; Iversen, S.A.; MacKenzie, K.; et al. Stock Identity of Horse Mackerel (Trachurus trachurus) in the Northeast Atlantic and Mediterranean Sea: Integrating the Results from Different Stock Identification Approaches. Fish. Res. 2008, 89, 196–209. [Google Scholar] [CrossRef]
- Cadrin, S.X.; Bernreuther, M.; Daníelsdóttir, A.K.; Hjörleifsson, E.; Johansen, T.; Kerr, L.; Kristinsson, K.; Mariani, S.; Nedreaas, K.; Pampoulie, C.; et al. Population Structure of Beaked Redfish, Sebastes mentella: Evidence of Divergence Associated with Different Habitats. ICES J. Mar. Sci. 2010, 67, 1617–1630. [Google Scholar] [CrossRef] [Green Version]
- Smedbol, R.K.; Wroblewski, J.S. Metapopulation Theory and Northern Cod Population Structure: Interdependency of Subpopulations in Recovery of a Groundfish Population. Fish. Res. 2002, 55, 161–174. [Google Scholar] [CrossRef]
- Park, L.; Moran, P. Developments in Molecular Genetic Techniques in Fisheries. Rev. Fish Biol. Fish. 1994, 4, 272–299. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Fernández Cirelli, A. Otolith Chemical Composition as a Useful Tool for Sciaenid Stock Discrimination in the South-Western Atlantic. Sci. Mar. 2006, 70, 325–334. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and Composition of Fish Otoliths: Pathways, Mechanisms and Applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef] [Green Version]
- Tuset, V.M.; Lozano, I.J.; Gonzĺez, J.A.; Pertusa, J.F.; García-Díaz, M.M. Shape Indices to Identify Regional Differences in Otolith Morphology of Comber, Serranus cabrilla (L., 1758). J. Appl. Ichthyol. 2003, 19, 88–93. [Google Scholar] [CrossRef]
- Avigliano, E.; Martinez, C.F.R.; Volpedo, A.V. Combined Use of Otolith Microchemistry and Morphometry as Indicators of the Habitat of the Silverside (Odontesthes bonariensis) in a Freshwater-Estuarine Environment. Fish. Res. 2014, 149, 55–60. [Google Scholar] [CrossRef]
- Biolé, F.G.; Thompson, G.A.; Vargas, C.V.; Leisen, M.; Barra, F.; Volpedo, A.V.; Avigliano, E. Fish Stocks of Urophycis brasiliensis Revealed by Otolith Fingerprint and Shape in the Southwestern Atlantic Ocean. Estuar. Coast. Shelf Sci. 2019, 229, 106406. [Google Scholar] [CrossRef]
- Tracey, S.R.; Lyle, J.M.; Duhamel, G. Application of Elliptical Fourier Analysis of Otolith Form as a Tool for Stock Identification. Fish. Res. 2006, 77, 138–147. [Google Scholar] [CrossRef]
- de Carvalho, B.M.; Volpedo, A.V.; Fávaro, L.F. Ontogenetic and Sexual Variation in the Sagitta Otolith of Menticirrhus americanus (Teleostei; Sciaenidae) (Linnaeus, 1758) in a Subtropical Environment. Pap. Avulsos Zool. 2020, 60, 1–12. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, K.M.; Horn, M.H. Phenotypic Variation among Populations of Atherinops affinis (Atherinopsidae) with Insights from a Geometric Morphometric Analysis. J. Fish Biol. 2004, 64, 1117–1135. [Google Scholar] [CrossRef]
- Tombari, A.D.; Gosztonyi, A.E.; Echeverria, D.D.; Volpedo, A.V. Otolith and Vertebral Morphology of Marine Atherinid Species (Atheriniformes, Atherinopsidae) Coexisting in the Southwestern Atlantic Ocean. Ciencias Mar. 2010, 36, 213–223. [Google Scholar] [CrossRef]
- Lattuca, M.E.; Lozano, I.E.; Brown, D.R.; Renzi, M.; Luizon, C.A. Natural Growth, Otolith Shape and Diet Analyses of Odontesthes nigricans Richardson (Atherinopsidae) from Southern Patagonia. Estuar. Coast. Shelf Sci. 2015, 166, 105–114. [Google Scholar] [CrossRef]
- Thompson, G.A.; Volpedo, A.V. Diet Composition and Feeding Strategy of the New World Silverside Odontesthes argentinensis in a Temperate Coastal Area (South America). Mar. Coast. Fish. 2018, 10, 80–88. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Akaba, T.; Ijima, K.; Yamaguchi, K.; Yoshizaki, G.; Takashima, F. Spontaneous Hybridization in the Laboratory and Genetic Markers for the Identification of Hybrids between Two Atherinid Species, Odontesthes bonariensis (Valenciennes, 1835) and Patagonina hatcheri (Eigenmann, 1909). Aquac. Res. 1997, 28, 291–300. [Google Scholar] [CrossRef]
- Conte-Grand, C.; Sommer, J.; Ortí, G.; Cussac, V. Populations of Odontesthes (Teleostei: Atheriniformes) in the Andean Region of Southern South America: Body Shape and Hybrid Individuals. Neotrop. Ichthyol. 2015, 13, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Colautti, D.C.; Miranda, L.; Gonzalez-Castro, M.; Villanova, V.; Strüssmann, C.A.; Mancini, M.; Maiztegui, T.; Berasain, G.; Hattori, R.; Grosman, F.; et al. Evidence of a Landlocked Reproducing Population of the Marine Pejerrey Odontesthes argentinensis (Actinopterygii; Atherinopsidae). J. Fish Biol. 2020, 96, 202–216. [Google Scholar] [CrossRef]
- Avigliano, E.; Miller, N.; Volpedo, A.V. Silversides (Odontesthes bonariensis) Reside within Freshwater and Estuarine Habitats, Not Marine Environments. Estuar. Coast. Shelf Sci. 2018, 205, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Levy, E.; Canel, D.; Rossin, M.A.; Hernández-Orts, J.S.; González-Castro, M.; Timi, J.T. Parasites as Indicators of Fish Population Structure at Two Different Geographical Scales in Contrasting Coastal Environments of the South-Western Atlantic. Estuar. Coast. Shelf Sci. 2019, 229, 106400. [Google Scholar] [CrossRef]
- Dyer, B.S. Systematic Revision of the South American Silversides (Teleostei, Atheriniformes). Biocell 2006, 30, 69–88. [Google Scholar]
- Di Dario, F.; Macedo dos Santos, V.L.; Maia de Souza Pereira, M. Range Extension of Odontesthes argentinensis (Valenciennes, 1835) (Teleostei: Atherinopsidae) in the Southwestern Atlantic, with Additional Records in the Rio de Janeiro State, Brazil. J. Appl. Ichthyol. 2014, 30, 421–423. [Google Scholar] [CrossRef]
- Levy, E.; Canel, D.; Rossin, M.A.; González-Castro, M.; Timi, J.T. Parasite Assemblages as Indicators of an Incipient Speciation Process of Odontesthes argentinensis in an Estuarine Environment. Estuar. Coast. Shelf Sci. 2021, 250, 107168. [Google Scholar] [CrossRef]
- De Buen, F. Los Pejerreyes (Familia Atherinidae) En La Fauna Uruguaya, Con Descripción de Nuevas Especies. Bol. do Inst. Ocean. 1953, 4, 3–80. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, L.A. Production of “Pejerrey” Odontesthes argentinensis Fingerlings: A Review of Current Techniques. Biocell 2006, 30, 121–123. [Google Scholar] [PubMed]
- Llompart, F.M.; Colautti, D.C.; Maiztegui, T.; Cruz-Jiménez, A.M.; Baigún, C.R.M. Biological Traits and Growth Patterns of Pejerrey Odontesthes argentinensis. J. Fish Biol. 2013, 82, 458–474. [Google Scholar] [CrossRef]
- Llompart, F.M.; Colautti, D.C.; Baigún, C.R.M. Conciliating Artisanal and Recreational Fisheries in Anegada Bay, Argentina. Fish. Res. 2017, 190, 140–149. [Google Scholar] [CrossRef]
- Guidi, C.; Baigún, C.R.M.; Ginter, L.G.; Soricetti, M.; Rivas, F.J.G.; Morawicki, S.; Quezada, F.; Bazzani, J.L.; Solimano, P.J. Characteristics, Preferences and Perceptions of Recreational Fishers in Northern Patagonia, Argentina. Reg. Stud. Mar. Sci. 2021, 45, 101828. [Google Scholar] [CrossRef]
- Llompart, F.M.; Colautti, D.C.; Baign, C.R.M. Assessment of a Major Shore-Based Marine Recreational Fishery in the Southwest Atlantic, Argentina. N. Z. J. Mar. Freshw. Res. 2012, 46, 57–70. [Google Scholar] [CrossRef]
- Tombari, A.D.; Volpedo, A.V.; Echeverria, D.D. Desarrollo de La Sagitta En Juveniles y Adultos de Odontesthes argentinensis (Valenciennes, 1835) y O. bonariensis (Valenciennes, 1835) de La Provincia de Buenos Aires, Argentina (Teleostei: Atheriniformes). Rev. Chil. Hist. Nat. 2005, 78, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Biolé, F.G.; Callicó Fortunato, R.; Thompson, G.A.; Volpedo, A.V. Application of Otolith Morphometry for the Study of Ontogenetic Variations of Odontesthes argentinensis. Environ. Biol. Fishes 2019, 102, 1301–1310. [Google Scholar] [CrossRef]
- Kjerfve, B.; Perillo, G.M.E.; Gardner, L.R.; Rine, J.M.; Dias, G.T.M.; Mochel, F.R. Morphodynamics of Muddy Environments along the Atlantic Coasts of North and South America. In Muddy Coasts of the World: Processes, Deposits and Function; Healy, T., Wang, Y., Healy, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Acha, E.; Orduna, M.; Rodrigues, K.; Militelli, M.; Braverman, M. Caracterización de La Zona de “El Rincón” (Provincia de Buenos Aires) Como Área de Reproducción de Peces Costeros. Rev. Invest. Desarr. Pesq. 2012, 21, 31–43. [Google Scholar]
- Piola, A.R.; Rivas, A.L. Corrientes En La Plataforma Continental. El Mar Argentino y sus Recur. Pesq. 1997, 1, 119–132. [Google Scholar]
- Mancini, M.; Grosman, F.; Dyer, B.; García, G.; Ponti, O.; Sanzano, P.; Salinas, V. Pejerreyes Del Sur de América: Aportes Al Estado de Conocimiento Con Especial Referencia a Odontesthes Bonariensis; UniRío Editora: Río Cuarto, Argentina, 2016; ISBN 9789876881968. [Google Scholar]
- Libungan, L.A.; Pálsson, S. ShapeR: An R Package to Study Otolith Shape Variation among Fish Populations. PLoS ONE 2015, 10, e0121102. [Google Scholar] [CrossRef]
- John Haines, A.; Crampton, J.S. Improvements to the Method of Fourier Shape Analysis as Applied in Morphometric Studies. Palaeontology 2000, 43, 765–783. [Google Scholar] [CrossRef]
- Nason, G. Wavethresh: Wavelets Statistics and Transforms, R Package. 2016. Available online: https://CRAN.R-project.org/package=wavethresh (accessed on 25 December 2021).
- Longmore, C.; Fogarty, K.; Neat, F.; Brophy, D.; Trueman, C.; Milton, A.; Mariani, S. A Comparison of Otolith Microchemistry and Otolith Shape Analysis for the Study of Spatial Variation in a Deep-Sea Teleost, Coryphaenoides rupestris. Environ. Biol. Fishes 2010, 89, 591–605. [Google Scholar] [CrossRef]
- Agüera, A.; Brophy, D. Use of Saggital Otolith Shape Analysis to Discriminate Northeast Atlantic and Western Mediterranean Stocks of Atlantic Saury, Scomberesox saurus saurus (Walbaum). Fish. Res. 2011, 110, 465–471. [Google Scholar] [CrossRef]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.H.A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schwartz, M.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package Version 2.13.0. 2014. Available online: https://CRAN.R-project.org/package=gplots (accessed on 25 December 2021).
- Volpedo, A.V.; Vaz-dos-Santos, A.M. Métodos de Estudios Con Otolitos: Principios y Aplicaciones; INPA CONICET UBA: Ciudad Autónoma de Buenos Aires, Argentina, 2015. [Google Scholar]
- Tuset, V.M.; Lombarte, A.; Assis, C.A. Otolith Atlas for the Western Mediterranean, North and Central Eastern Atlantic. Sci. Mar. 2008, 72, 7–198. [Google Scholar] [CrossRef]
- Lleonart, J.; Salat, J.; Torres, G.J. Removing Allometric Effects of Body Size in Morphological Analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Package ‘MASS’: Support Functions and Datasets for Venables and Ripley’s “Modern Applied Statistics with S”. 2021. Available online: https://CRAN.R-project.org/package=MASS (accessed on 23 November 2021).
- Neves, J.; Silva, A.A.; Moreno, A.; Veríssimo, A.; Santos, A.M.; Garrido, S. Population Structure of the European Sardine Sardina pilchardus from Atlantic and Mediterranean Waters Based on Otolith Shape Analysis. Fish. Res. 2021, 243, 106050. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. ISBN 9781118445112. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B. Vegan: Community Ecology Package, Version 2.0–7. R Package. 2021. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 December 2021).
- Peters, A.; Hothorn, T.; Ripley, B.D.; Therneau, T.; Atkinson, B. Ipred: Improved Predictors. R Package. 2021. Available online: https://CRAN.R-project.org/package=ipred (accessed on 25 December 2021).
- Donelson, J.M.; Sunday, J.M.; Figueira, W.F.; Gaitán-Espitia, J.D.; Hobday, A.J.; Johnson, C.R.; Leis, J.M.; Ling, S.D.; Marshall, D.; Pandolfi, J.M.; et al. Understanding Interactions between Plasticity, Adaptation and Range Shifts in Response to Marine Environmental Change. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180186. [Google Scholar] [CrossRef] [Green Version]
- Callicó Fortunato, R.; Benedito Durà, V.; Volpedo, A.V. Otolith Morphometry and Microchemistry as Habitat Markers for Juvenile Mugil cephalus Linnaeus 1758 in Nursery Grounds in the Valencian Community, Spain. J. Appl. Ichthyol. 2017, 33, 163–167. [Google Scholar] [CrossRef]
- Callicó Fortunato, R.; González-Castro, M.; Reguera Galán, A.; García Alonso, I.; Kunert, C.; Benedito Durà, V.; Volpedo, A. Identification of Potential Fish Stocks and Lifetime Movement Patterns of Mugil liza Valenciennes 1836 in the Southwestern Atlantic Ocean. Fish. Res. 2017, 193, 164–172. [Google Scholar] [CrossRef]
- Thompson, G.; Callico Fortunato, R.; Chiesa, I.; Volpedo, A. Trophic Ecology of Mugil liza at the Southern Limit of Its Distribution (Buenos Aires, Argentina). Brazilian J. Oceanogr. 2015, 63, 271–278. [Google Scholar] [CrossRef]
- González-Castro, M.; Rosso, J.J.; Delpiani, S.M.; Mabragaña, E.; Díaz de Astarloa, J.M. Inferring Boundaries among Fish Species of the New World Silversides (Atherinopsidae; Genus Odontesthes): New Evidences of Incipient Speciation between Marine and Brackish Populations of Odontesthes argentinensis. Genetica 2019, 147, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Di Giácomo, E.E.; Calvo, J.; Perier, M.R.; Morriconi, E. Spawning Aggregations of Merluccius hubbsi, in Patagonian Waters: Evidence for a Single Stock? Fish. Res. 1993, 16, 9–16. [Google Scholar] [CrossRef]
- Sardella, N.H.; Timi, J.T. Parasites of Argentine Hake in the Argentine Sea: Population and Infracommunity Structure as Evidence for Host Stock Discrimination. J. Fish Biol. 2004, 65, 1472–1488. [Google Scholar] [CrossRef]
- Deville, D.; Sanchez, G.; Barahona, S.; Yamashiro, C.; Oré-Chávez, D.; Bazán, R.Q.; Umino, T. Morphological Variation of the Sea Silverside Odontesthes regia in Regions with Dissimilar Upwelling Intensity along the Humboldt Current System. Ocean Sci. J. 2020, 55, 33–48. [Google Scholar] [CrossRef]
- D’Anatro, A.; González-Bergonzoni, I.; Vidal, N.; Tesitore, G.; de Mello, F.T. Confirmation of the Occurrence of Odontesthes argentinensis (Valenciennes, 1835) (Atheriniformes, Atherinopsidae) in the Rio Uruguay. Panam. J. Aquat. Sci. 2020, 15, 100–105. [Google Scholar]
- Hughes, L.C.; Cardoso, Y.P.; Sommer, J.A.; Cifuentes, R.; Cuello, M.; Somoza, G.M.; González-Castro, M.; Malabarba, L.R.; Cussac, V.; Habit, E.M.; et al. Biogeography, Habitat Transitions and Hybridization in a Radiation of South American Silverside Fishes Revealed by Mitochondrial and Genomic RAD Data. Mol. Ecol. 2020, 29, 738–751. [Google Scholar] [CrossRef]
- Heras, S.; Roldán, M.I. Phylogenetic Inference in Odontesthes and Atherina (Teleostei: Atheriniformes) with Insights into Ecological Adaptation. C. R.—Biol. 2011, 334, 273–281. [Google Scholar] [CrossRef]
- Volpedo, A.V.; Miretzky, P.; Fernández-Cirelli, A. Stocks Pesqueros de Cynoscion guatucupa y Micropogonias furnieri (Pisces, Sciaenidae ), En La Costa Atlántica de Sudamérica: Comparación Entre Métodos de Identificación. Mem. De La Fund. La Salle De Cienc. Nat. 2007, 2007, 115–130. [Google Scholar]
- Tuset, V.M.; Farré, M.; Otero-Ferrer, J.L.; Vilar, A.; Morales-Nin, B.; Lombarte, A. Testing Otolith Morphology for Measuring Marine Fish Biodiversity. Mar. Freshw. Res. 2016, 67, 1037–1048. [Google Scholar] [CrossRef] [Green Version]
- Tuset, V.M.; Otero-Ferrer, J.L.; Siliprandi, C.; Manjabacas, A.; Marti-Puig, P.; Lombarte, A. Paradox of Otolith Shape Indices: Routine but Overestimated Use. Can. J. Fish. Aquat. Sci. 2021, 78, 681–692. [Google Scholar] [CrossRef]
- Volpedo, A.; Echeverría, D.D. Ecomorphological Patterns of the Sagitta in Fish on the Continental Shelf off Argentine. Fish. Res. 2003, 60, 551–560. [Google Scholar] [CrossRef]
- De Carvalho, B.M.; Spach, H.L.; Vaz-Dos-Santos, A.M.; Volpedo, A.V. Otolith Shape Index: Is It a Tool for Trophic Ecology Studies? J. Mar. Biol. Assoc. U. K. 2019, 99, 1675–1682. [Google Scholar] [CrossRef]
- Assis, I.O.; da Silva, V.E.L.; Souto-Vieira, D.; Lozano, A.P.; Volpedo, A.V.; Fabré, N.N. Ecomorphological Patterns in Otoliths of Tropical Fishes: Assessing Trophic Groups and Depth Strata Preference by Shape. Environ. Biol. Fishes 2020, 103, 349–361. [Google Scholar] [CrossRef]
- de Carvalho, B.M.; Vaz-dos-Santos, A.M.; Spach, H.L.; Volpedo, A.V. Ontogenetic Development of the Sagittal Otolith of the Anchovy, Anchoa tricolor, in a Subtropical Estuary. Sci. Mar. 2015, 79, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Almeida, P.R.C.; Monteiro-Neto, C.; Tubino, R.A.; Costa, M.R. Variations in the Shape of the Sagitta Otolith of Coryphaena hippurus (Actinopterygii: Coryphaenidae) in a Resurgence Area on the Southwest Coast of the Atlantic Ocean. Iheringia—Ser. Zool. 2020, 110, 1–11. [Google Scholar] [CrossRef]
- Kikuchi, E.; García, S.; da Costa, P.A.S.; Cardoso, L.G.; Haimovici, M. Discrimination of Red Porgy Pagrus pagrus (Sparidae) Potential Stocks in the South-Western Atlantic by Otolith Shape Analysis. J. Fish Biol. 2021, 98, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sadighzadeh, Z.; Tuset, V.M.; Valinassab, T.; Dadpour, M.R.; Lombarte, A. Comparison of Different Otolith Shape Descriptors and Morphometrics for the Identification of Closely Related Species of Lutjanus spp. from the Persian Gulf. Mar. Biol. Res. 2012, 8, 802–814. [Google Scholar] [CrossRef]
- Soeth, M.; Spach, H.L.; Daros, F.A.; Adelir-Alves, J.; de Almeida, A.C.O.; Correia, A.T. Stock Structure of Atlantic Spadefish Chaetodipterus faber from Southwest Atlantic Ocean Inferred from Otolith Elemental and Shape Signatures. Fish. Res. 2019, 211, 81–90. [Google Scholar] [CrossRef]
- Colautti, D.; Suquele, P.; Calvo, S. La Pesca Artesanal En La Zona Sur de La Bahía Anegada, Provincia de Buenos Aires; Ministerio de Asuntos Agrarios Dirección Provincial de Pesca: La Plata, Argentina, 2009. [Google Scholar]
- Venerus, L.A.; Cedrola, P.V. Review of Marine Recreational Fisheries Regulations in Argentina. Mar. Policy 2017, 81, 202–210. [Google Scholar] [CrossRef]
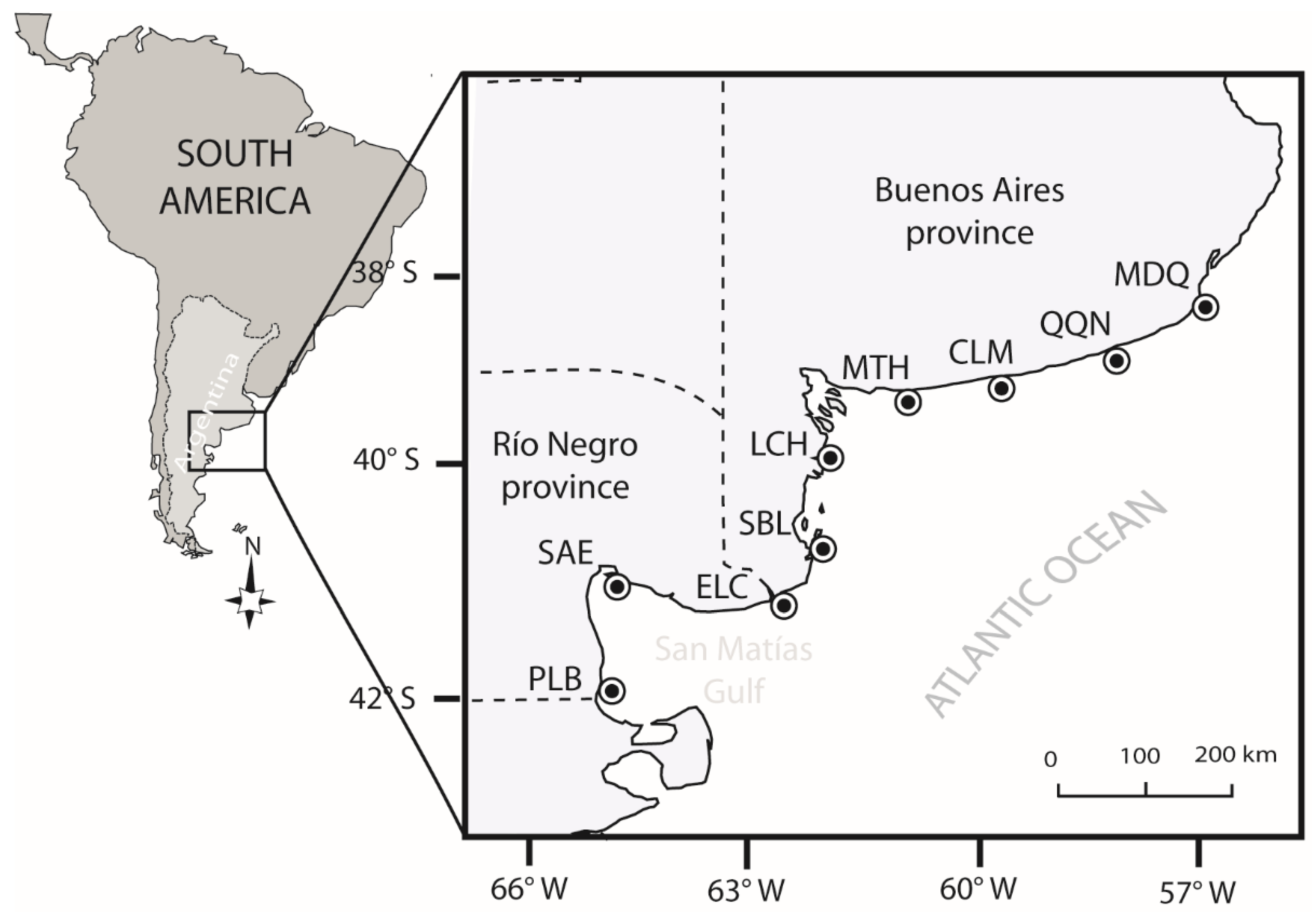



| Localities | Latitude | Longitude | N | TL (mm) | STL (mm) | |
|---|---|---|---|---|---|---|
| Mar Del Plata | MDQ | 38°02′ | 57°31′ | 57 | 209 ± 30 | 175 ± 25 |
| Quequén | QQN | 38°24′ | 58°40′ | 75 | 200 ± 30 | 168 ± 25 |
| Claromecó | CLM | 38°51′ | 60°04′ | 47 | 208 ± 67 | 178 ± 57 |
| Monte Hermoso | MTH | 38°59′ | 61°15′ | 39 | 226 ± 68 | 191 ± 60 |
| La Chiquita | LCH | 39°35′ | 62°05′ | 13 | 255 ± 99 | 214 ± 85 |
| San Blas | SBL | 40°31′ | 62°16′ | 64 | 269 ± 45 | 227 ± 36 |
| El Cóndor | ELC | 41°00′ | 62°48′ | 20 | 317 ± 53 | 267 ± 49 |
| San Antonio Este | SAE | 40°50′ | 64°50′ | 30 | 280 ± 46 | 245 ± 41 |
| Puerto Lobos | PLB | 41°51′ | 65°02′ | 19 | 334 ± 17 | 285 ± 14 |
| Shape Indices | MDQ | QQN | CLM | MTH | LCH | SBL | ELC | SAE | PLB | F | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Circularity | 15.06 ± 0.6 a,b | 14.73 ± 0.47 a | 14.81 ± 1.1 a,b | 15.31 ± 0.89 b,c | 14.88 ± 1.02 b,c,d | 15.82 ± 0.67 c,d,e | 16.42 ± 1.04 f | 16.19 ± 0.88 d,e,f | 16.49 ± 0.6 e,f | 23.69 | <0.001 ** |
| Rectangularity | 0.73 ± 0.02 a | 0.74 ± 0.01 a | 0.74 ± 0.02 a | 0.73 ± 0.02 a | 0.73 ± 0.01 a | 0.74 ± 0.02 a | 0.72 ± 0.03 a | 0.74 ± 0.02 a | 0.74 ± 0.02 a | 1.93 | 0.0544 |
| Aspect ratio | 0.63 ± 0.04 a | 0.64 ± 0.03 a | 0.63 ± 0.05 a | 0.62 ± 0.05 a | 0.65 ± 0.08 a,b | 0.59 ± 0.04 b | 0.56 ± 0.06 b,c | 0.53 ± 0.05 b | 0.53 ± 0.03 c | 20.41 | <0.001 ** |
| Roundness | 0.59 ± 0.04 a | 0.59 ± 0.03 a | 0.6 ± 0.05 a | 0.58 ± 0.05 a | 0.61 ± 0.08 a,b | 0.55 ± 0.04 b | 0.52 ± 0.05 b,c | 0.53 ± 0.04 b | 0.5 ± 0.03 c | 20.06 | <0.001 ** |
| Localities | MDQ | QQN | CLM | MTH | LCH | SBL | ELC | SAE | PLB | Error (%) | Total Error (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Morphometric Indices (MI) (Wilks’ λ = 0.652, p < 0.001 **) | |||||||||||
| MDQ | 14 | 19 | 10 | 4 | 4 | 0 | 4 | 1 | 1 | 75 | |
| QQN | 9 | 42 | 18 | 4 | 0 | 1 | 1 | 0 | 0 | 44 | |
| CLM | 3 | 23 | 10 | 2 | 0 | 0 | 4 | 2 | 3 | 79 | |
| MTH | 6 | 7 | 8 | 6 | 1 | 1 | 5 | 3 | 2 | 85 | |
| LCH | 2 | 2 | 3 | 0 | 0 | 0 | 4 | 0 | 2 | 100 | 72.25 |
| SBL | 7 | 3 | 7 | 8 | 3 | 7 | 13 | 7 | 9 | 89 | |
| ELC | 2 | 1 | 0 | 1 | 1 | 2 | 7 | 2 | 4 | 65 | |
| SAE | 3 | 2 | 2 | 1 | 2 | 2 | 8 | 3 | 7 | 90 | |
| PLB | 0 | 0 | 0 | 0 | 2 | 1 | 4 | 0 | 12 | 37 | |
| (b) Wavelet coefficients (WC) (Wilks’ λ = 0.059, p < 0.001 **) | |||||||||||
| MDQ | 45 | 3 | 1 | 2 | 1 | 4 | 0 | 1 | 0 | 21 | |
| QQN | 3 | 67 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 11 | |
| CLM | 0 | 2 | 41 | 1 | 0 | 1 | 0 | 2 | 0 | 13 | |
| MTH | 0 | 0 | 2 | 34 | 1 | 1 | 0 | 1 | 0 | 13 | |
| LCH | 0 | 0 | 0 | 1 | 12 | 0 | 0 | 0 | 0 | 8 | 17.86 |
| SBL | 6 | 2 | 0 | 4 | 4 | 43 | 2 | 3 | 0 | 33 | |
| ELC | 1 | 0 | 1 | 0 | 1 | 1 | 15 | 1 | 0 | 25 | |
| SAE | 2 | 0 | 1 | 0 | 3 | 1 | 0 | 23 | 0 | 23 | |
| PLB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 0 | |
| (c) Fourier descriptors (FD) (Wilks’ λ = 0.016, p < 0.001 **) | |||||||||||
| MDQ | 47 | 0 | 1 | 4 | 1 | 2 | 0 | 2 | 0 | 18 | |
| QQN | 0 | 63 | 0 | 3 | 4 | 4 | 0 | 1 | 0 | 16 | |
| CLM | 0 | 2 | 38 | 1 | 2 | 2 | 0 | 2 | 0 | 19 | |
| MTH | 2 | 3 | 0 | 26 | 4 | 3 | 0 | 1 | 0 | 33 | |
| LCH | 0 | 0 | 0 | 0 | 9 | 1 | 2 | 1 | 0 | 31 | 25.27 |
| SBL | 1 | 1 | 2 | 14 | 3 | 33 | 4 | 6 | 0 | 48 | |
| ELC | 0 | 0 | 1 | 0 | 1 | 2 | 16 | 0 | 0 | 20 | |
| SAE | 0 | 3 | 2 | 2 | 0 | 2 | 0 | 21 | 0 | 30 | |
| PLB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 0 | |
| (d) MI + FD + WC (Wilks’ λ = 0.002, p < 0.001 **) | |||||||||||
| MDQ | 55 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | |
| QQN | 0 | 72 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | |
| CLM | 0 | 0 | 45 | 0 | 0 | 1 | 0 | 1 | 0 | 4 | |
| MTH | 1 | 0 | 0 | 36 | 0 | 2 | 0 | 0 | 0 | 8 | |
| LCH | 0 | 0 | 0 | 0 | 11 | 2 | 0 | 0 | 0 | 15 | 7.42 |
| SBL | 2 | 0 | 0 | 2 | 1 | 57 | 0 | 2 | 0 | 11 | |
| ELC | 0 | 0 | 0 | 0 | 0 | 4 | 16 | 0 | 0 | 20 | |
| SAE | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 26 | 0 | 13 | |
| PLB | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 19 | 0 | |
| Variables | Localities Groups | F. Model | p Value |
|---|---|---|---|
| Combined Morphometric indices, Fourier descriptors and Wavelet coefficients | MDQ, QQN, CLM vs. MTH, LCH, SBL, ELC, SAE, PLB | 36.76 | <0.0001 ** |
| MDQ, QQN, CLM vs. MTH, LCH, SBL, ELC, SAE vs. PLB | 28.54 | <0.0001 ** | |
| MDQ, QQN, CLM vs. MTH, LCH, SBL, ELC, SAE | 29.57 | <0.0001 ** | |
| MDQ, QQN, CLM vs. PLB | 44.95 | <0.0001 ** | |
| MTH, LCH, SBL, ELC, SAE vs. PLB | 15.28 | <0.0001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawicki, S.; Solimano, P.J.; Volpedo, A.V. Unravelling Stock Spatial Structure of Silverside Odontesthes argentinensis (Valenciennes, 1835) from the North Argentinian Coast by Otoliths Shape Analysis. Fishes 2022, 7, 155. https://doi.org/10.3390/fishes7040155
Morawicki S, Solimano PJ, Volpedo AV. Unravelling Stock Spatial Structure of Silverside Odontesthes argentinensis (Valenciennes, 1835) from the North Argentinian Coast by Otoliths Shape Analysis. Fishes. 2022; 7(4):155. https://doi.org/10.3390/fishes7040155
Chicago/Turabian StyleMorawicki, Santiago, Patricio J. Solimano, and Alejandra V. Volpedo. 2022. "Unravelling Stock Spatial Structure of Silverside Odontesthes argentinensis (Valenciennes, 1835) from the North Argentinian Coast by Otoliths Shape Analysis" Fishes 7, no. 4: 155. https://doi.org/10.3390/fishes7040155
APA StyleMorawicki, S., Solimano, P. J., & Volpedo, A. V. (2022). Unravelling Stock Spatial Structure of Silverside Odontesthes argentinensis (Valenciennes, 1835) from the North Argentinian Coast by Otoliths Shape Analysis. Fishes, 7(4), 155. https://doi.org/10.3390/fishes7040155