Epigenetics and Probiotics Application toward the Modulation of Fish Reproductive Performance
Abstract
1. Introduction
2. Fish Reproductive Dysfunctions

3. Reproductive Dysfunction Caused by Endocrine Disruptors
3.1. Chemical Stressors

3.2. Stress, Temperature, and Photoperiod
4. Existing Methods for Eradicating Reproductive Dysfunctions of Brood Fish
4.1. Broodstock Management
4.2. Manipulation of the Reproductive System in Fish
5. Key Genes and Hormones Related to Reproductive Function
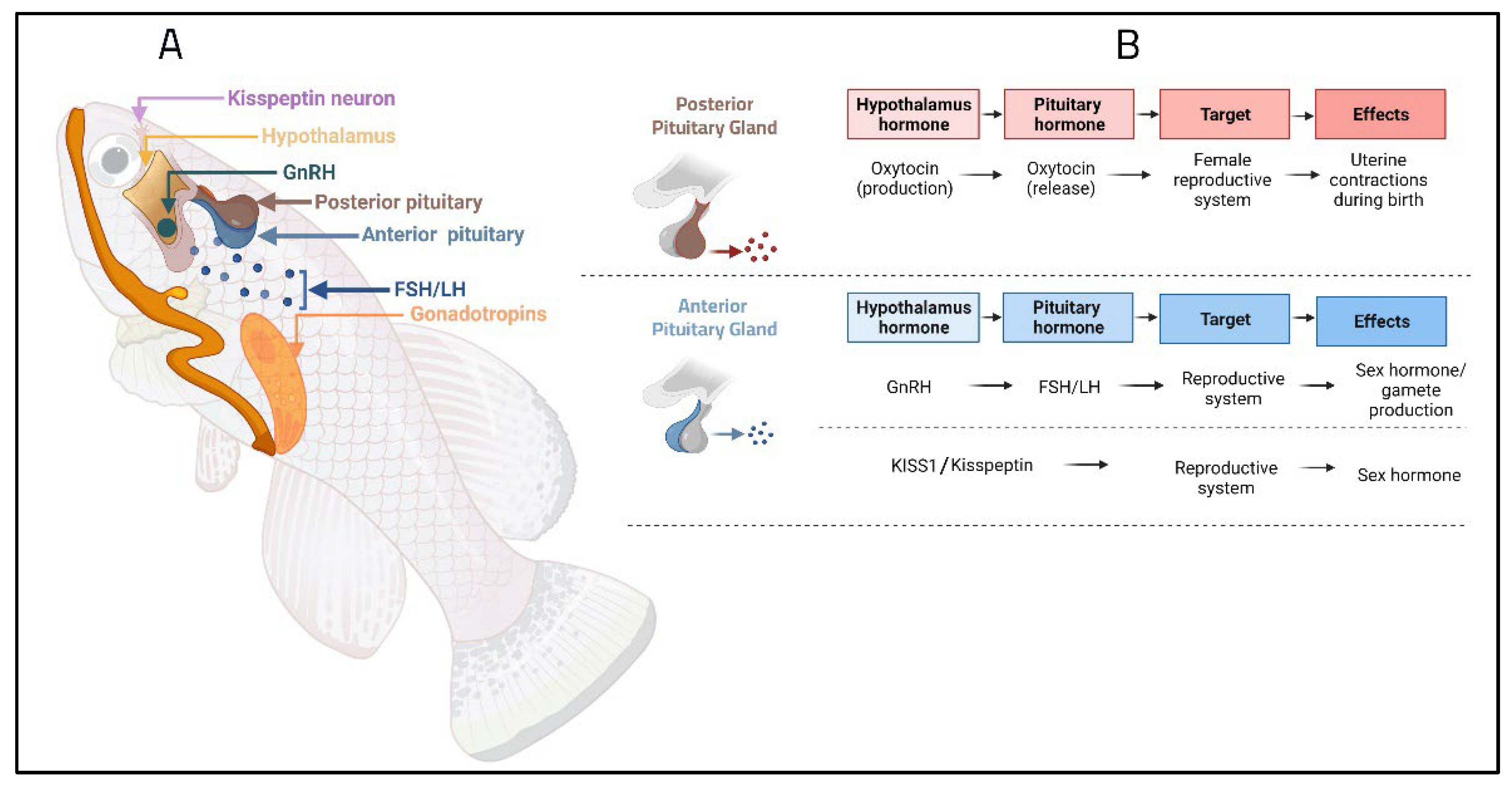
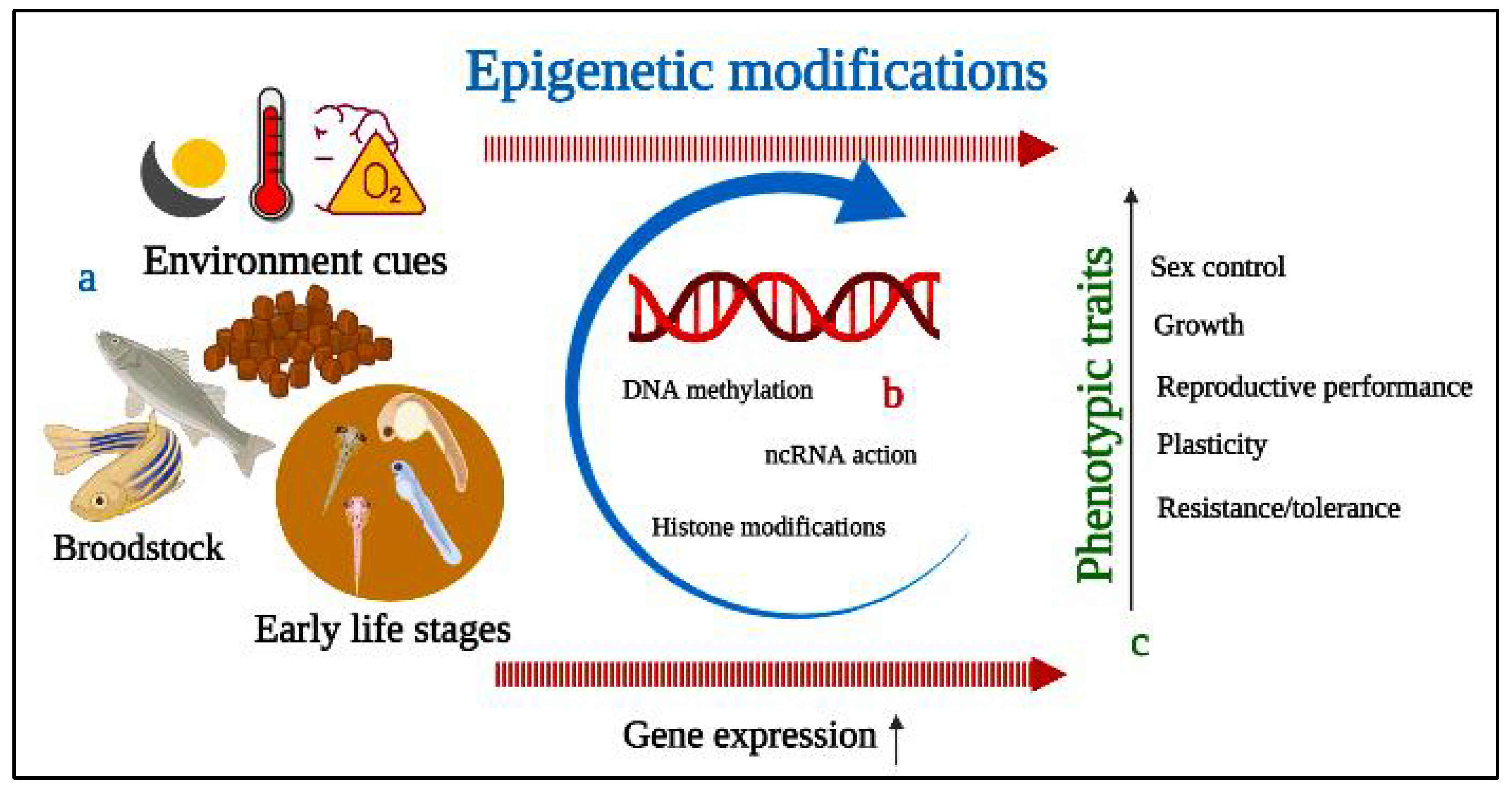
6. Epigenetics Mechanism and Modifications of Fish Reproductive Performance
| Species | Conditions of Exposure and Stressors | Epigenetics Marker | Methodology | Epigenetics Results | Phenotypic Outcomes | References |
|---|---|---|---|---|---|---|
| Dicentrarchus labrax(European sea bass) | High temperature during the thermosensitive phase | Methylation of gonadal promoters of cyp19a and β-actin | Bisulphite sequencing (BS Seq) | Methylation of cyp19a ↑ | Masculinization | [147] |
| Cynoglossus semilaevis(Halfsmooth tongue sole) | Exposure to higher temperatures during early developmental period | DNA methylation of gonadal tissue and methylation status of dmrt1 | BS-Seq | Genes involved in sex determination during sexual reversal ↑ | Masculinization | [18,19] |
| Solea senegalensis (Senegalese sole) | Larvae undergoing transformation were raised at various temperatures | Cytosine methylation of the Myogenin (myog) promoter | BS-seq | Methylation of myog gene promoterat ↑, Myog transcription ↓ and dnmt1 and dnmt3s ↑ at 15 °C | Plasticity of myogenesis | [148] |
| Gadus morhua (Atlantic cod) | Juvenile exposure to two distinct photoperiods | Expression of DNA methyltransferases (dnmts) in fast muscle tissue | Semi quantitative and Quantitative real-time PCR (qRT-PCR) | Expression of dnmt1 and dnmt3a in muscle ↑ | Muscle growth and final weight | [161] |
| Salmo trutta (Brown trout) | Freshwater fish are exposed to seawater after consuming a salt-enriched diet | DNA methylation at CpG sites in gill tissue samples | Methyl sensitive amplification and polymorphism (msAP) | Salt-induced reversible and temporary changes in global DNA methylation | Physiological adaptation of freshwater to seawater | [152] |
| Anguillla anguilla (European eel) | Chronic cadmium (Cd) exposure of immature organisms | Global and site-specific CpG methylation in liver tissue | Enzyme-linked immunosorbent assay (ELISA) and msAP-PCR | Global CpG methylation↑; Hypermethylation of genes related to intracellular trafficking and phospholipid biosynthesis ↑ | [162] | |
| Danio rerio (Zebrafish) | Copper and heat stress exposure during embryogenesis | Global DNA methylation; stress-related geneexpression | Colorimetric technique—MethylFlash Methylated DNA Quantification Kit; qRT-PCR | Global cytosine methylation →, Expression of dnmt3 ↑; mRNA expression of mt2 and hsp70 ↑ | High mortality, late embryo hatching, and low hatching rate | [163] |
| Oryzias melastigma (Medaka fish) | Exposure to hypoxia in mature stage | DNA methylation of sperm cells (F0 F2) | qRT-PCR and Methylated DNA | DNA methylation in sperm (F2 fish) ↑; Hypomethylation of ehmt2 and ptk2b ↑; Hypermethylation of foxp2 exonic regions ↑ | Aberrant sperm motility and decreased spermatid and fertility rate | [153] |
| D. rerio | Temperature and Cd exposure during whole life | DNA methylation, environmental sex determination | - | Methylation of cyp19a1a gene ↑; foxl2a/dmrt1 methylation levels ↑ | Masculinization | [131] |
| D. rerio | Tris (1,3-dichloro-2-propyl) phosphate exposure during early development | Global DNA methylation | ELISA and qRT-PCR | Transcription mbpa, gap43 and syn2a ↑ | Sex-dependent behavior in adults | [132] |
| Cyprinus carpio(Common carp) | - | Global and gene specific DNA methylation in sperm aging | Whole-genome BS-Seq | Global CpG methylation ↑ | Sperm motility, velocity, concentration, and viability | [154] |
| Oryzias latipes(Medaka) | - | microRNAs (miRNA) expression in gonad | qRT-PCR and Agilent 8x60K microarray | The knockout of mir-202 gene resulted higher fecundity ↑; vitellogenic follicles number ↓ | Number and quality of eggs | [160] |
| Menidia beryllina(Inland silverside) | Exposure to endocrine-disrupting chemicals like Bifenthrin, EE2, levonorgestrel, trenbolone | DNA methylation patterns for F0, F1, and and F2 generation | BS-seq | Promoter and/or gene body methylation multigenerational (F1) and transgenerational (F2) ↑ | Fish phenotypic variation in future generations | [164] |
| Oncorhynchus mykiss(Steelhead trout) | Nutrition and stress in captive and natural conditions, and exposure to toxicants | DNA methylation in sperm and RBC DNA | qRT-PCR -Seq | DNA methylation in sperm and RBC ↑; Epigenetic transgenerational and phenotypic variation of next generations ↑ | Growth/maturation | [165] |
| D. rerio | Early exposure of 5-aza-2′-deoxycytidine | DNA methylation during sexual development | Microarray and qRT-PCR | Expression of cyp11a1, esr2b and fgla ↑, Fanconi anemia or the Wnt signaling pathways ↓ | Altered embryonic development, delayed hatching and increased teratology and mortality | [157] |
| Salmo salar(Atlantic salmon) | - | miRNAs related to the immature, prepubertal, and pubertal testis | miRNA and mRNA-seq | Expression of miRNAs and their mRNA targets during early puberty ↑ | Pubertal maturation | [158] |
| Oreochromis niloticus(Nile Tilapia) | Exposure to Aromatase inhibitors | DNA methylation in the head kidney, testis, and ovary | qRT-PCR | The expression of dnmts ↓and cyp19a1a and dmrt1 ↑ | Gonadal development | [155] |
| O. niloticus | Exposure to higher temperature | DNA methylation in three age groups of fish | BS-seq and qRT-PCR | cyp19a1a promoter DNA methylation levels ↑; cyp19a1a mRNA expression ↓ | Masculinization | [156] |
| Morone saxatilis(Striped bass) | In vitro fertilization | DNA methylation in sperm fertility | Methyl-CpG-binding domain sequencing | WDR3/UTP12 and GPCR ↑ involved in sperm flagella formation, hormonal signaling and tissue development regulation | Sperm fertility | [166] |
7. Influence of Probiotics on Reproductive Performance of Fish
7.1. Ornamental Fish
7.1.1. Male
7.1.2. Female
| Supplemented Probiotics | Fish Species | Fish Number | Duration | Concentration | Effects on Fish | References |
|---|---|---|---|---|---|---|
| Bacillus subtilis | Poecilia reticulata (Guppy), P.sphenops (Valenciennes), Xiphophorus helleri (Swordtail fish) and X. maculatus (Platyfish) | 60 virgin females of each species | 365 days | 5 × 107–5 × 108 CFU g−1 and 5 × 105–5 × 106 CFU g−1 | EP Fec and GSI ↑; SR (fry) ↑; Fry death and deformities ↓ | [27] |
| Lactobacillus rhamnosus IMC 501 | Danio rerio (Zebrafish) | 10 females | 10 days | 106 CFU g−1 | EP Fec, GSI, and Ovolution rate ↑; Oocyte maturation G and FD ↑; | [29,31,176,185] |
| Oocyte maturation FD and FM ↑ | [170] | |||||
| Follicular survival ↑ and apoptosis ↓ | [30] | |||||
| Lab. rhamnosus IMC 501 | D. rerio | 40 males and females | 10 days | 106 CFU g−1 | Embryo development ↑; HR ↑ | [134] |
| Pediococcus acidilactici (Bactocell®) | D. rerio | 5 wild males | 10 days | 106 CFU g−1 | SP testicular cells ↑ | [164] |
| Lab. rhamnosus CECT8361 and Bifidobacterium longum CECT7347 | D. rerio | 36 Males | 21 days | 109 CFU g−1 | SP SQ, SDn, SM ↑ | [182] |
| PrimaLac® (Lab. acidophilus, Lab. casei, Enterococcus faecium, Bifidobacterium thermophilum) | X. helleri | 10 females and 3 males | 182 days | 0.04%, 0.09% and 0.14% | EP Fec and GSI ↑; SR (fry) ↑ | [28] |
| P. acidilactici | Carassius auratus (Goldfish) | 720 fishes | 180 days | 0.1, 0.2, and 0.3% | EP GSI, HSI, AF, RF, ED, OD, FR, and HR ↑; SP SM, SD, SDn, and Stc ↑ | [133] |
| Lab. rhamnosus IMC 501 | Fundulus heteroclitus (Killifish) | 10 females and 10 males | 8 days | 106 CFU mL−1 | EP GSI, Fec ↑ and HR →; SR (fry) ↑; GP L and W ↑ | [36] |
7.2. Commercial Fish
7.2.1. Male
7.2.2. Female
| Probiotics Used | Fish Species | Fish Number | Duration | Application Mode | Effects on Fish | References |
|---|---|---|---|---|---|---|
| Enterococcus xiangfangensis, Pseudomonas stutzeri, Bacillus subtilis, Citrobacter freundii, and Pseudomonas aeruginos | Barbonymus gonionotus (Silver barb) | 96 males and females | 60 days | Dietary supplementation at 1.35 × 109 CFU kg−1 | EP GSI, Fec, FR, and HR ↑ | [168] |
| B. subtilis | Oreochromis niloticus (Nile tilapia) | 118 females and 48 males | 14 days | Dietary supplementation at 1010 CFU g−1 | EP Fec ↑; SR (fry) ↑; EFi TcN, GR and ToP ↑ | [22] |
| Bio-Aqua® (P. acidilactici, Enterococcus faecium, B. subtilis, Lactobacillus acidophilus, Lab. plantarum, Lab. casei, Lab. rhamnosus, Bifidobacterium bifidum and Saccharomyces cerevisiae) | Oncorhynchus mykiss (Rainbow trout) | 60 females | 8 weeks | Dietary supplementation at 4 × 109 CFU g−1 | EP AF, RF, FR, HR, and ES ↑; SR (fry) ↑ | [38] |
| Lab. rhamnosus | Ompok pabda (Butter catfish) | 240 males and females | 60 days | Dietary supplementation at 5 × 106−8 CFU g−1 | EP GSI, Fec, FR, and HR ↑; SR (fry) ↑ | [39] |
| Probio-7 (Saccharomyces cerevisiae, Aspergillus oryzae, Lab. acidophilus, B. subtilis, Rhodopseudomonas, Actinomycetes, and Nitrobacter) | Clarias gariepinus (African catfish) | - | 80 days | Fermented dose at 5 mL kg−1 | EP FR, HR, and SR ↑; Maturity time ↑ | [195] |
| Lab. rhamnosus IMC 501 | Anguilla anguilla (European eel) | 40 males | 63 days | Added water at 103, 105 and 106 CFU mL−1 | SP SM, SDn, and Spermatogenesis ↑ | [41] |
8. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, I.C.; Chao, N.-H. Aquaculture and food crisis: Opportunities and constraints. Asia Pac. J. Clin. Nutr. 2009, 18, 564–569. [Google Scholar] [PubMed]
- Merrifield, D.L.; Ringo, E. Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Akhtar, M.; Ciji, A.; Sarma, D.; Rajesh, M.; Kamalam, B.; Sharma, P.; Singh, A. Reproductive dysfunction in females of endangered golden mahseer (Tor putitora) in captivity. Anim. Reprod. Sci. 2017, 182, 95–103. [Google Scholar] [CrossRef]
- Mañanós, E.; Duncan, N.; Mylonas, C. Reproduction and control of ovulation, spermiation and spawning in cultured fish. In Methods in Reproductive Aquaculture: Marine and Freshwater Species; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 3–80. [Google Scholar]
- Ottolenghi, F.; Silvestri, C.; Giordano, P.; Lovatelli, A.; New, M.B. Capture-Based Aquaculture: The Fattening of Eels, Groupers, Tunas and Yellowtails; FAO: Mexico City, Mexico, 2004. [Google Scholar]
- Guzmán, J.; Luckenbach, A.; Goetz, F.W.; Fairgrieve, W.T.; Middleton, M.A.; Swanson, P. Reproductive dysfunction in cultured sablefish (Anoplopoma fimbria). Bull. Fish. Res. Agency 2015, 40, 111–119. [Google Scholar]
- Gioacchini, G.; Giorgini, E.; Vaccari, L.; Carnevali, O. Can Probiotics Affect Reproductive Processes of Aquatic Animals? In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 328–346. [Google Scholar]
- Martínez Cruz, P.; Ibáñez, A.L.; Monroy Hermosillo, O.A.; Ramírez Saad, H.C. Use of probiotics in aquaculture. Int. Sch. Res. Not. 2012, 2012, 916845. [Google Scholar] [CrossRef] [PubMed]
- Granada, L.; Lemos, M.F.; Cabral, H.N.; Bossier, P.; Novais, S.C. Epigenetics in aquaculture—The last frontier. Rev. Aquac. 2018, 10, 994–1013. [Google Scholar] [CrossRef]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Herráez, M.P.; Lombó, M.; González-Rojo, S. The Role of Epigenetics in Fish Biology and Reproduction: An Insight into the Methods Applied to Aquaculture. In Cellular and Molecular Approaches in Fish Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 69–104. [Google Scholar]
- Gavery, M.R.; Roberts, S.B. Epigenetic considerations in aquaculture. PeerJ. 2017, 5, e4147. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Skinner, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenet. 2016, 2, dvw002. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.L.; Tobler, M.; Beck, D.; Sadler-Riggleman, I.; Quackenbush, C.R.; Rodriguez, L.A.; Skinner, M.K. Epigenetic inheritance of DNA methylation changes in fish living in hydrogen sulfide—Rich springs. Proc. Natl. Acad. Sci. USA 2021, 118, e2014929118. [Google Scholar] [CrossRef]
- González-Recio, O. Epigenetics: A new challenge in the post-genomic era of livestock. Front. Genet. 2012, 2, 106. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.C., III; Li, Y.; Ding, Y.; Liu, J.; Reading, B.J.; Fuller, S.A.; Song, J. DNA methylation profiles correlated to striped bass sperm fertility. BMC Genom. 2018, 19, 244. [Google Scholar] [CrossRef]
- Renn, S.C.; Hurd, P.L. Epigenetic regulation and environmental sex determination in cichlid fishes. Sex. Dev. 2021, 15, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, G.; Shao, C.; Huang, Q.; Liu, G.; Zhang, P.; Song, W.; An, N.; Chalopin, D.; Volff, J.-N.; et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat. Genet. 2014, 46, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Li, Q.; Chen, S.; Zhang, P.; Lian, J.; Hu, Q.; Sun, B.; Jin, L.; Liu, S.; Wang, Z.; et al. Epigenetic modification and inheritance in sexual reversal of fish. Genome Res. 2014, 24, 604–615. [Google Scholar] [CrossRef]
- Labbé, C.; Robles, V.; Herraez, M.P. Epigenetics in fish gametes and early embryo. Aquaculture 2017, 472, 93–106. [Google Scholar] [CrossRef]
- Hasan, K.N.; Banerjee, G. Recent studies on probiotics as beneficial mediator in aquaculture: A review. J. Basic Appl. Zool. 2020, 81, 53. [Google Scholar] [CrossRef]
- Hasan, M.T.; Jang, W.J.; Kim, H.; Lee, B.-J.; Kim, K.W.; Hur, S.W.; Lim, S.G.; Bai, S.C.; Kong, I.-S. Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 82, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.T.; Sumon, A.A.; Pugazhendi, A.; Al Harbi, M.; Hussain, A.; Haque, F. Use of probiotics in commercially important finfish aquaculture. Int. J. Probiotics Prebiotics 2020, 15, 7–21. [Google Scholar] [CrossRef]
- Hasan, M.T.; Jang, W.J.; Lee, B.-J.; Hur, S.W.; Lim, S.G.; Kim, K.W.; Han, H.-S.; Lee, E.-W.; Bai, S.C.; Kong, I.-S. Dietary Supplementation of Bacillus sp. SJ-10 and Lactobacillus plantarum KCCM 11322 Combinations enhance growth and cellular and humoral immunity in olive flounder (Paralichthys olivaceus). Probiotics Antimicrob. Proteins 2021, 13, 1277–1291. [Google Scholar] [CrossRef]
- Hasan, M.T.; Je Jang, W.; Lee, J.M.; Lee, B.-J.; Hur, S.W.; Gu Lim, S.; Kim, K.W.; Han, H.-S.; Kong, I.-S. Effects of immunostimulants, prebiotics, probiotics, synbiotics, and potentially immunoreactive feed additives on olive flounder (Paralichthys olivaceus): A review. Rev. Fish. Sci. Aquac. 2019, 27, 417–437. [Google Scholar] [CrossRef]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. In Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO Food and Nutrition Paper—Series Number: 0254-4725|1014-2908; FAO/WHO: Cordoba, Argentina, 2001; p. 56. [Google Scholar]
- Ghosh, S.; Sinha, A.; Sahu, C. Effect of probiotic on reproductive performance in female livebearing ornamental fish. Aquac. Res. 2007, 38, 518–526. [Google Scholar] [CrossRef]
- Abasali, H.; Mohamad, S. Effect of dietary supplementation with probiotic on reproductive performance of female livebearing ornamental fish. Res. J. Anim. Sci. 2010, 4, 103–107. [Google Scholar]
- Gioacchini, G.; Bizzaro, D.; Giorgini, E.; Ferraris, P.; Sabbatini, S.; Carnevali, O. P-230 Oocytes maturation induction by Lactobacillus rhamnosus in Danio rerio: In vivo and in vitro studies. Hum. Reprod. 2010, 25, I205–I206. [Google Scholar]
- Gioacchini, G.; Dalla Valle, L.; Benato, F.; Fimia, G.M.; Nardacci, R.; Ciccosanti, F.; Piacentini, M.; Borini, A.; Carnevali, O. Interplay between autophagy and apoptosis in the development of Danio rerio follicles and the effects of a probiotic. Reprod. Fertil. Dev. 2013, 25, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, G.; Giorgini, E.; Ferraris, P.; Tosi, G.; Bizzaro, D.; Silvi, S. Could probiotics improve fecundity? Danio rerio as case of study. J. Biotechnol. 2010, 150, 59–60. [Google Scholar] [CrossRef]
- Giorgini, E.; Conti, C.; Ferraris, P.; Sabbatini, S.; Tosi, G.; Rubini, C.; Vaccari, L.; Gioacchini, G.; Carnevali, O. Effects of Lactobacillus rhamnosus on zebrafish oocyte maturation: An FTIR imaging and biochemical analysis. Anal. Bioanal. Chem. 2010, 398, 3063–3072. [Google Scholar] [CrossRef]
- Miccoli, A.; Gioacchini, G.; Maradonna, F.; Benato, F.; Skobo, T.; Carnevali, O. Beneficial bacteria affect Danio rerio development by the modulation of maternal factors involved in autophagic, apoptotic and dorsalizing processes. Cell. Physiol. Biochem. 2015, 35, 1706–1718. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Pardo, M.; Riesco, M.; Cruz, Z.; Robles, V. Effect of diet supplementation with a commercial probiotic containing Pediococcus acidilactici (Lindner, 1887) on the expression of five quality markers in zebrafish (Danio rerio (Hamilton, 1822)) testis. J. Appl. Ichthyol. 2015, 31, 18–21. [Google Scholar] [CrossRef]
- Mehdinejad, N.; Imanpour, M.R.; Jafari, V. Combined or individual effects of dietary probiotic, Pediococcus acidilactici and nucleotide on reproductive performance in goldfish (Carassius auratus). Probiotics Antimicrob. Proteins 2019, 11, 233–238. [Google Scholar] [CrossRef]
- Lombardo, F.; Gioacchini, G.; Carnevali, O. Probiotic-based nutritional effects on killifish reproduction. Fish. Aquacult J. FAJ-33 2011, 2011, FAJ-33. [Google Scholar] [CrossRef]
- Dias, D.d.C.; Furlaneto, F.d.P.B.; Sussel, F.R.; Tachibana, L.; Gonçalves, G.S.; Ishikawa, C.M.; Natori, M.M.; Ranzani-Paiva, M.J.T. Economic feasibility of probiotic use in the diet of Nile tilapia, Oreochromis niloticus, during the reproductive period. Acta Scientiarum. Anim. Sci. 2020, 42, e47960. [Google Scholar] [CrossRef]
- Akbari Nargesi, E.; Falahatkar, B.; Sajjadi, M.M. Dietary supplementation of probiotics and influence on feed efficiency, growth parameters and reproductive performance in female rainbow trout (Oncorhynchus mykiss) broodstock. Aquac. Nutr. 2020, 26, 98–108. [Google Scholar] [CrossRef]
- Rahman, M.L.; Akhter, S.; Mallik, M.K.M.; Rashid, I. Probiotic enrich dietary effect on the reproduction of butter catfish, Ompok pabda (Hamilton, 1872). Int. J. Curr. Res. Life Sci. 2018, 7, 866–873. [Google Scholar]
- Ariole, C.N.; Okpokwasili, G.C. The effect of indigenous probiotics on egg hatchability and larval viability of Clarias gariepinus. Rev. Ambiente Água 2012, 7, 81–88. [Google Scholar] [CrossRef][Green Version]
- Vílchez, M.C.; Santangeli, S.; Maradonna, F.; Gioacchini, G.; Verdenelli, C.; Gallego, V.; Peñaranda, D.S.; Tveiten, H.; Pérez, L.; Carnevali, O.; et al. Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 2015, 84, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.T.; Jang, W.J.; Lee, S.; Kim, K.W.; Lee, B.J.; Han, H.S.; Bai, S.C.; Kong, I.S. Effect of β-glucooligosaccharides as a new prebiotic for dietary supplementation in olive flounder (Paralichthys olivaceus) aquaculture. Aquac. Res. 2018, 49, 1310–1319. [Google Scholar] [CrossRef]
- Hasan, M.T.; Jang, W.J.; Lee, B.-J.; Kim, K.W.; Hur, S.W.; Lim, S.G.; Bai, S.C.; Kong, I.-S. Heat-killed Bacillus sp. SJ-10 probiotic acts as a growth and humoral innate immunity response enhancer in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2019, 88, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y. Promoting oocyte maturation, ovulation and spawning in farmed fish. In The Fish Oocyte; Springer: Berlin/Heidelberg, Germany, 2007; pp. 437–474. [Google Scholar]
- Papadaki, M.; Peleteiro, J.B.; Alvarez-Blázquez, B.; Villanueva, J.L.R.; Linares, F.; Vilar, A.; Rial, E.P.; Lluch, N.; Fakriadis, I.; Sigelaki, I.; et al. Description of the annual reproductive cycle of wreckfish Polyprion americanus in captivity. Fishes 2018, 3, 43. [Google Scholar] [CrossRef]
- Fernando, A.; Phang, V.; Chan, S. Diets and feeding regimes of poeciliid fishes in Singapore. Asian Fish. Sci 1991, 4, 99–107. [Google Scholar]
- Izquierdo, M.; Fernandez-Palacios, H.; Tacon, A. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Rawls, J.F. Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol. Ecol. 2012, 21, 3100–3102. [Google Scholar] [CrossRef] [PubMed]
- Maruska, K.P.; Fernald, R.D. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology 2011, 26, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, B.; Ge, W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015, 29, 76–98. [Google Scholar] [CrossRef]
- Selvaraj, S.; Chidambaram, P.; Ezhilarasi, V.; Kumar, P.P.; Moses, T.; Antony, C.; Ahilan, B. A Review on the Reproductive Dysfunction in Farmed Finfish. Annu. Res. Rev. Biol. 2021, 36, 65–81. [Google Scholar] [CrossRef]
- Abellan, E.; Basurco, B. Marine Finfish Species Diversification: Current Situation and Prospects in Mediterranean Aquaculture; CIHEAM-IAMZ: Zaragoza, Spain; FAO: Rome, Italy, 1999. [Google Scholar]
- Monbrison, D.D.; Tzchori, I.; Holland, M.C.; Zohar, Y.; Yaron, Z.; Elizur, A. Acceleration of gonadal development and spawning induction in the Mediterranean grey mullet, Mugil cephalus: Preliminary studies. Isr. J. Aquac. 1997, 49, 214–221. [Google Scholar]
- Mylonas, C.C.; Magnus, Y.; Klebanov, Y.; Gissis, A.; Zohar, Y. Reproductive biology and endocrine regulation of final oocyte maturation of captive white bass. J. Fish. Biol. 1997, 51, 234–250. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Woods, L., III; Zohar, Y. Cyto-histological examination of post-vitellogenesis and final oocyte maturation in captive-reared striped bass. J. Fish. Biol. 1997, 50, 34–49. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef]
- Podhorec, P.; Kouřil, J. Hypothalamic factors (GnRH and DA) and their utilization to elimination of reproductive dysfunction in Cyprinidae fish (a review). Bull. VÚRH Vodňany 2009, 45, 10–17. [Google Scholar]
- Zohar, Y.; Mylonas, C. Endocrine manipulations of spawning in cultured fish: From hormones to genes, Reproductive Biotechnology in Finfish Aquaculture. Aquaculture 2001, 197, 99–136. [Google Scholar] [CrossRef]
- Bromage, N.; Jones, J.; Randall, C.; Thrush, M.; Davies, B.; Springate, J.; Duston, J.; Barker, G. Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 1992, 100, 141–166. [Google Scholar] [CrossRef]
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and testing for endocrine disruption in fish—Biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Grim, K.C.; Wolfe, M.; Hawkins, W.; Johnson, R.; Wolf, J. Intersex in Japanese medaka (Oryzias latipes) used as negative controls in toxicologic bioassays: A review of 54 cases from 41 studies. Environ. Toxicol. Chem. 2007, 26, 1636–1643. [Google Scholar] [CrossRef]
- Arslan, P.; Özeren, S.C.C.; Dikmen, B.Y. The effects of endocrine disruptors on fish. Environ. Res. Technol. 2021, 4, 145–151. [Google Scholar] [CrossRef]
- Sabra, F.S.; Mehana, E.-S.E.-D. Pesticides toxicity in fish with particular reference to insecticides. Asian J. Agric. Food Sci. 2015, 3. [Google Scholar]
- Murty, A.S. Toxicity of Pesticides to Fish; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Lal, B. Pesticide—Induced reproductive dysfunction in Indian fishes. Fish. Physiol. Biochem. 2007, 33, 455–462. [Google Scholar] [CrossRef]
- Khan, M.Z.; Law, F.C. Adverse effects of pesticides and related chemicals on enzyme and hormone systems of fish, amphibians and reptiles: A review. Proc. Pak. Acad. Sci. 2005, 42, 315–323. [Google Scholar]
- Singh, T.; Lal, B.; Yadav, A. Pesticides and Fish. In Pesticides, Man and Biosphere; Board of Editors, Ed.; Hindustan Publishing Corporation: Delhi, India, 1997; pp. 197–248. [Google Scholar]
- Murthy, K.S.; Kiran, B.; Venkateshwarlu, M. A review on toxicity of pesticides in Fish. Int. J. Open Sci. Res. 2013, 1, 15–36. [Google Scholar]
- Tazeen, S.; Kulkarni, R. Histopathological impact of profenofos on ovary of the freshwater fish Notopterus notopterus. Asian J. Res. Zool. 2018, 1, 1–7. [Google Scholar] [CrossRef][Green Version]
- Arcand-Hoy, L.D.; Benson, W.H. Fish reproduction: An ecologically relevant indicator of endocrine disruption. Environ. Toxicol. Chem. Int. J. 1998, 17, 49–57. [Google Scholar] [CrossRef]
- El-Gawad, E.; Abbass, A.; Shaheen, A. Risks Induced by Pesticides on Fish Reproduction. In Proceedings of the 5th Global Fisheries and Aquaculture Research Conference, Faculty of Agriculture, Cairo University, Giza, Egypt, 1–3 October 2012; Massive Conferences and Trade Fairs: Cairo, Egypt, 2012; pp. 329–338. [Google Scholar]
- Delbes, G.; Blázquez, M.; Fernandino, J.; Grigorova, P.; Hales, B.; Metcalfe, C.; Navarro-Martín, L.; Parent, L.; Robaire, B.; Rwigemera, A.; et al. Effects of endocrine disrupting chemicals on gonad development: Mechanistic insights from fish and mammals. Environ. Res. 2022, 204, 112040. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.G.; Kime, D.E. Early life exposure to environmental levels of the aromatase inhibitor tributyltin causes masculinisation and irreversible sperm damage in zebrafish (Danio rerio). Aquat. Toxicol. 2003, 65, 309–316. [Google Scholar] [CrossRef]
- Hanson, R.; Dodoo, D.; Essumang, D.; Blay, J.; Yankson, K. The effect of some selected pesticides on the growth and reproduction of fresh water Oreochromis niloticus, Chrysicthys nigrodigitatus and Clarias gariepinus. Bull. Environ. Contam. Toxicol. 2007, 79, 544–547. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Bilal, R.M.; Gewida, A.G.; Dhama, K.; Abdel-Latif, H.M.; Amer, M.S.; Rivero-Perez, N.; Zaragoza-Bastida, A.; Binnaser, Y.S.; et al. An overview on the potential hazards of pyrethroid insecticides in fish, with special emphasis on cypermethrin toxicity. Animals 2021, 11, 1880. [Google Scholar] [CrossRef]
- Coats, J.; Symonik, D.; Bradbury, S.; Dyer, S.; Timson, L.; Atchison, G. Toxicology of synthetic pyrethroids in aquatic organisms: An overview. Environ. Toxicol. Chem. Int. J. 1989, 8, 671–679. [Google Scholar] [CrossRef]
- Jaensson, A.; Scott, A.P.; Moore, A.; Kylin, H.; Olsén, K.H. Effects of a pyrethroid pesticide on endocrine responses to female odours and reproductive behaviour in male parr of brown trout (Salmo trutta L.). Aquat. Toxicol. 2007, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Werner, I.; Schneeweiss, A.; Segner, H.; Junghans, M. Environmental risk of pesticides for fish in small-and medium-sized streams of Switzerland. Toxics 2021, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.; Kadlec, S.; Kurker, V.; Etterson, M. Effects of a 28-day early life stage exposure to carbaryl on fathead minnow long-term growth and reproduction. Aquat. Toxicol. 2022, 242, 106018. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Alavi, S.M.H. Androgen signaling in male fishes: Examples of anti-androgenic chemicals that cause reproductive disorders. Theriogenology 2019, 139, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.; Pottinger, T.; Sumpter, J. On the use of dexamethasone to block the pituitary-interrenal axis in the brown trout, Salmo trutta L. Gen. Comp. Endocrinol. 1987, 65, 346–353. [Google Scholar] [CrossRef]
- Pankhurst, N.; Van Der Kraak, G. Evidence that acute stress inhibits ovarian steroidogenesis in rainbow trout in vivo, through the action of cortisol. Gen. Comp. Endocrinol. 2000, 117, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.L.; Ruby, S.M.; Idler, D.R.; So, Y. Plasma vitellogenin levels in pre-spawning rainbow trout, Oncorhynchus mykiss, during acid exposure. Arch. Environ. Contam. Toxicol. 1990, 19, 803–806. [Google Scholar] [CrossRef]
- Carragher, J.; Sumpter, J.; Pottinger, T.; Pickering, A. The deleterious effects of cortisol implantation on reproductive function in two species of trout, Salmo trutta L. and Salmo gairdneri Richardson. Gen. Comp. Endocrinol. 1989, 76, 310–321. [Google Scholar] [CrossRef]
- Pataueg, A.; Larson, E.T.; Brown, C.L. Evolution of Thyroid Enhancement of Embryogenesis and Early Survival. In Hypothyroidism—New Aspects of an Old Disease; Cvitan, K.K., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Polat, H.; Ozturk, R.C.; Terzi, Y.; Aydin, I.; Kucuk, E. Effect of photoperiod manipulation on spawning time and performance of turbot (Scophthalmus maximus). Aquac. Stud. 2021, 21, 109–115. [Google Scholar] [CrossRef]
- Rideout, R.M.; Rose, G.A.; Burton, M.P. Skipped spawning in female iteroparous fishes. Fish Fish. 2005, 6, 50–72. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Bromage, N.R.; Elliott, J.; Springate, J.; Whitehead, C. The effects of constant photoperiods on the timing of spawning in the rainbow trout. Aquaculture 1984, 43, 213–223. [Google Scholar] [CrossRef]
- Carrillo, M.; Bromage, N.; Zanuy, S.; Serrano, R.; Prat, F. The effect of modifications in photoperiod on spawning time, ovarian development and egg quality in the sea bass (Dicentrarchus labrax L.). Aquaculture 1989, 81, 351–365. [Google Scholar] [CrossRef]
- Onumah, E.E.; Wessels, S.; Wildenhayn, N.; Brümmer, B.; Hörstgen-Schwark, G. Stocking density and photoperiod manipulation in relation to estradiol profile to enhance spawning activity in female Nile tilapia. Turk. J. Fish. Aquat. Sci. 2010, 10, 463–470. [Google Scholar]
- Abdollahpour, H.; Falahatkar, B.; Lawrence, C. The effect of photoperiod on growth and spawning performance of zebrafish. Danio Rerio. Aquac. Rep. 2020, 17, 100295. [Google Scholar]
- Hong, B.S.; Lee, H.B.; Park, J.Y.; Yoon, J.H.; Lee, I.Y.; Lim, H.K. Effects of Photoperiod, water temperature, and exogenous hormones on spawning and plasma gonadal steroid in starry flounder, Platichthys stellatus. Isr. J. Aquac. 2021, 73, 1–12. [Google Scholar] [CrossRef]
- Chakravarty, S.; Chadha, N.; Mahapatra, B.; Sawant, P.B.; Dasgupta, S. Temperature accelerates photoperiod mediated testicular maturity in Clarias magur (Hamilton, 1822). Int. J. Curr. Microbiol. Appl. Sci 2021, 10, 500–509. [Google Scholar]
- Beirão, J.; Egeland, T.B.; Purchase, C.F.; Nordeide, J.T. Fish sperm competition in hatcheries and between wild and hatchery origin fish in nature. Theriogenology 2019, 133, 201–209. [Google Scholar] [CrossRef]
- Moran, D.; Smith, C.K.; Gara, B.; Poortenaar, C.W. Reproductive behaviour and early development in yellowtail kingfish (Seriola lalandi Valenciennes 1833). Aquaculture 2007, 262, 95–104. [Google Scholar] [CrossRef]
- Yue, K.; Shen, Y. An overview of disruptive technologies for aquaculture. Aquac. Fish. 2021, 7, 111–120. [Google Scholar] [CrossRef]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of Stress on Fish Reproduction, Gamete Quality, and Progeny. In Reproductive Biotechnology in Finfish Aquaculture; Elsevier: Amsterdam, The Netherlands, 2001; pp. 3–24. [Google Scholar]
- McNair, A.; Lokman, P.M.; Closs, G.P.; Nakagawa, S. Ecological and evolutionary applications for environmental sex reversal of fish. Q. Rev. Biol. 2015, 90, 23–44. [Google Scholar] [CrossRef]
- Megbowon, I.; Mojekwu, T. Tilapia sex reversal using methyl testosterone (MT) and its effect on fish, man and environment. Biotechnology 2013, 13, 213–216. [Google Scholar] [CrossRef][Green Version]
- Setiawan, A.; Muncaster, S.; Pether, S.; King, A.; Irvine, G.; Lokman, P.; Symonds, J. The effects of gonadotropin-releasing hormone analog on yellowtail kingfish Seriola lalandi (Valenciennes, 1833) spawning and egg quality. Aquac. Rep. 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Avella, M.A.; Place, A.; Du, S.-J.; Williams, E.; Silvi, S.; Zohar, Y.; Carnevali, O. Lactobacillus rhamnosus accelerates zebrafish backbone calcification and gonadal differentiation through effects on the GnRH and IGF systems. PLoS ONE 2012, 7, e45572. [Google Scholar] [CrossRef] [PubMed]
- Sekkin, S.; Kum, C. Antibacterial Drugs in Fish Farms: Application and Its Effects. In Recent Advances in Fish Farms; IntechOpen: London, UK, 2011; pp. 217–250. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Awad, E.; Awaad, A. Role of medicinal plants on growth performance and immune status in fish. Fish. Shellfish. Immunol. 2017, 67, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Pourmohammadi Fallah, H.; Yousefi, M.; Dawood, M.A.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Doan, H.V. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Balam, F.H.; Ahmadi, Z.S.; Ghorbani, A. Inhibitory effect of chrysin on estrogen biosynthesis by suppression of enzyme aromatase (CYP19): A systematic review. Heliyon 2020, 6, e03557. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, M.; Nihei, Y.; Kaneko, T.; Fukada, H.; Hirano, K.; Hara, A.; Watabe, S. Plasma vitellogenin levels in male common carp Cyprinus carpio and crucian carp Carassius cuvieri of Lake Kasumigaura. Fish. Sci. 2002, 68, 1055–1066. [Google Scholar] [CrossRef][Green Version]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Nuzaiba, P.M.; Varghese, T.; Gupta, S.; Sahu, N.P.; Srivastava, P.P. Estrogenic and vitellogenic responses in genistein fed adult male Cyprinus carpio. Aquaculture 2022, 548, 737559. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Cholewińska, P.; Palić, D.; Bednarska, M.; Jarosz, M.; Wiśniewska, I. Estrogen Receptors Mediated Negative Effects of Estrogens and Xenoestrogens in Teleost Fishes. Int. J. Mol. Sci. 2022, 23, 2605. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; London, S. Nutrition and reproduction in fish. Encycl. Reprod. 2018, 9, 743–748. [Google Scholar]
- Winemiller, K.O.; Jepsen, D.B. Effects of seasonality and fish movement on tropical river food webs. J. Fish. Biol. 1998, 53, 267–296. [Google Scholar] [CrossRef]
- Ghaedi, A.; Kabir, M.A.; Hashim, R. Effect of lipid levels on the reproductive performance of Snakehead murrel, Channa striatus. Aquac. Res. 2016, 47, 983–991. [Google Scholar] [CrossRef]
- Kamenskaya, D.; Pankova, M.; Atopkin, D.; Brykov, V. Fish growth-hormone genes: Evidence of functionality of paralogous genes in Levanidov’s charr Salvelinus levanidovi. Mol. Biol. 2015, 49, 687–693. [Google Scholar] [CrossRef]
- Bock, S.L.; Chow, M.I.; Forsgren, K.L.; Lema, S.C. Widespread alterations to hypothalamic-pituitary-gonadal (HPG) axis signaling underlie high temperature reproductive inhibition in the eurythermal sheepshead minnow (Cyprinodon variegatus). Mol. Cell. Endocrinol. 2021, 537, 111447. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Ogawa, S.; Parhar, I.S. Role of serotonin in fish reproduction. Front. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef]
- Biran, J.; Levavi-Sivan, B. Endocrine Control of Reproduction, Fish. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, pp. 362–368. [Google Scholar]
- Filby, A.L.; Aerle, R.v.; Duitman, J.; Tyler, C.R. The kisspeptin/gonadotropin-releasing hormone pathway and molecular signaling of puberty in fish. Biol. Reprod. 2008, 78, 278–289. [Google Scholar] [CrossRef]
- Ohga, H.; Selvaraj, S.; Matsuyama, M. The roles of kisspeptin system in the reproductive physiology of fish with special reference to chub mackerel studies as main axis. Front. Endocrinol. 2018, 9, 147. [Google Scholar] [CrossRef]
- Reddon, A.R.; O’Connor, C.M.; Marsh-Rollo, S.E.; Balshine, S. Effects of isotocin on social responses in a cooperatively breeding fish. Anim. Behav. 2012, 84, 753–760. [Google Scholar] [CrossRef]
- Chaves-Pozo, E.; García-Ayala, A.; Cabas, I. Effects of sex steroids on fish leukocytes. Biology 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50, S195–S219. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Miura, C.I. Molecular control mechanisms of fish spermatogenesis. Fish Physiol. Biochem. 2003, 28, 181–186. [Google Scholar] [CrossRef]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Knowles, J.; Vysloužil, J.; Policar, T.; Milla, S.; Holická, M.; Podhorec, P. Spawning performance and sex steroid levels in female pikeperch Sander lucioperca treated with poly (lactic-co-glycolic acid) microparticles. Animals 2022, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Lorioux, S.; Héroin, D.; Daffe, G.; Etcheverria, B.; Cachot, J.; Morin, B.; Dufour, S.; Gonzalez, P. Transgenerational epigenetic sex determination: Environment experienced by female fish affects offspring sex ratio. Environ. Pollut. 2021, 277, 116864. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, L.; Han, J.; Zou, Y.; Wang, Y.; Feng, C.; Zhou, B. Early-life exposure to tris (1, 3-dichloro-2-propyl) phosphate caused multigenerational neurodevelopmental toxicity in zebrafish via altering maternal thyroid hormones transfer and epigenetic modifications. Environ. Pollut. 2021, 285, 117471. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.E.; Miaskowski, C.A.; Levine, J.D.; Pullinger, C.R.; Aouizerat, B.E. Epigenetic regulation and measurement of epigenetic changes. Biol. Res. Nurs. 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Breton-Larrivée, M.; Elder, E.; McGraw, S. DNA methylation, environmental exposures and early embryo development. Anim. Reprod. 2019, 16, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Li, Y.; Robertson, K.D. DNA methylation: Superior or subordinate in the epigenetic hierarchy? Genes Cancer 2011, 2, 607–617. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Brykczynska, U.; Hisano, M.; Erkek, S.; Ramos, L.; Oakeley, E.J.; Roloff, T.C.; Beisel, C.; Schübeler, D.; Stadler, M.B.; Peters, A.H. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010, 17, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-F.; Zhang, H.; Cairns, B.R. Genes for embryo development are packaged in blocks of multivalent chromatin in zebrafish sperm. Genome Res. 2011, 21, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Bizuayehu, T.T.; Babiak, I. MicroRNA in teleost fish. Genome Biol. Evol. 2014, 6, 1911–1937. [Google Scholar] [CrossRef] [PubMed]
- Pauli, A.; Rinn, J.L.; Schier, A.F. Non-coding RNAs as regulators of embryogenesis. Nat. Rev. Genet. 2011, 12, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, R.; Worren, M.M.; Høyheim, B. Discovery and characterization of miRNA genes in Atlantic salmon (Salmo salar) by use of a deep sequencing approach. BMC Genom. 2013, 14, 482. [Google Scholar] [CrossRef]
- Juanchich, A.; Bardou, P.; Rué, O.; Gabillard, J.-C.; Gaspin, C.; Bobe, J.; Guiguen, Y. Characterization of an extensive rainbow trout miRNA transcriptome by next generation sequencing. BMC Genom. 2016, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of reproductive disease. Biol Reprod 2015, 93, 145. [Google Scholar] [CrossRef] [PubMed]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Donelson, J.M.; Wong, M.; Booth, D.J.; Munday, P.L. Transgenerational plasticity of reproduction depends on rate of warming across generations. Evol. Appl. 2016, 9, 1072–1081. [Google Scholar] [CrossRef]
- Navarro-Martín, L.; Viñas, J.; Ribas, L.; Díaz, N.; Gutiérrez, A.; Di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef]
- Campos, C.; Valente, L.; Conceição, L.; Engrola, S.; Fernandes, J. Temperature affects methylation of the myogenin putative promoter, its expression and muscle cellularity in Senegalese sole larvae. Epigenetics 2013, 8, 389–397. [Google Scholar] [CrossRef]
- Akhtar, W.; Veenstra, G.J.C. TBP-related factors: A paradigm of diversity in transcription initiation. Cell Biosci. 2011, 1, 23. [Google Scholar] [CrossRef] [PubMed]
- Le Luyer, J.; Laporte, M.; Beacham, T.D.; Kaukinen, K.H.; Withler, R.E.; Leong, J.S.; Rondeau, E.B.; Koop, B.F.; Bernatchez, L. Parallel epigenetic modifications induced by hatchery rearing in a Pacific salmon. Proc. Natl. Acad. Sci. USA 2017, 114, 12964–12969. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Barreto, D.; Garcia de Leaniz, C.; Verspoor, E.; Sobolewska, H.; Coulson, M.; Consuegra, S. DNA methylation changes in the sperm of captive-reared fish: A route to epigenetic introgression in wild populations. Mol. Biol. Evol. 2019, 36, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Morán, P.; Marco-Rius, F.; Megías, M.; Covelo-Soto, L.; Pérez-Figueroa, A. Environmental induced methylation changes associated with seawater adaptation in brown trout. Aquaculture 2013, 392, 77–83. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lau, K.; Lai, K.-P.; Zhang, J.-W.; Tse, A.C.-K.; Li, J.-W.; Tong, Y.; Chan, T.-F.; Wong, C.K.-C.; Chiu, J.M.-Y.; et al. Hypoxia causes transgenerational impairments in reproduction of fish. Nat. Commun. 2016, 7, 12114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Vechtova, P.; Fussy, Z.; Sterba, J.; Linhartová, Z.; Rodina, M.; Tučková, V.; Gela, D.; Samarin, A.M.; Lebeda, I. Changes in phenotypes and DNA methylation of in vitro aging sperm in common carp Cyprinus carpio. Int. J. Mol. Sci. 2021, 22, 5925. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-L.; Yan, L.-X.; Shi, H.-J.; Liu, X.-Y.; Zheng, Q.-Y.; Sun, L.-N.; Wang, D.-S. Genome-wide identification, evolution of DNA methyltransferases and their expression during gonadal development in Nile tilapia. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 226, 73–84. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Sun, L.X.; Zhu, J.J.; Zhao, Y.; Wang, H.; Liu, H.J.; Ji, X.S. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization. J. Therm. Biol. 2017, 69, 76–84. [Google Scholar] [CrossRef]
- Ribas, L.; Vanezis, K.; Imués, M.A.; Piferrer, F. Treatment with a DNA methyltransferase inhibitor feminizes zebrafish and induces long-term expression changes in the gonads. Epigenet. Chromatin 2017, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Skaftnesmo, K.O.; Edvardsen, R.B.; Furmanek, T.; Crespo, D.; Andersson, E.; Kleppe, L.; Taranger, G.L.; Bogerd, J.; Schulz, R.W.; Wargelius, A. Integrative testis transcriptome analysis reveals differentially expressed miRNAs and their mRNA targets during early puberty in Atlantic salmon. BMC Genom. 2017, 18, 801. [Google Scholar] [CrossRef] [PubMed]
- Domingues, W.B.; Silveira, T.L.; Nunes, L.S.; Blodorn, E.B.; Schneider, A.; Corcine, C.D.; Varela Junior, A.S.; Acosta, I.B.; Kütter, M.T.; Greif, G.; et al. GH Overexpression alters spermatic cells microRNAome profile in transgenic zebrafish. Front. Genet. 2021, 12, 1712. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Bugeon, J.; Bouchareb, A.; Henry, L.; Delahaye, C.; Legeai, F.; Montfort, J.; Le Cam, A.; Siegel, A.; Bobe, J.; et al. MiR-202 controls female fecundity by regulating medaka oogenesis. PLoS Genet. 2018, 14, e1007593. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Nagasawa, K.; Fasulo, S.; Fernandes, J.M. Influence of photoperiod on expression of DNA (cytosine-5) methyltransferases in Atlantic cod. Gene 2013, 519, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Baillon, L.; Sow, M.; Gotreau, S.; Gonzalez, P. Effect of low-dose cadmium exposure on DNA methylation in the endangered European eel. Environ. Sci. Technol. 2014, 48, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Dorts, J.; Falisse, E.; Schoofs, E.; Flamion, E.; Kestemont, P.; Silvestre, F. DNA methyltransferases and stress-related genes expression in zebrafish larvae after exposure to heat and copper during reprogramming of DNA methylation. Sci. Rep. 2016, 6, 34254. [Google Scholar] [CrossRef] [PubMed]
- Major, K.M.; DeCourten, B.M.; Li, J.; Britton, M.; Settles, M.L.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Early life exposure to environmentally relevant levels of endocrine disruptors drive multigenerational and transgenerational epigenetic changes in a fish model. Front. Mar. Sci. 2020, 7, 471. [Google Scholar] [CrossRef]
- Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Differential DNA methylation in somatic and sperm cells of hatchery vs wild (natural-origin) steelhead trout populations. Environ. Epigenet. 2021, 7, dvab002. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial manipulations to improve fish health and production—A Mediterranean perspective. Fish Shellfish Immunol. 2011, 30, 1–16. [Google Scholar] [CrossRef]
- Aydin, F.; Şehriban, Ç.-Y. Effect of probiotics on reproductive performance of fish. Nat. Eng. Sci. 2019, 4, 153–162. [Google Scholar] [CrossRef]
- Salam, M.A.; Islam, M.; Paul, S.I.; Rahman, M.; Rahman, M.L.; Islam, F.; Rahman, A.; Shaha, D.C.; Alam, M.S.; Islam, T. Gut probiotic bacteria of Barbonymus gonionotus improve growth, hematological parameters and reproductive performances of the host. Sci. Rep. 2021, 11, 10692. [Google Scholar] [CrossRef] [PubMed]
- Jayasankar, V.; Tomy, S.; Wilder, M.N. Insights on molecular mechanisms of ovarian development in decapod crustacea: Focus on vitellogenesis-stimulating factors and pathways. Front. Endocrinol. 2020, 11, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Gioacchini, G.; Giorgini, E.; Merrifield, D.L.; Hardiman, G.; Borini, A.; Vaccari, L.; Carnevali, O. Probiotics can induce follicle maturational competence: The Danio rerio case. Biol. Reprod. 2012, 86, 65. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, J.; Wang, X.; Liu, D. Genetic manipulation in zebrafish. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2017, 33, 1674–1692. [Google Scholar]
- Koulish, S.; Kramer, C.R.; Grier, H.J. Organization of the male gonad in a protogynous fish, Thalassoma bifasciatum (Teleostei: Labridae). J. Morphol. 2002, 254, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Sumon, T.A.; Hussain, H.M.A.; Sumon, M.A.A.; Jang, W.J.; Guardiola, F.A.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, E.W.; Kim, C.H.; Hasan, M.T. Functionality and prophylactic role of probiotics in shellfish aquaculture. Aquac. Rep. 2022, 25, 101220. [Google Scholar] [CrossRef]
- Sumon, M.A.A.; Sumon, S.T.A.; Hussain, M.A.; Lee, S.J.; Jang, W.J.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, E.W.; Hasan, M.T. Single and multi-strain probiotics supplementation in commercially prominent finfish aquaculture: Review of the current knowledge. J. Microbiol. Biotechnol. 2022, 32, 681–698. [Google Scholar] [CrossRef]
- Giri, S.S.; Yun, S.; Jun, J.W.; Kim, H.J.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Han, S.J.; Sukumaran, V.; Park, S.C. Therapeutic effect of intestinal autochthonous Lactobacillus reuteri P16 against waterborne lead toxicity in Cyprinus carpio. Front. Immunol. 2018, 9, 1824. [Google Scholar] [CrossRef]
- Gioacchini, F.; Lombardo, F.; Merrifield, D.; Silvi, S.; Cresci, A.; Avella, M.; Carnevali, O. Effects of probiotic on zebrafish reproduction. J. Aquac. Res. Dev. 2011, S1, 1–6. [Google Scholar] [CrossRef]
- Grunwald, D.J.; Eisen, J.S. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nat. Rev. Genet. 2002, 3, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chen, Y. Zebrafish as an emerging model to study gonad development. Comput. Struct. Biotechnol. J. 2020, 18, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yu, E.; Lu, M.; Xie, J. Effects of probiotic supplementation on gut microbiota as well as metabolite profiles within Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 527, 735428. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, D.; Liu, W.; Wang, J.; Liu, X.; Zhou, C.; Gui, J.; Xiao, W. Zebrafish androgen receptor is required for spermatogenesis and maintenance of ovarian function. Oncotarget 2018, 9, 24320–24334. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Riesco, M.F.; Martínez-Vázquez, J.M.; Robles, V. Diet supplemented with antioxidant and anti-inflammatory probiotics improves sperm quality after only one spermatogenic cycle in zebrafish model. Nutrients 2019, 11, 843. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, O.; Avella, M.; Gioacchini, G. Effects of probiotic administration on zebrafish development and reproduction. Gen. Comp. Endocrinol. 2013, 188, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Xu, L.; Yang, Y.; He, S.; Dai, Y.; Zhao, H.; Zhou, Z. Comparison of fecundity and offspring immunity in zebrafish fed Lactobacillus rhamnosus CICC 6141 and Lactobacillus casei BL23. Reproduction 2014, 147, 53–64. [Google Scholar] [CrossRef]
- Gioacchini, F.; Maradonna, F.; Lombardo, F.; Bizzaro, D.; Olivotto, I.; Carnevali, O. Increase of fecundity by probiotic administration in zebrafish (Danio rerio). Reproduction 2010, 140, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The gut microbiota of marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Giahi, L.; Mohammadmoradi, S.; Javidan, A.; Sadeghi, M.R. Nutritional modifications in male infertility: A systematic review covering 2 decades. Nutr. Rev. 2016, 74, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Ekasari, J.; Rivandi, D.R.; Firdausi, A.P.; Surawidjaja, E.H.; Zairin Jr, M.; Bossier, P.; De Schryver, P. Biofloc technology positively affects Nile tilapia (Oreochromis niloticus) larvae performance. Aquaculture 2015, 441, 72–77. [Google Scholar] [CrossRef]
- Gbemisola, O.B. Sperm quality and reproductive performance of male Clarias gariepinus induced with synthetic hormones (ovatide and ovaprim). Int. J. Fish. Aquac. 2014, 6, 9–15. [Google Scholar]
- Koh, I.C.C.; Badrul Nizam, B.H.; Muhammad Abduh, Y.; Abol Munafi, A.B.; Iehata, S. Molecular characterization of microbiota associated with sperm of Malaysian mahseer Tor tambroides. Evol. Bioinform. 2019, 15, 1176934319850821. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Naik, S.; Vakil, B. Probiotics, prebiotics and synbiotics-a review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, M.D. Evolution of probiotics in aquatic world: Potential effects, the current status in Egypt and recent prospectives. J. Adv. Res. 2015, 6, 765–791. [Google Scholar] [CrossRef]
- Ishthiaq, I.B.; Ahmed, J.; Ramalingam, K. Probiotics in Brackish Water Fish Farming: A Special Focus on Encapsulated Probiotics. Proc. Int. 2021, 3, 74. [Google Scholar]
- Kusuma, P.S.W.; Hariani, D.; Mukti, A.T. Evaluation of probiotic-fermented feed addition and laser-firing to accelerate mature broodstocks and seed productions of African Catfish (Clarias gariepinus). Turk. J. Fish. Aquat. Sci. 2021, 22, TRJFAS19303. [Google Scholar] [CrossRef]
- Chen, X.; Yi, H.; Liu, S.; Zhang, Y.; Su, Y.; Liu, X.; Bi, S.; Lai, H.; Zeng, Z.; Li, G. Probiotics improve eating disorders in mandarin fish (Siniperca chuatsi) induced by a pellet feed diet via stimulating immunity and regulating gut microbiota. Microorganisms 2021, 9, 1288. [Google Scholar] [CrossRef]
- Shen, Y.; Yue, G. Current status of research on aquaculture genetics and genomics-information from ISGA 2018. Aquac. Fish. 2019, 4, 43–47. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumon, M.A.A.; Molla, M.H.R.; Hakeem, I.J.; Ahammad, F.; Amran, R.H.; Jamal, M.T.; Gabr, M.H.; Islam, M.S.; Alam, M.T.; Brown, C.L.; et al. Epigenetics and Probiotics Application toward the Modulation of Fish Reproductive Performance. Fishes 2022, 7, 189. https://doi.org/10.3390/fishes7040189
Sumon MAA, Molla MHR, Hakeem IJ, Ahammad F, Amran RH, Jamal MT, Gabr MH, Islam MS, Alam MT, Brown CL, et al. Epigenetics and Probiotics Application toward the Modulation of Fish Reproductive Performance. Fishes. 2022; 7(4):189. https://doi.org/10.3390/fishes7040189
Chicago/Turabian StyleSumon, Md Afsar Ahmed, Mohammad Habibur Rahman Molla, Israa J. Hakeem, Foysal Ahammad, Ramzi H. Amran, Mamdoh T. Jamal, Mohamed Hosny Gabr, Md. Shafiqul Islam, Md. Tariqul Alam, Christopher L. Brown, and et al. 2022. "Epigenetics and Probiotics Application toward the Modulation of Fish Reproductive Performance" Fishes 7, no. 4: 189. https://doi.org/10.3390/fishes7040189
APA StyleSumon, M. A. A., Molla, M. H. R., Hakeem, I. J., Ahammad, F., Amran, R. H., Jamal, M. T., Gabr, M. H., Islam, M. S., Alam, M. T., Brown, C. L., Lee, E.-W., Moulay, M., Asseri, A. H., Opo, F. A. D. M., Alsaiari, A. A., & Hasan, M. T. (2022). Epigenetics and Probiotics Application toward the Modulation of Fish Reproductive Performance. Fishes, 7(4), 189. https://doi.org/10.3390/fishes7040189










