Designing a Multi-Parameter Method to Assess the Adaptation Period of Crucian Carp under Stress Conditions of the Bionic Robot Fish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
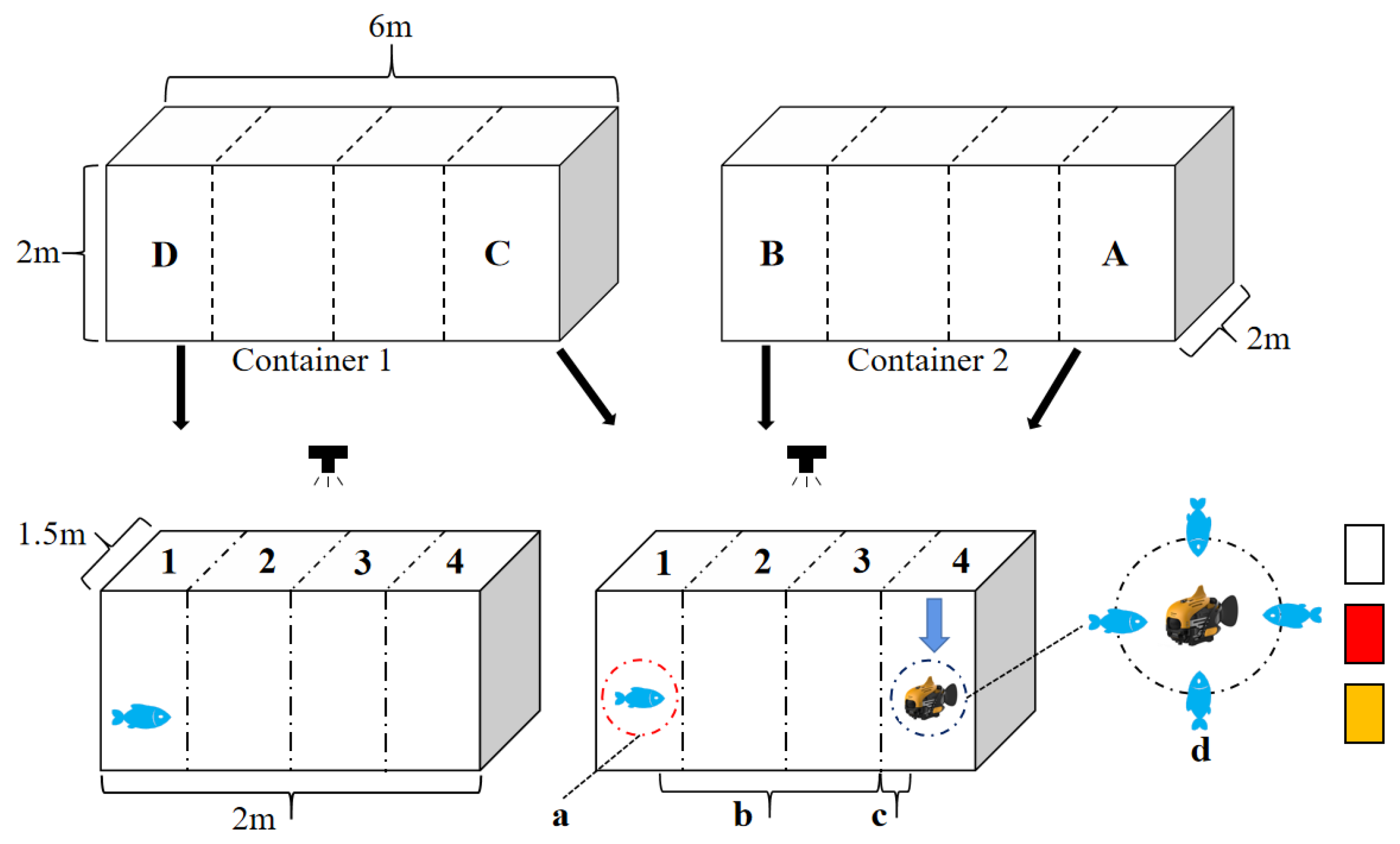
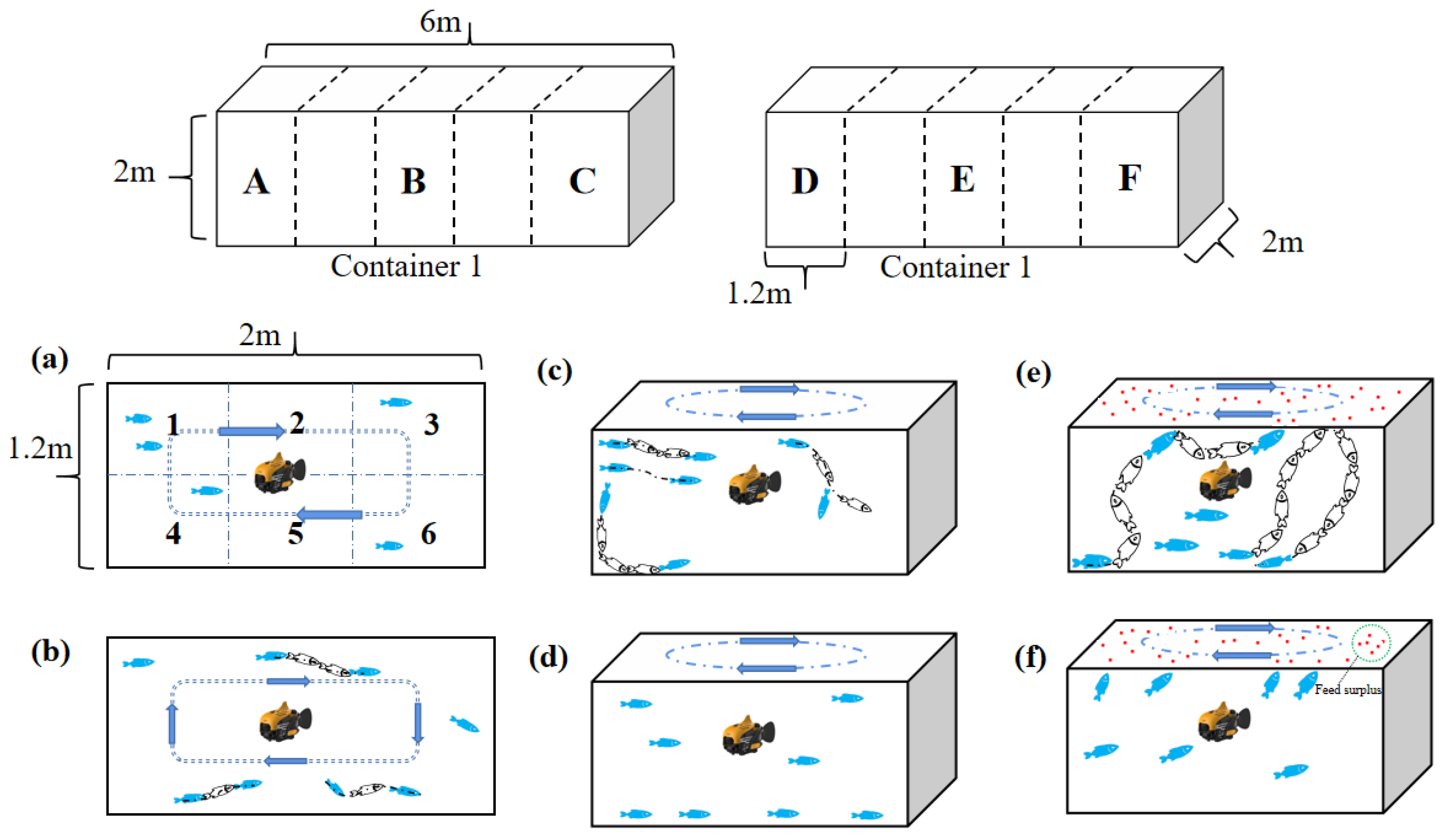
2.2. Experimental Design
2.3. Behavioral Location and Body-Color Analysis
2.4. Feeding State Analysis
2.5. Sampling Link
2.6. Method for Determination of Blood and Plasma Parameters
2.7. Skin Melanin Ratio
2.8. Statistical Analyses
3. Results
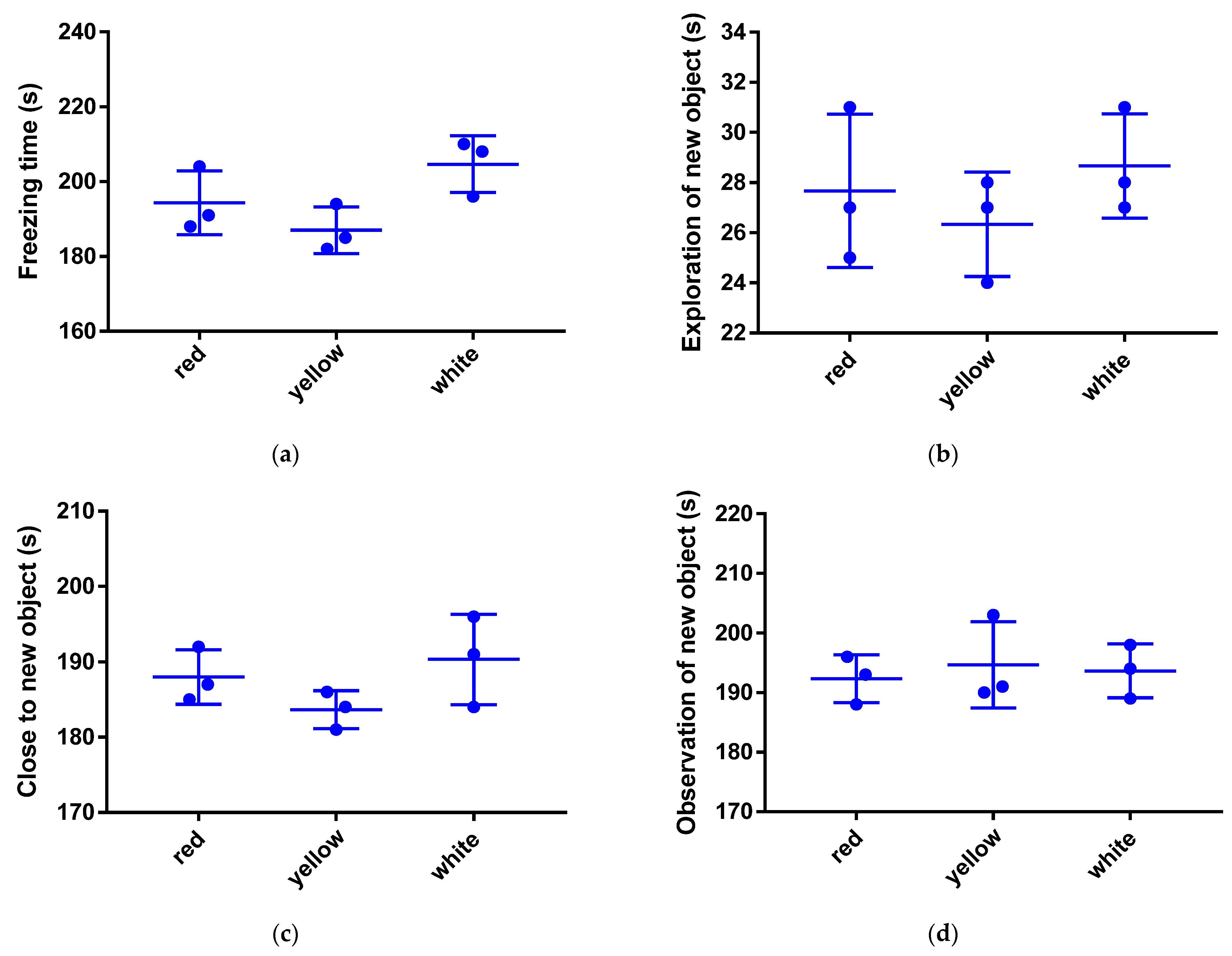
3.1. New Object Test
3.2. Behavior and Color Analysis
3.2.1. Position Analysis
3.2.2. Activity
3.2.3. Position in the Water
3.2.4. Abnormal Swimming
3.2.5. Body Color
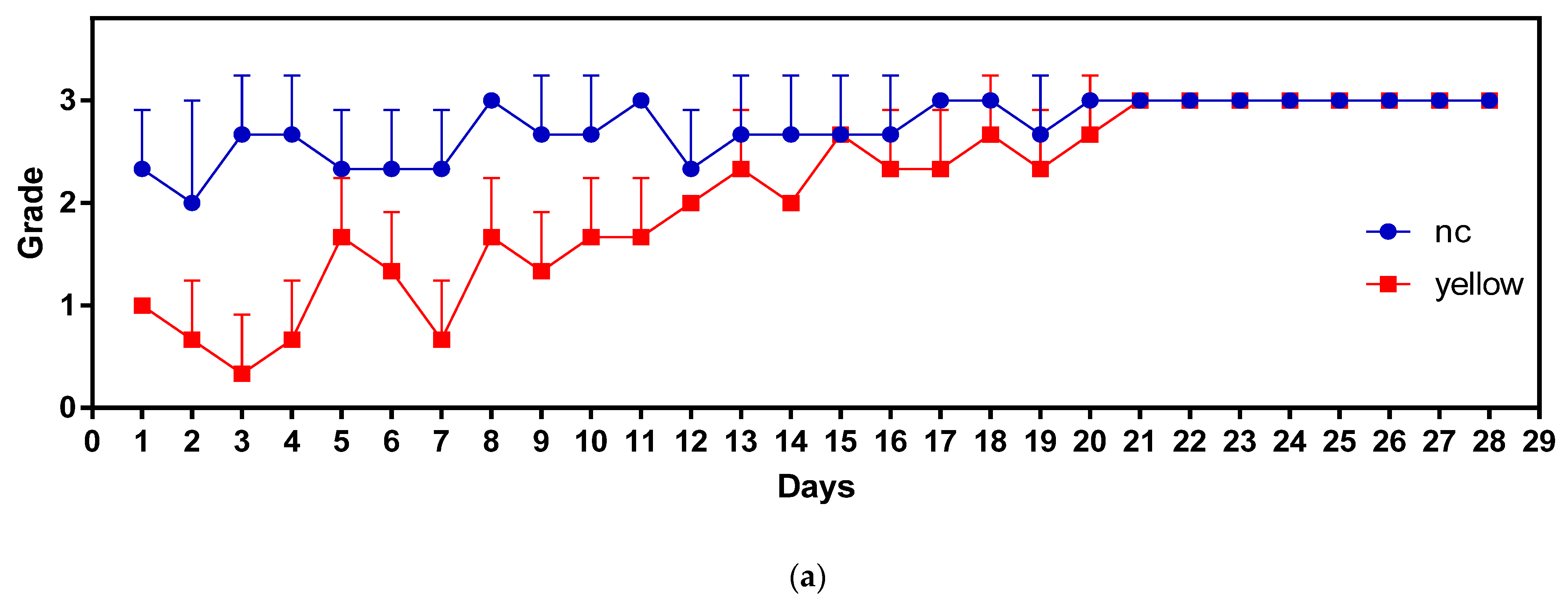
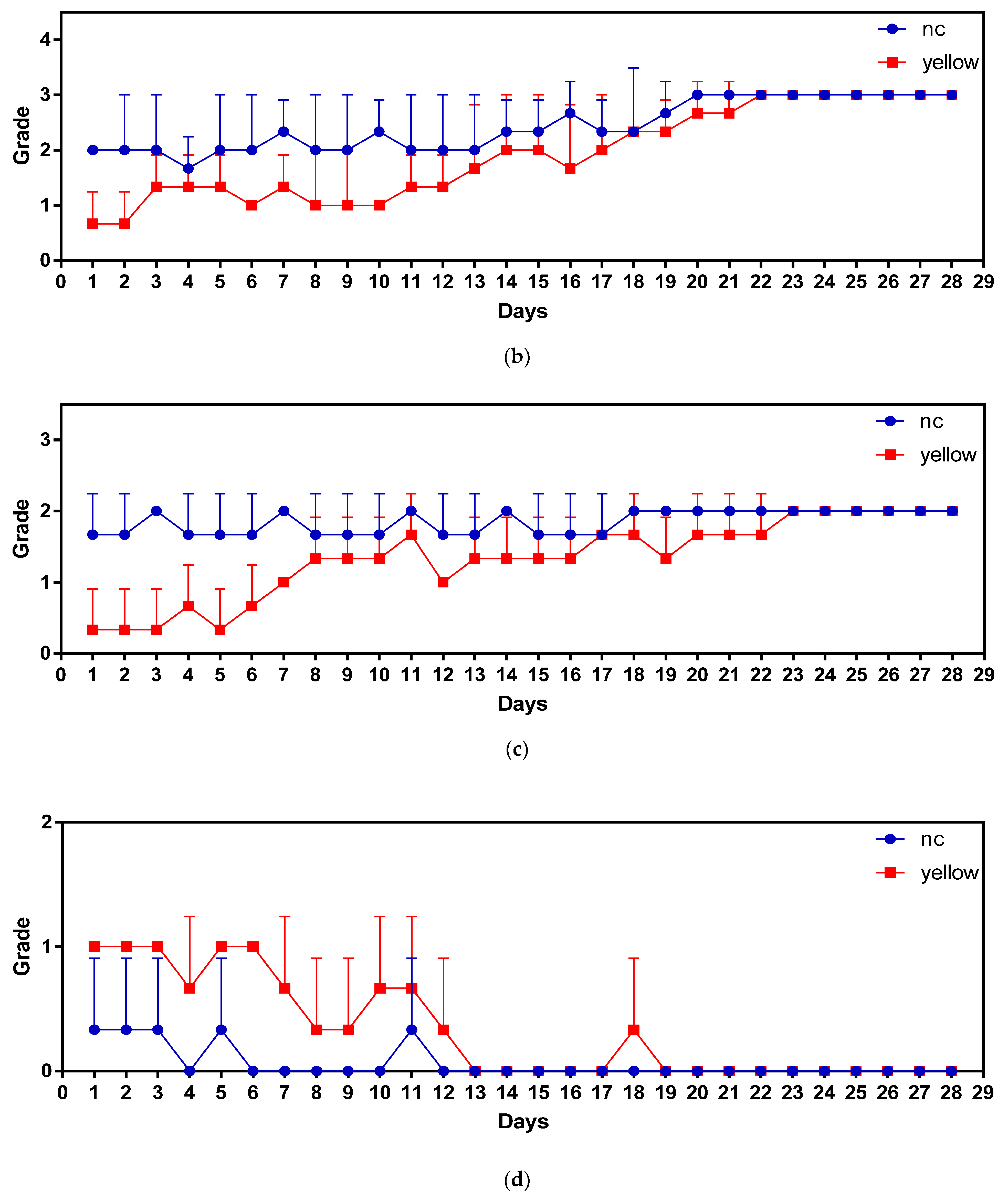

3.3. Feeding Behavior Analyses
3.3.1. Reaction to Food
3.3.2. Food Consumption
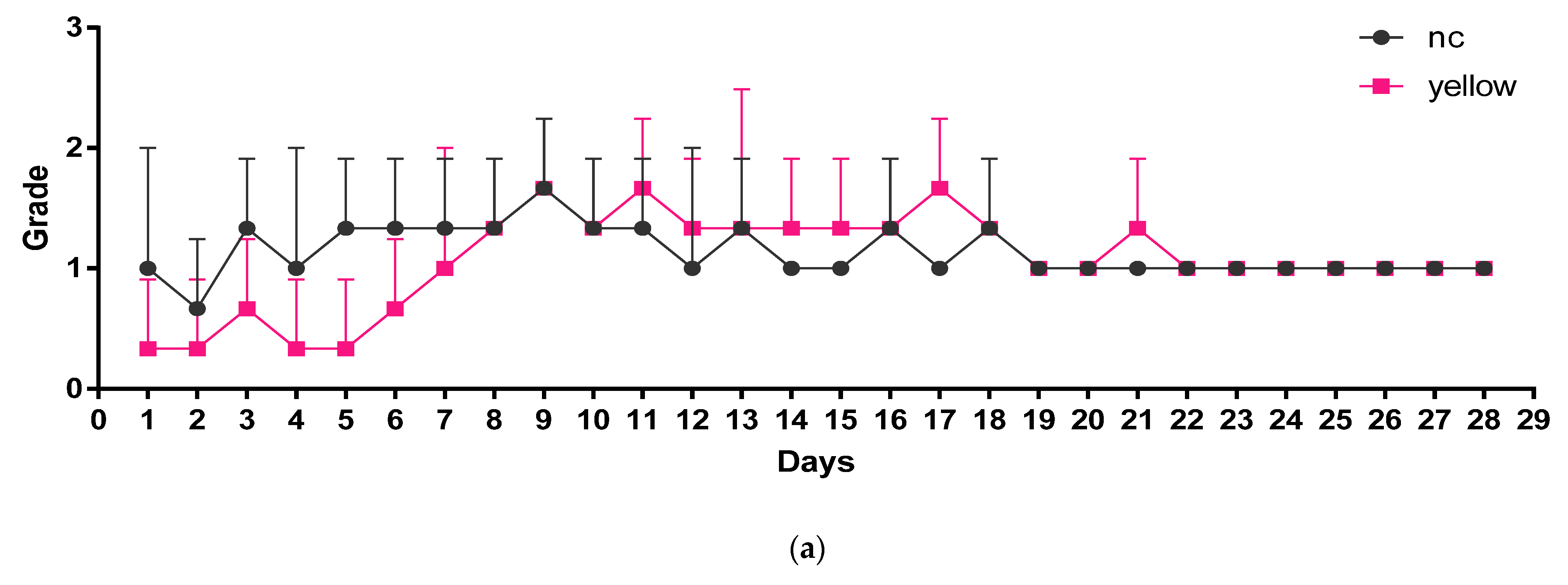

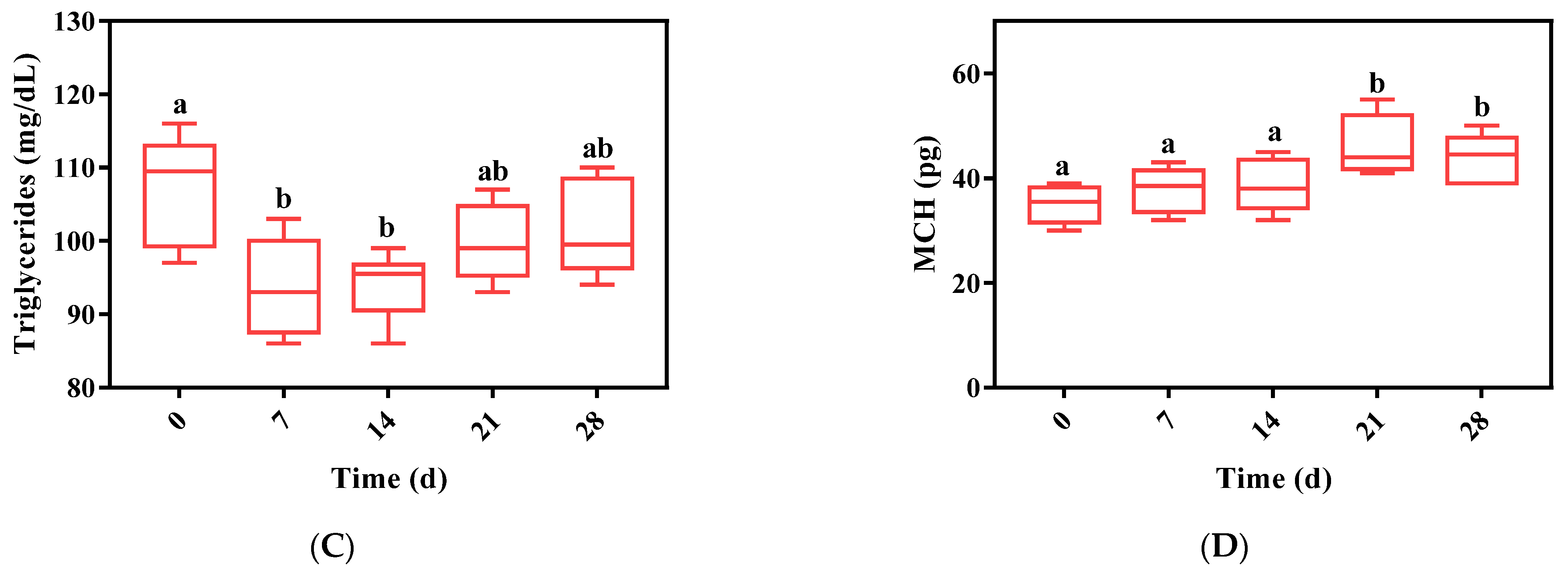
3.4. Determination of Blood and Plasma
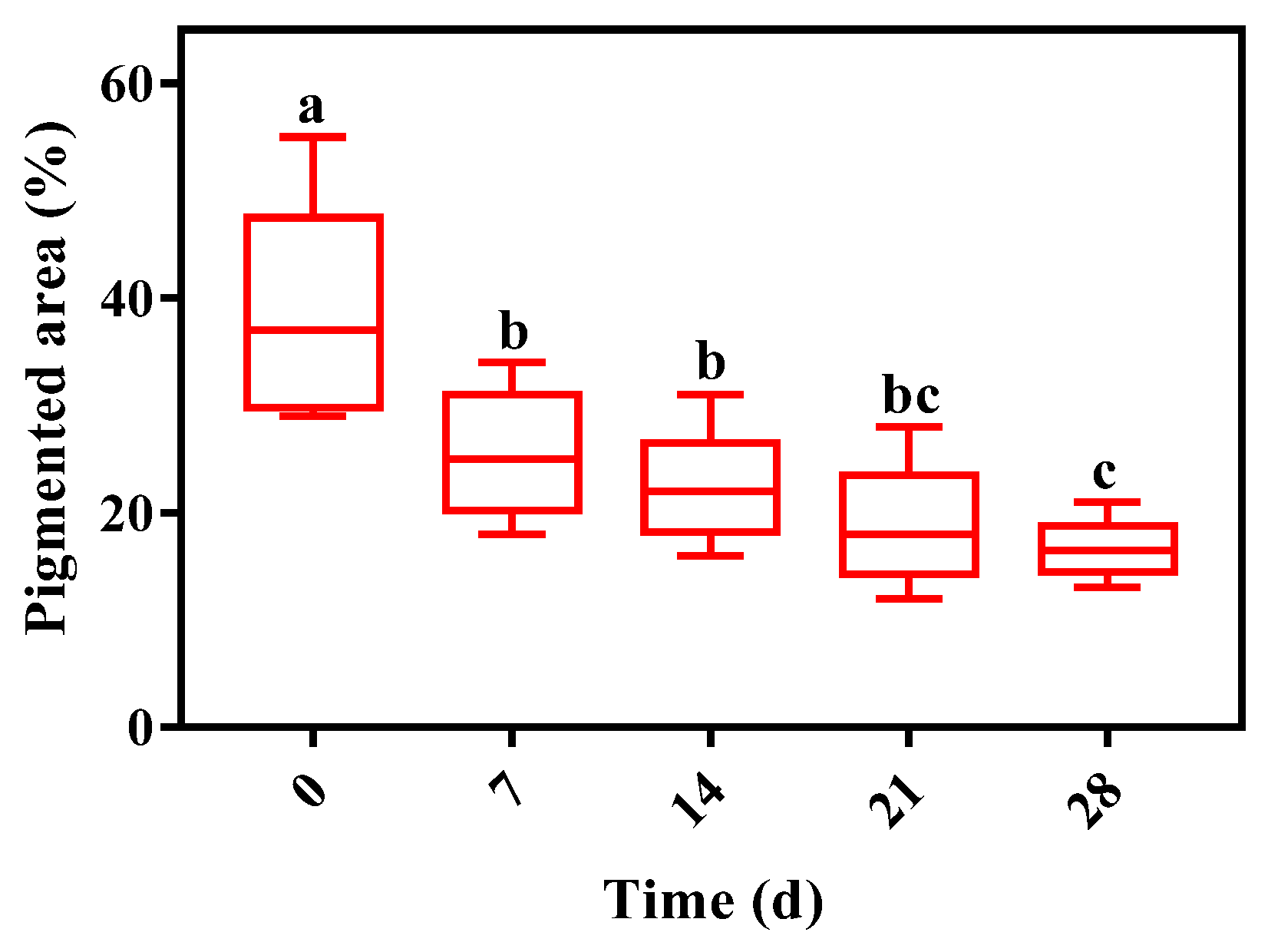
3.5. Skin Melanin Area Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bortoletti, M.; Maccatrozzo, L.; Radaelli, G.; Caberlotto, S.; Bertotto, D. Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress. Animals 2021, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, P.; Valença-Silva, G.; Queiroz, M.R.; Broom, D.M. Living with low environmental complexity increases fear indicators in Nile tilapia. Anim. Behav. 2021, 174, 169–174. [Google Scholar] [CrossRef]
- Wang, B.; Ma, G.; Liu, Y.; Wang, Y.; Du, X.; Shi, Q.; Mao, H. Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus. Fishes 2021, 6, 66. [Google Scholar] [CrossRef]
- Paunescu, A.; Soare, L.C.; Fierascu, I.; Fierascu, R.C.; Mihaescu, C.F.; Tofan, L.; Ponepal, C.M. Ecotoxicological Studies on the Action of Actara 25 WG Insecticide on Prussian Carp (Carassius gibelio) and Marsh Frog (Pelophylax ridibundus). Toxics 2022, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Zhao, J.; Ji, B.; Li, H.; Zhang, Y.; Peng, Z.; Zhou, F.; Ding, X.; Ye, Z. Impact of underwater noise on the growth, physiology and behavior of Micropterus salmoides in industrial recirculating aquaculture systems. Environ. Pollut. 2021, 291, 118152. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Cornish, A.; Elwin, A.; D’Cruze, N. A Kettle of Fish: A Review of the Scientific Literature for Evidence of Fish Sentience. Animals 2022, 12, 1182. [Google Scholar] [CrossRef]
- Chen, B.K.; Murawski, N.J.; Cincotta, C.; McKissick, O.; Finkelstein, A.; Hamidi, A.B.; Merfeld, E.; Doucette, E.; Grella, S.L.; Shpokayte, M.; et al. Artifificially Enhancing and Suppressing Hippocampus-Mediated Memories. Curr. Biol. 2019, 29, 1885–1894. [Google Scholar] [CrossRef]
- MacIver, M.A.; Finlay, B.L. The neuroecology of the water-to-land transition and the evolution of the vertebrate brain. Phil. Trans. R. Soc. B 2021, 377. [Google Scholar] [CrossRef]
- Brunet, V.; Kleiber, A.; Patinote, A.; Sudan, P.-L.; Duret, C.; Gourmelen, G.; Moreau, E.; Fournel, C.; Pineau, L.; Calvez, S.; et al. Positive welfare effects of physical enrichments from the nature-, functions and feeling- based approaches in farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 550, 737825. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An Important Tool to Assess the Welfare of Aquatic Animals. Biology 2021, 10, 61. [Google Scholar] [CrossRef]
- Bruzsik, B.; Biro, L.; Sarosdi, K.R.; Zelena, D.; Sipos, E.; Szebik, H.; Török, B.; Mikics, E.; Toth, M. Neurochemically distinct populations of the bed nucleus of stria terminalis modulate innate fear response to weak threat evoked by predator odor stimuli. Neurobiol. Stress 2021, 15, 100415. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. As soon as there was life, there was danger: The deep history of survival behaviours and the shallower history of consciousness. Philos. Trans. R. Soc. B Biol. Sci. 2021, 377. [Google Scholar] [CrossRef] [PubMed]
- Zidan, E.M.; Goma, A.A.; Tohamy, H.G.; Soliman, M.M.; Shukry, M. Insight study on the impact of different salinity levels on behavioural responses, biochemical stress parameters and growth performance of African catfish (Clarias gariepinus). Aquac. Res. 2022, 53, 2750–2759. [Google Scholar] [CrossRef]
- Chekani, R.; Akrami, R.; Ghiasvand, Z.; Chitsaz, H.; Jorjani, S. Effect of dietary dehydrated lemon peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 2021, 539, 736597. [Google Scholar] [CrossRef]
- Franco-Martinez, L.; Brandts, I.; Reyes-López, F.; Tort, L.; Tvarijonaviciute, A.; Teles, M. Skin Mucus as a Relevant Low-Invasive Biological Matrix for the Measurement of an Acute Stress Response in Rainbow Trout (Oncorhynchus mykiss). Water 2022, 14, 1754. [Google Scholar] [CrossRef]
- Yousefi, M.; Hoseini, S.M.; Aydın, B.; Mirghaed, A.T.; Kulikov, E.V.; Drukovsky, S.G.; Seleznev, S.B.; Rudenko, P.A.; Hoseinifar, S.H.; Van Doan, H. Anesthetic efficacy and hemato-biochemical effects of thymol on juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2022, 547, 737540. [Google Scholar] [CrossRef]
- Muñoz, L.; Aspillaga, E.; Palmer, M.; Saraiva, J.; Arechavala-Lopez, P. Acoustic Telemetry: A Tool to Monitor Fish Swimming Behavior in Sea-Cage Aquaculture. Front. Mar. Sci. 2020, 7, 645. [Google Scholar] [CrossRef]
- Souza, R.C.R.; Pompeu, P.S. Ecological separation by ecomorphology and swimming performance between two congeneric fish species. Zoologia 2020, 37, 1–8. [Google Scholar] [CrossRef]
- de Alba, G.; Carrillo, S.; Sánchez-Vázquez, F.J.; López-Olmeda, J.F. Combined blue light and daily thermocycles enhance zebrafish growth and development. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 501–515. [Google Scholar] [CrossRef]
- Talijančić, I.; Žužul, I.; Kiridžija, V.; Šiljić, J.; Pleadin, J.; Grubišić, L.; Šegvić-Bubić, T. Plastic Responses of Gilthead Seabream Sparus aurata to Wild and Aquaculture Pressured Environments. Front. Mar. Sci. 2021, 8, 694627. [Google Scholar] [CrossRef]
- Tunçelli, G.; Pirhonen, J. Effects of weekend starvation and the duration of daily feeding on production parameters of rainbow trout Oncorhynchus mykiss. Aquaculture 2021, 543, 737028. [Google Scholar] [CrossRef]
- Madureira, T.V.; Costa, J.L.; Malhão, F.; Lopes, C.; Gonçalves, J.F.; Rocha, E. Design of a multi-parametric profile for assessing the acclimation period of juvenile brown trout after an acute transport to new housing environment. Appl. Anim. Behav. Sci. 2019, 219. [Google Scholar] [CrossRef]
- Tsakoumis, E.; Tsoulia, T.; Feidantsis, K.; Mouchlianitis, F.A.; Berillis, P.; Bobori, D.; Antonopoulou, E. Cellular Stress Responses of the Endemic Freshwater Fish Species Alburnus vistonicus Freyhof & Kottelat, 2007 in a Constantly Changing Environment. Appl. Sci. 2021, 11, 11021. [Google Scholar]
- Artero, C.; Marchetti, S.; Bauer, E.; Viala, C.; Noël, C.; Koenig, C.; Berzins, R.; Lampert, L. High-Resolution Acoustic Cameras Provide Direct and Efficient Assessments of Large Demersal Fish Populations in Extremely Turbid Waters. Appl. Sci. 2021, 11, 1899. [Google Scholar] [CrossRef]
- Walli, A.; Teo, S.L.H.; Boustany, A.; Farwell, C.J.; Williams, T.; Dewar, H.; Prince, E.; Block, B.A. Seasonal Movements, Aggregations and Diving Behavior of Atlantic Bluefin Tuna (Thunnus thynnus) Revealed with Archival Tags. PLoS ONE 2009, 4, e6151. [Google Scholar] [CrossRef]
- Gutierrez-Estrada, J.C.; Pulido-Calvo, I.; Castro-Gutierrez, J.; Peregrín, A.; Domínguez, S.L.; Gomez-Bravo, F.; Garrocho-Cruz, A.; de la Rosa-Lucas, I. Fish abundance estimation with imaging sonar in semi-intensive aquaculture ponds. Aquac. Eng. 2022, 97, 102235. [Google Scholar] [CrossRef]
- Calduch-Giner, J.; Holhorea, P.G.; Ferrer, M.Á.; Naya-Català, F.; Rosell-Moll, E.; García, C.V.; Prunet, P.; Espmark, Å.M.; Leguen, I.; Kolarevic, J.; et al. Revising the Impact and Prospects of Activity and Ventilation Rate Bio-Loggers for Tracking Welfare and Fish-Environment Interactions in Salmonids and Mediterranean Farmed Fish. Front. Mar. Sci. 2022, 9, 854888. [Google Scholar] [CrossRef]
- De Pasquale, C.; Kemerer, N.; White, N.; Yost, M.; Wolfkill, J.; Sturgill, J.; Li, X. The Influence of an Enriched Environment in Enhancing Recognition Memory in Zebrafish (Danio rerio). Front. Vet. Sci. 2021, 8, 749746. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; Sánchez, B.; Barroso, F.G.; Toniolo, M.; Trenzado, C.E.; Rus, A.S. Effects of rearing conditions on behavioural responses, social kinetics and physiological parameters in gilthead sea bream Sparus aurata. Appl. Anim. Behav. Sci. 2017, 197, 120–128. [Google Scholar] [CrossRef]
- Zhao, J.; Bao, W.J.; Zhang, F.D.; Ye, Z.Y.; Liu, Y.; Shen, M.W.; Zhu, S.M. Assessing appetite of the swimming fish based on spontaneous collective behaviors in a recirculating aquaculture system. Aquac. Eng. 2017, 78, 196–204. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Audira, G.; Liang, S.-T.; Siregar, P.; Suryanto, M.; Lin, H.-C.; Villalobos, O.; Villaflores, O.; Hao, E.; Lim, K.-H.; et al. Duplicated dnmt3aa and dnmt3ab DNA Methyltransferase Genes Play Essential and Non-Overlapped Functions on Modulating Behavioral Control in Zebrafish. Genes 2020, 11, 1322. [Google Scholar] [CrossRef]
- Suriyampola, P.S.; Iruri-Tucker, A.A.; Padilla-Velez, L.; Enriquez, A.; Shelton, D.S.; Martins, E.P. Small increases in group size improve small shoals’ response to water flow in zebrafish. J. Zool. 2022, 316, 271–281. [Google Scholar] [CrossRef] [PubMed]
- McLean, E. Fish tank color: An overview. Aquaculture 2021, 530, 735750. [Google Scholar] [CrossRef]
- Wu, H.; Murata, M.; Matsumoto, H.; Ohnuki, H.; Endo, H. Integrated Biosensor System for Monitoring and Visualizing Fish Stress Response. Sensors Mater. 2020, 32, 4095. [Google Scholar] [CrossRef]
- Höglund, E.; Fernandes, P.; Rojas-Tirado, P.; Rundberget, J.T.; Hess-Erga, O.-K. Assessing Stress Resilience After Smolt Transportation by Waterborne Cortisol and Feeding Behavior in a Commercial Atlantic Salmon (Salmo salar) Grow-Out Recirculating Aquaculture System. Front. Physiol. 2022, 12, 771951. [Google Scholar] [CrossRef]
- Carbonara, P.; Dioguardi, M.; Cammarata, M.; Zupa, W.; Vazzana, M.; Spedicato, M.T.; Lembo, G. Basic knowledge of social hierarchies and physiological profile of reared sea bass Dicentrarchus labrax (L.). PLoS ONE 2019, 14, e0208688. [Google Scholar] [CrossRef]
- Dasgupta, P. Temporal variations in glucose level, oxidative stress and antioxidant profle of Koi carps (Cyprinus carpio var koi) in response to hypoxic tolerance. Biol. Futur. 2021, 72, 421–429. [Google Scholar] [CrossRef]
- Demska-Zakęś, K.; Gomułka, P.; Rożyński, M.; Zakęś, Z. Effect of a short-term sodium chloride bath on juvenile pikeperch (Sander lucioperca) welfare. Aquac. Rep. 2021, 19, 100569. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, Y.; Adányi, N.; Zhang, X. Effects of waterless live transportation on survivability, physiological responses and flesh quality in Chinese farmed sturgeon (Acipenser schrenckii). Aquaculture 2020, 518, 734834. [Google Scholar] [CrossRef]
- Barton, B.A. Salmonid Fishes Differ in Their Cortisol and Glucose Responses to Handling and Transport Stress. North Am. J. Aquac. 2000, 62, 12–18. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Baes, M.; Hoseini, S.M. Humoral immune responses and gill antioxidant-related gene expression of common carp (Cyprinus carpio) exposed to lufenuron and flonicamide. Fish Physiol. Biochem. 2020, 46, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Souza-Bastos, L.R.; Bastos, L.P.; Carneiro, P.C.F.; Freire, C.A. Acute salt exposure of the freshwater Characiformes: Pacu (Piaractus mesopotamicus, Holmberg 1887), Tambaqui (Colossoma macropomum, Cuvier 1818), and their hybrid “Tambacu”. Aquaculture 2016, 465, 352–358. [Google Scholar] [CrossRef]
- Sadoul, B.; Alfonso, S.; Cousin, X.; Prunet, P.; Begout, M.L.; Leguen, I. Global assessment of the response to chronic stress in European sea bass. Aquaculture 2021, 544, 737072. [Google Scholar] [CrossRef]
- Kawakami, Y. Characterization of triglycerides during early development of the Japanese eel (Anguilla japonica). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 265, 111125. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Qi, C.; Li, E.; Du, Z.; Qin, J.G.; Chen, L. Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 2018, 495, 187–195. [Google Scholar] [CrossRef]
- Borowiec, B.G.; Scott, G.R. Rapid and reversible modulation of blood haemoglobin content during diel cycles of hypoxia in killifish (Fundulus heteroclitus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 261, 111054. [Google Scholar] [CrossRef]
- Costa, D.C.; Mattioli, C.C.; Silva, W.S.; Takata, R.; Leme, F.O.P.; Oliveira, A.L.; Luz, R.K. The effect of environmental colour on the growth, metabolism, physiology and skin pigmentation of the carnivorous freshwater catfish Lophiosilurus alexandri. J. Fish Biol. 2017, 90, 922–935. [Google Scholar] [CrossRef]
- Gozdowska, M.; Sokołowska, E.; Pomianowski, K.; Kulczykowska, E. Melatonin and cortisol as components of the cutaneous stress response system in fish: Response to oxidative stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 268. [Google Scholar] [CrossRef]
- Øverli, Ø.; Nordgreen, J.; Mejdell, C.M.; Janczak, A.M.; Kittilsen, S.; Johansen, I.B.; Horsberg, T.E. Ectoparasitic sea lice (Lepeophtheirus salmonis) affect behavior and brain serotonergic activity in Atlantic salmon (Salmo salar L.): Perspectives on animal welfare. Physiol. Behav. 2014, 132, 44–50. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Mao, H.; Zhao, J.; Liu, Y.; Wang, Y.; Du, X. Designing a Multi-Parameter Method to Assess the Adaptation Period of Crucian Carp under Stress Conditions of the Bionic Robot Fish. Fishes 2022, 7, 198. https://doi.org/10.3390/fishes7040198
Wang B, Mao H, Zhao J, Liu Y, Wang Y, Du X. Designing a Multi-Parameter Method to Assess the Adaptation Period of Crucian Carp under Stress Conditions of the Bionic Robot Fish. Fishes. 2022; 7(4):198. https://doi.org/10.3390/fishes7040198
Chicago/Turabian StyleWang, Bin, Hanping Mao, Jian Zhao, Yong Liu, Yafei Wang, and Xiaoxue Du. 2022. "Designing a Multi-Parameter Method to Assess the Adaptation Period of Crucian Carp under Stress Conditions of the Bionic Robot Fish" Fishes 7, no. 4: 198. https://doi.org/10.3390/fishes7040198
APA StyleWang, B., Mao, H., Zhao, J., Liu, Y., Wang, Y., & Du, X. (2022). Designing a Multi-Parameter Method to Assess the Adaptation Period of Crucian Carp under Stress Conditions of the Bionic Robot Fish. Fishes, 7(4), 198. https://doi.org/10.3390/fishes7040198








