Abstract
In this study, we aimed to estimate the age, growth, and population structure to explore the life history of purpleback flying squid (Sthenoteuthis oualaniensis) by statolith microstructure in the waters of the Xisha Islands of the South China Sea. The purpleback squid, S. oualaniensis, has been the most important economic cephalopod resource of the South China Sea; however, little is known about its life history, especially its age and population structure. The age and growth pattern have been explored via the statolith microstructure of this species of squid, specimens of which were caught randomly between January and March and between May and August of 2018, 2019, and 2020 in the waters surrounding the Xisha Islands of the South China Sea. The results indicated that the range of the mantle length (ML) was 63–229 mm for females and 59–184 for males, and the body weight (BW) ranged from 13 to 435 g for females and from 7 to 152 g for males; the ages were estimated as being between 81 and 298 days for females and between 67 and 286 days for males, respectively. The hatching date extended from January to December, with a peak between November and March of the following year, suggesting the presence of one spawning group (winter–spring group). Significant differences existed between the ML growth and the BW growth. The relationships between ML and age were best described by the linear function for females and the power function for males; the relationship between BW and age were best described by the exponential function for females and the power function for males, based on our AIC models, respectively. S. oualaniensis is a fast-growing squid; the growth rate is the fastest during the young life stage, and it decreases after the subadult stage (120–150 days). After the first spawning behavior, the inflection point of the growth was recognized at the age of 180–210 days (6–7 months). This study provided basic, favorable information for the fishery biology, ecology, and resource management of purpleback flying squid (S. oualaniensis) of the South China Sea.
1. Introduction
The purpleback flying squid, Sthenoteuthis oualaniensis is an oceanic, pelagic cephalopod species with a wide distribution throughout the tropical and subtropical open waters of the Indo-Pacific region [1,2], such as the South China Sea [3] and the north Indian Ocean [4]. S. oualaniensis is not only the most common bycatch of Dosidicus gigas fisheries in the southeastern Pacific Ocean [1], but it is also the most economically important cephalopod species in the South China Sea, supporting thousands of Chinese light-falling-net fishery fleets from Guangxi, Guangdong, and Hainan provinces [5]. Previous studies have been carried out on the life history of the squid, including the age and growth, population structure, and stock biomass [5,6,7,8,9,10]. The annual life cycle of S. oualaniensis is approximately 1 year, similar to most oceanic cephalopods, based on the microstructure of the hard structures, in the Northwest Indian Ocean [4], the Eastern Tropical Pacific Ocean [11,12] and the North Pacific Ocean [13,14]. S. oualaniensis grows fast, with a significant sexual difference in body growth. (Females grow usually much faster than males.) [4,6,15] The geographic variations in the mature size of this squid have been found; the size of the female mantle length (ML) at maturity is 220 mm, and that of males is 190 mm in Australian waters [16], and the smallest, fully mature females (stage V) have an ML of 244 mm in the Northwest Indian Ocean [4]. Females mature at 184–200 mm ML, with a beginning maturity size of 137 mm in ML for males in the Eastern Tropical Pacific Ocean [12]; however, S. oualaniensis observed in New Caledonia [17] and in Taiwan province of China [18] reach maturity at similar sizes. Maximum sizes recorded in Hawaiian waters for females are 335 mm in ML (1.6 kg), and 210 mm in ML for males [19].
A complex population structure, including three major and two minor forms with different distributions of S. oualaniensis has been reported [20]. The giant form, with ML sizes of 400–500 mm and a maximum size of 650 mm occurs only in the Northern Indian Ocean, a region in the Red Sea, in the Gulf of Aden, and in the Arabian Sea [4,21]. The medium form (the typical one, without a dorsal mantle photophore patch), with sizes of 120–150 mm and 190–250 mm for mature males and females, respectively, occurs in equatorial waters [1,20]. The dwarf equatorial form, with ML sizes of 90–120 mm at maturity, is found roughly within 10° latitude of the equator, where it co-occurs with the typical form [20,22], which can be differentiated from the typical one (by the absence of the dorsal photophore patch, a slightly different hectocotylus, and slight differences in the spermatophore and gladius structures) [20]. The extent of subpopulation mixing and genetic isolation are not well known, and several size classes have been observed in the same waters at times [16].
With the ongoing development of the marine fishing industry all over the world, cephalopod species have been attracting more and more attention [23], especially oceanic ommastrephidae, which seem to be the only unique, abundant reserve in the world’s ocean for the increase in the global commercial catch of high-quality food proteins [1]. S. oualaniensis is the most important economic ommastrephid resource in the South China Sea [24], caught as the target cephalopod species by the Chinese, Philippine, and Indonesian fishery fleets, as well as others [24,25]. Previous research has indicated that the biomass of S. oualaniensis is extremely high, estimated at 8–11.2 million tons globally [1], 3–4 million tons in the Indian Ocean [1], 5–7 million tons in the Pacific Ocean [1,26], and 1.1–2.3 million tons in the South China Sea [24,27], with a large potential for the development of these fisheries in the future [5,28].
S. oualaniensis plays an important role in the tropical marine ecosystem; the feeding spectrum of this squid changes with its body size; amphipods, euphausiids, and fish larvae are the food of young squid; myctophids and squid are preyed upon by adults in the Southeastern Pacific Ocean and the Indian Ocean [4,29]. Cannibalism is also usually observed in the Tropical Pacific and the Indian Ocean [4,6,22,29,30]. S. oualaniensis is preyed upon by blue marlins, sooty terns, brown noddies, skipjack and yellowfin tuna, wahoos, and scalloped hammerhead sharks [30]. It is also a large component of the diet of tropical oceanic seabirds, and it is the primary prey in the diet of some sperm whales [31].
S. oualaniensis is the most important cephalopod fishery resource of the South China Sea [5,6]; understanding the biology of the fisheries is essential to the sustainable exploitation and management of this type of squid; however, no information on the age, growth, and population structure in the waters of the Xisha Islands of the South China Sea has reported to date. The purpose of this study is to provide basic information on the age, growth, and population structure of S. oualaniensis in these waters, based on the analysis of the statolith microstructure according to data collected by the Chinese light-falling-net fishery from January to March and from May to August of 2018, 2019, and 2020. This study provides the available information regarding age and growth research as well as an assessment of the management of S. oualaniensis, which is an important resource in the South China Sea.
2. Materials and Methods
2.1. Sampling and Ageing
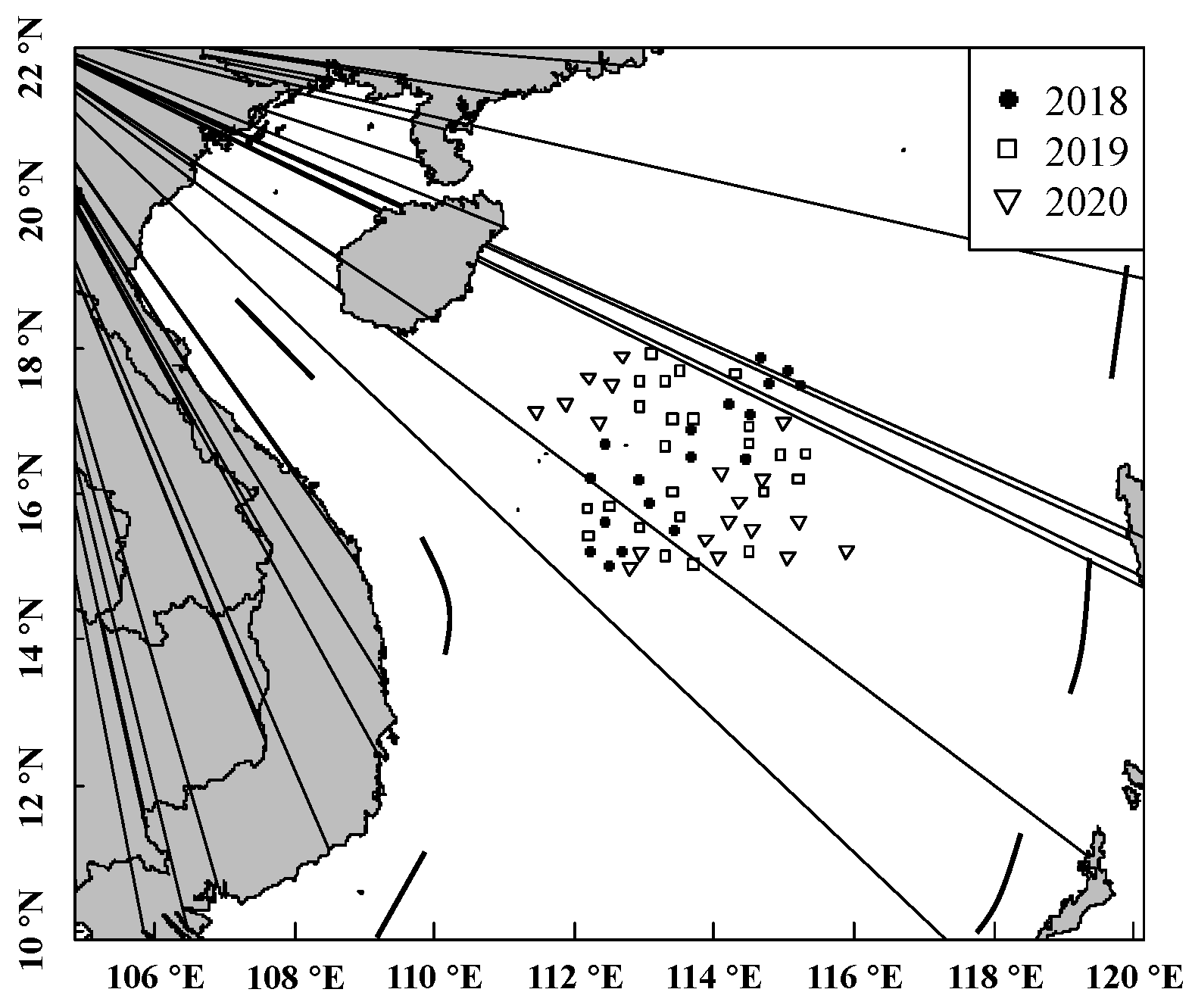
A total of 1460 samples of S. oualaniensis were collected randomly from 6 scientific surveys performed by the Chinese light-falling-net fishery from January to March and from May to August of 2018, 2019, and 2020 (location: 12° 18′ 00″ N–18° 46′ 00″ N, 110° 10′ 00″ E–115° 58′ 00″ E) in the waters of the Xisha Islands of the South China Sea (Figure 1). The samples were frozen on board immediately and transferred to the laboratory for further research.
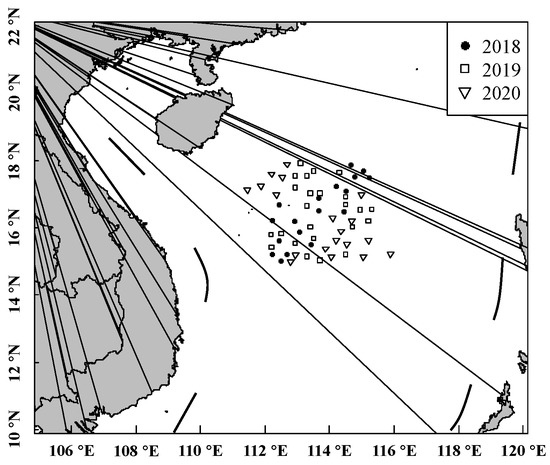
Figure 1.
Site of investigations and sample collection for S. oualaniensis.
Each squid was checked for the presence of a photophore on the dorsal. Measurements of ML (in mm) were taken to the nearest 1 mm, and body weight (BW, in g) to the nearest g for 1108 individuals that were randomly selected from the 1460 samples; the statoliths were extracted, washed, and stored in a centrifugal tube with 90% alcohol for age determination [32]. The standard statolith ageing methodology was used in this study [33]. The number of increments for each statolith was recorded independently by two readers, and the average was used if the range of the numbers was within 10% of the mean [34]. The daily deposition of the growth increments of the Ommastrephidae were verified [33,35,36]; the counts of the increments determined the age of each S. oualaniensis, and the hatching date was back-calculated from the capture date and age.
2.2. Data Analysis
According to the distribution of hatching dates, different seasonal spawning groups were defined. The following models were used to quantify the relationships between age and ML or BW, respectively; an analysis of covariance (ANCOVA) was performed to distinguish the difference between growth curves by spawning group or sex [34] (Table 1).

Table 1.
Types of growth functions.
Lt is mantle length (ML, mm) or body weight (BW, g); t is age (days); a, b, K, and t0 are estimated parameters; L∞ is the asymptotic ML or BW; and t0 is a hypothetical age when Lt equals zero.
For the above seven models, the Akaike Information Criterion (AIC) of each model was calculated [40,41,42,43]. The absolute daily growth rate (AGR, mm/d, or g/d) and instantaneous growth rate (IGR, %/d) of ML and BW were estimated for each 30-day interval. The AGRs and IGRs were calculated using the following functions [32]:
S1 and S2 represent the mean ML or BW at the beginning t1 and end t2 of the time interval, respectively.
3. Results
3.1. Size–Structure
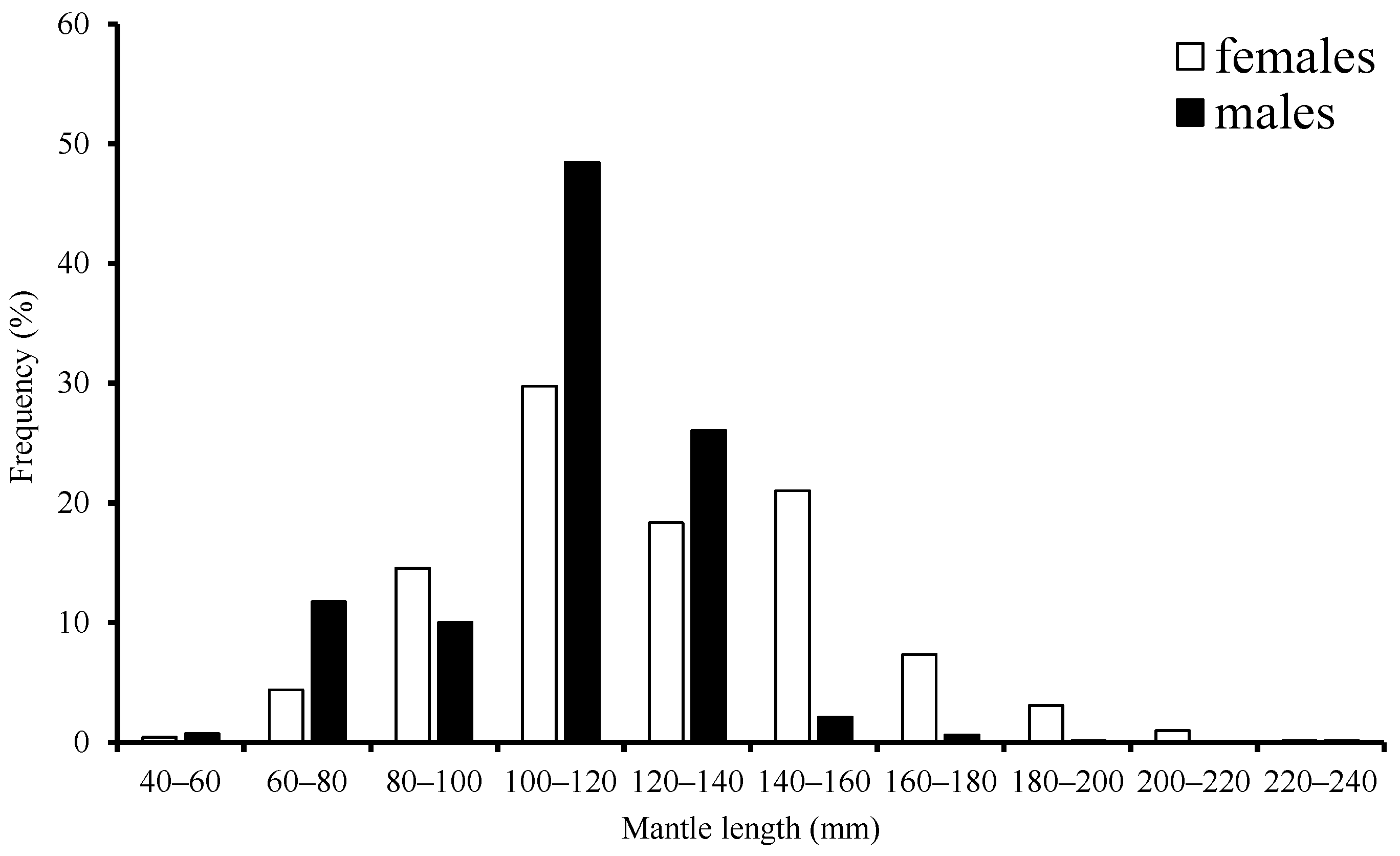
The range for ML was 63–229 mm with a mean of 124.97 mm, accounting for about 80.7% of females that were in the 80–160 mm length class. Male ML ranged from 59–184 mm with a mean of 114.21 mm, accounting for 90.1% that were in the 80–140 mm length class (Figure 2).
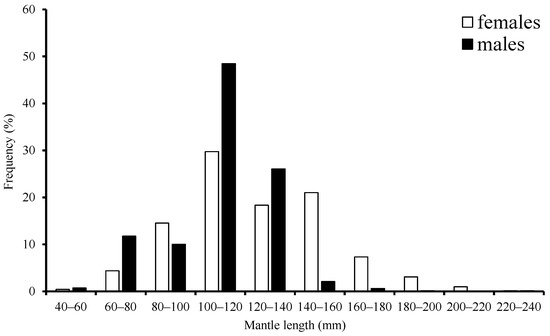
Figure 2.
Frequency distribution of mantle length for S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
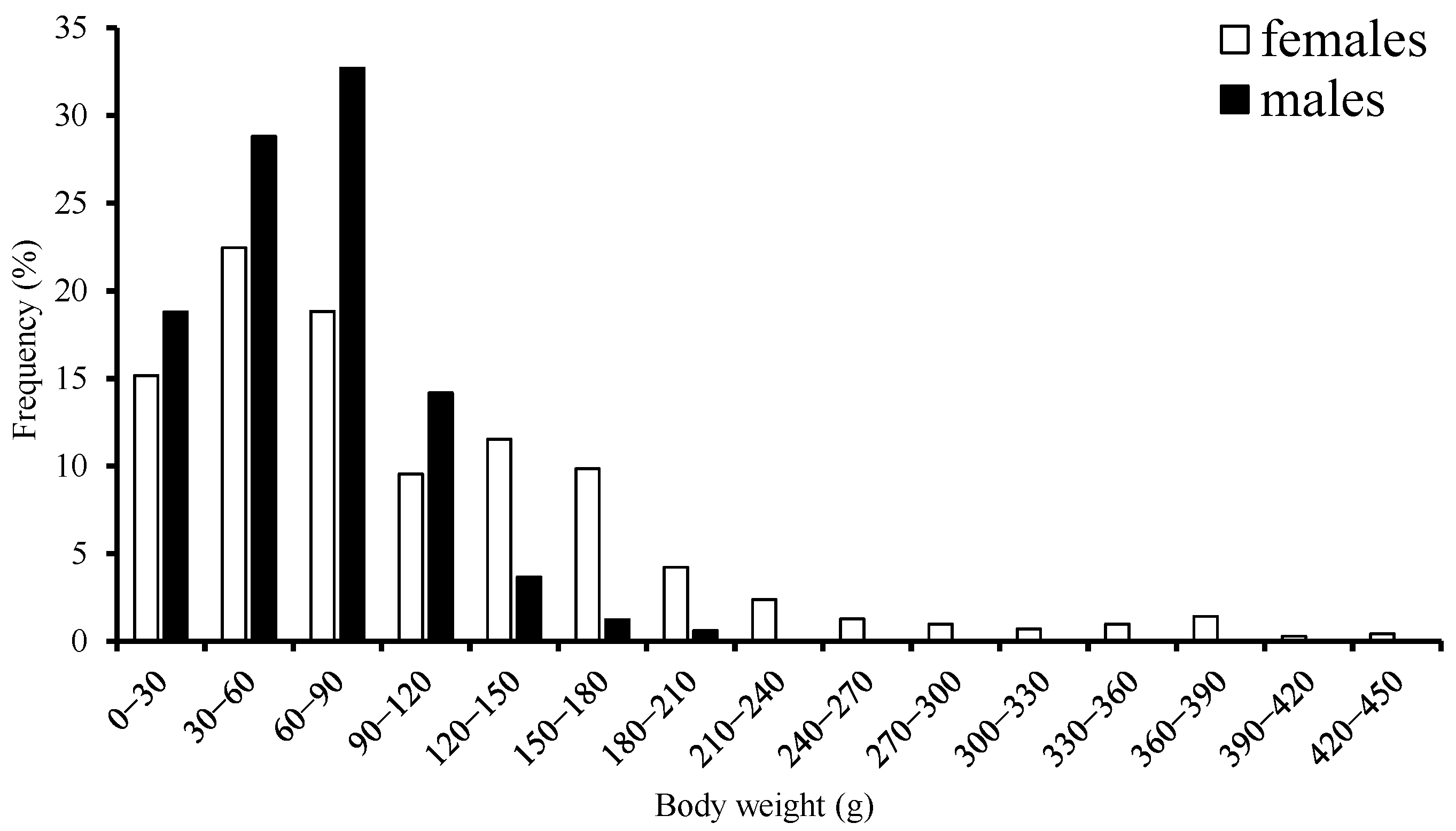
The range for BW was 13–435 g with a mean of 104.05 g, accounting for about 77.5% of females that were in the 0–150 g body-weight class. Male BW ranged from 8–152 g with a mean of 92.33 g, accounting for 91.3% that were in the 0–120 g body-weight class (Figure 3). According to the range of ML suggested by a previous study [1], most samples belonged to the dwarf form.
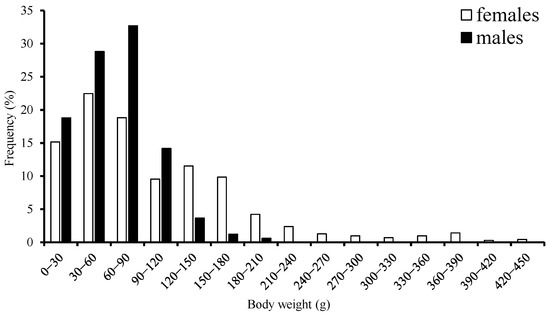
Figure 3.
Frequency distribution of body weight for S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
3.2. Age Structure and Population Structure
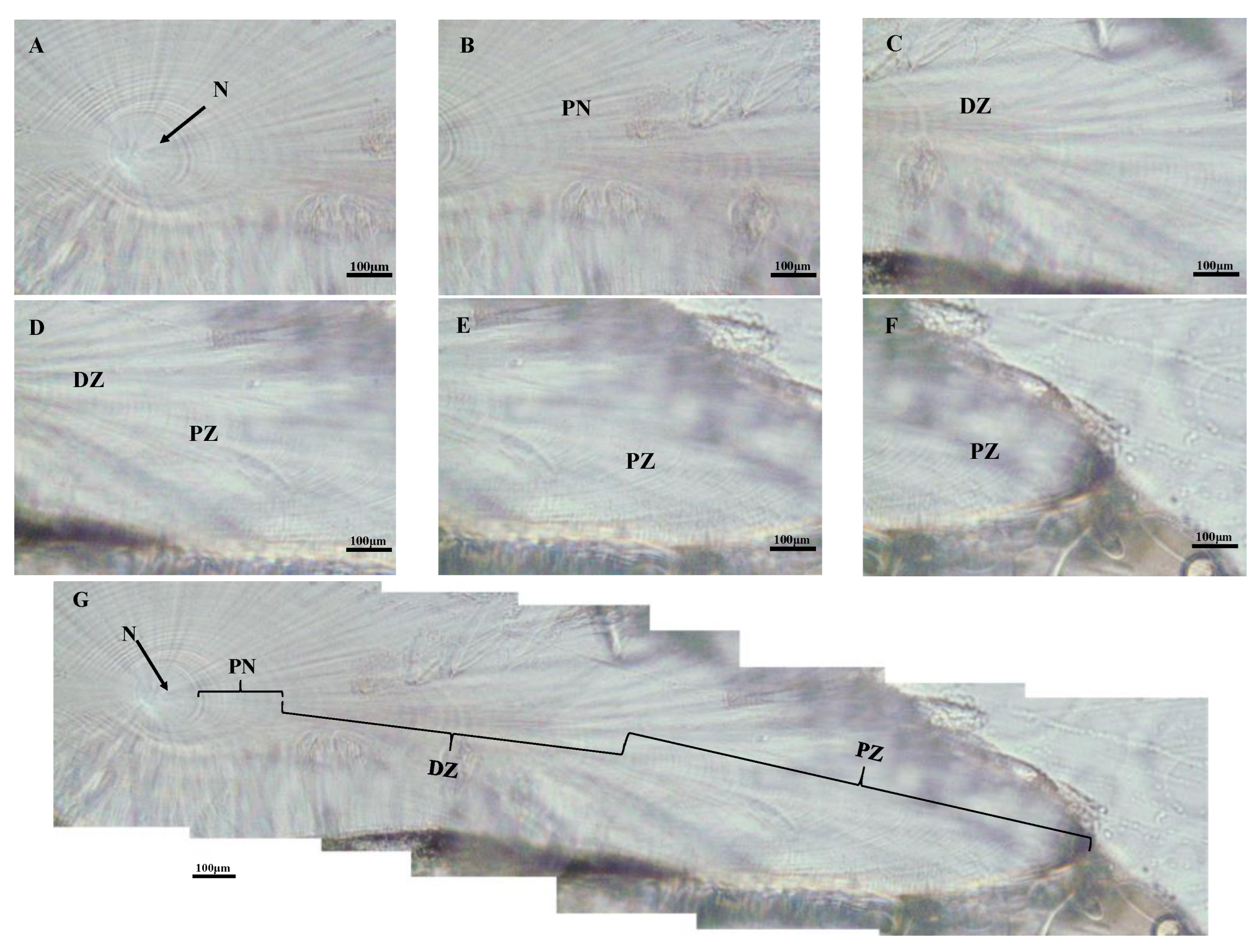
Three main growth zones were found in the dorsal dome of S. oualaniensis from the nuclear zone (Figure 4A) towards the edge of the dorsal dome: post-nuclear zone (PN, Figure 4B), dark zone (DZ, Figure 4C,D), and the peripheral zone (PZ, Figure 4E,F) for all individuals examined. Growth increments in the dark zone were significantly wider than those in the postnuclear and peripheral zones (Figure 4G).
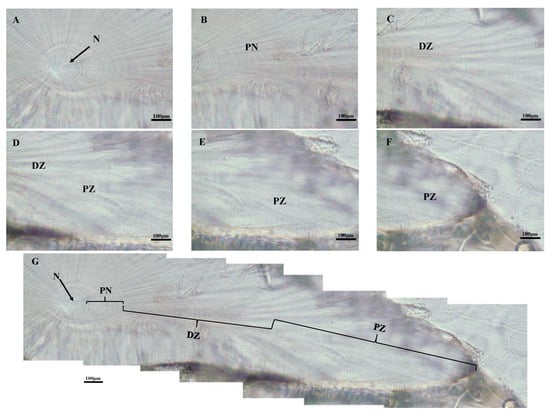
Figure 4.
Statolith microstructure of S. oualaniensis from the waters of the Xisha Islands of the South China Sea (female with mantle length 138 mm, body weight 173 g, age 169 d). (A) The nuclear zone (N); (B) the post-nuclear zone (PN); (C,D) the dark zone (DZ); (E,F) the peripheral zone (PZ); (G) growth increments in microstructure.
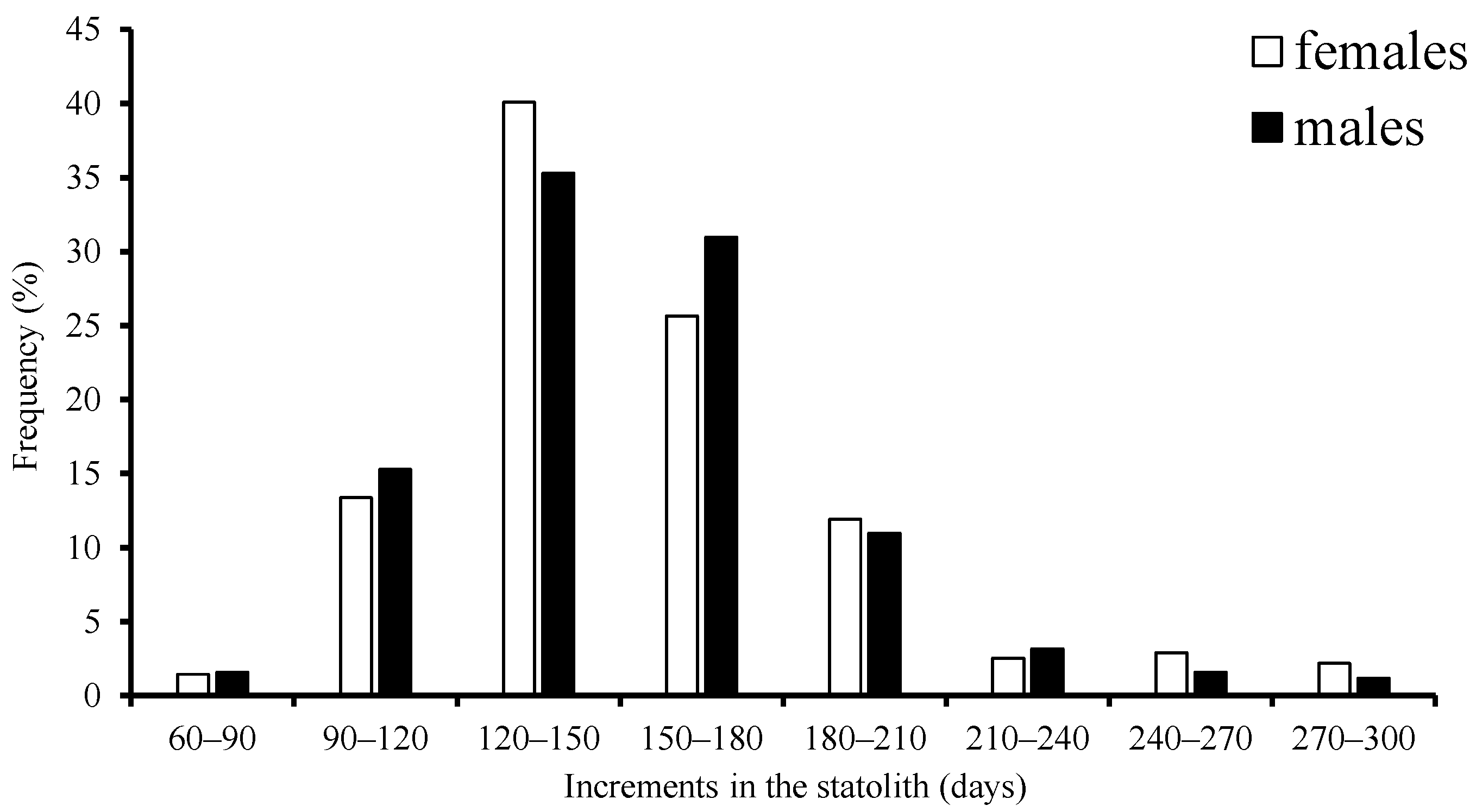
The age ranged from 81 to 298 days, with a high concentration in the 90–210 d group, accounting for 92.0% of females, and from 67 to 286 days, with the same high concentration in the 90–210 d group, accounting for 81.6% of males, respectively (Figure 5).
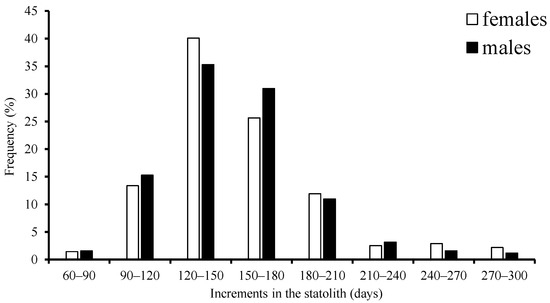
Figure 5.
Frequency distribution of age structure for S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
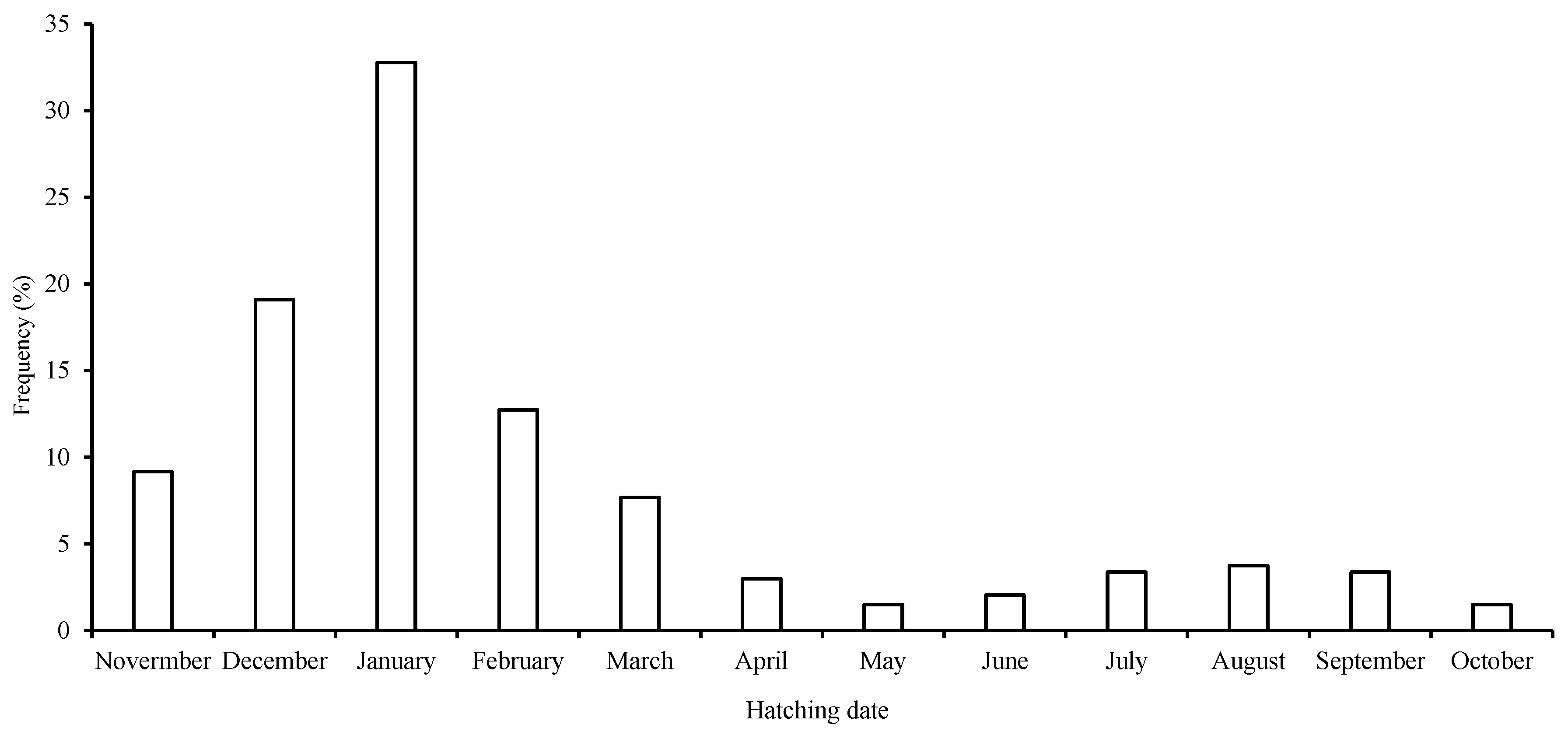
All of the samples in this study had hatched between January and December of 2018, 2019, and 2020, suggesting that S. oualaniensis spawned throughout the year, and the peak hatching dates ranged from November to March of the following year (in the northerly winter and spring), which comprised 72.8% of all the samples. This demonstrated the existence of 1 dominant winter–spring group for S. oualaniensis (Figure 6). Additionally, 83.5% of all samples lacked a dorsal photophore on the mantle.
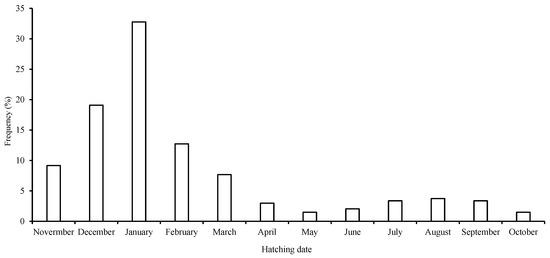
Figure 6.
Frequency distribution of back-calculated hatching dates for S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
3.3. Growth Models
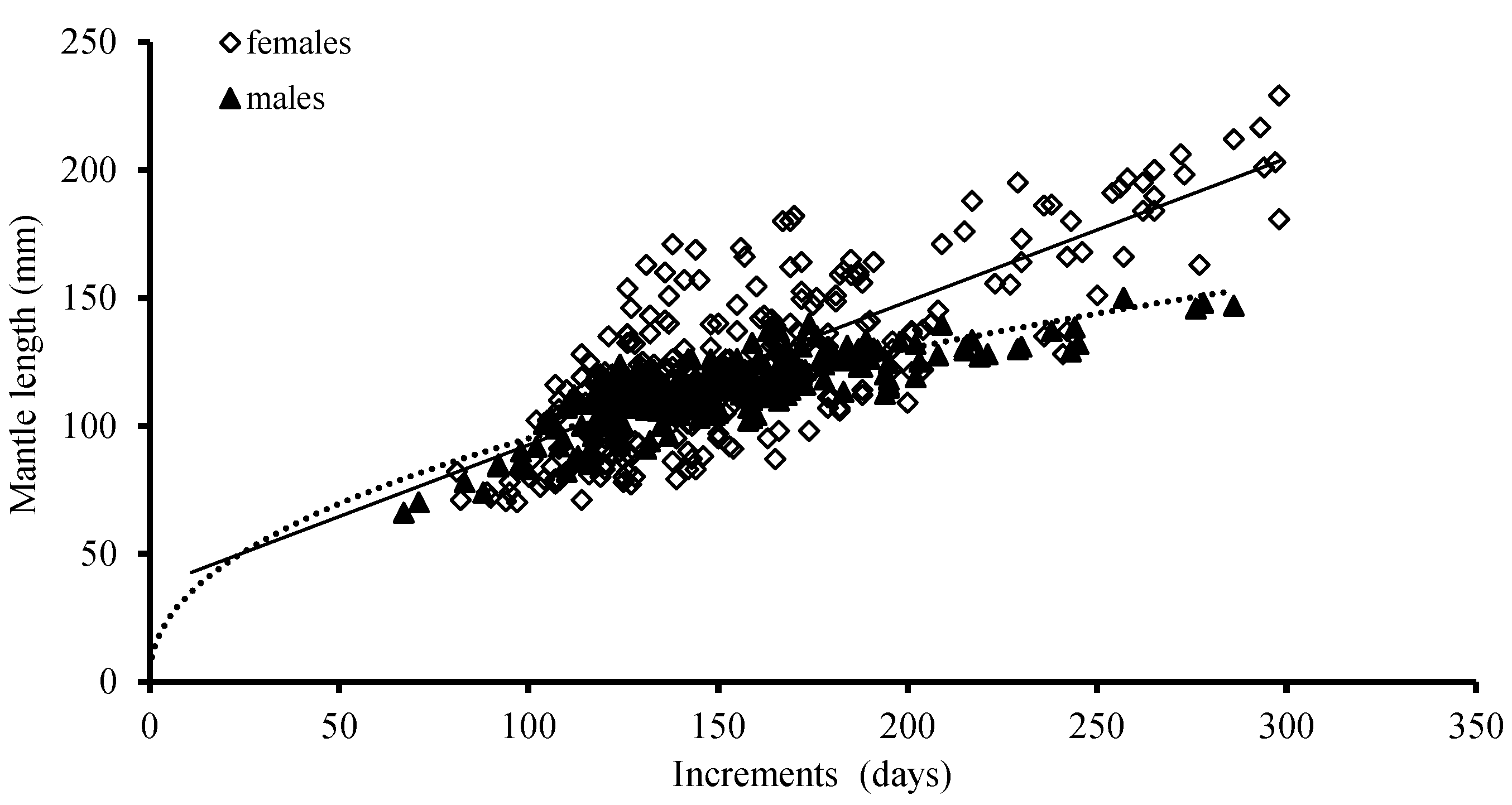
The ANCOVA results showed that there were significant differences between males and females in the growth of ML (p = 0.02 < 0.05) and BW (p = 0.01 < 0.05); the best growth models for ML was a linear function for females and a power function for males, based on the AIC model, respectively (Table 2; Figure 7).

Table 2.
Parameters of the growth models fitted to S. oualaniensis mantle length (ML)–age, body weight (BW)–age.
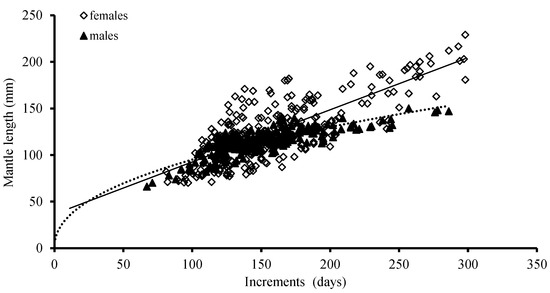
Figure 7.
Relationships between mantle length (ML) and age for S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
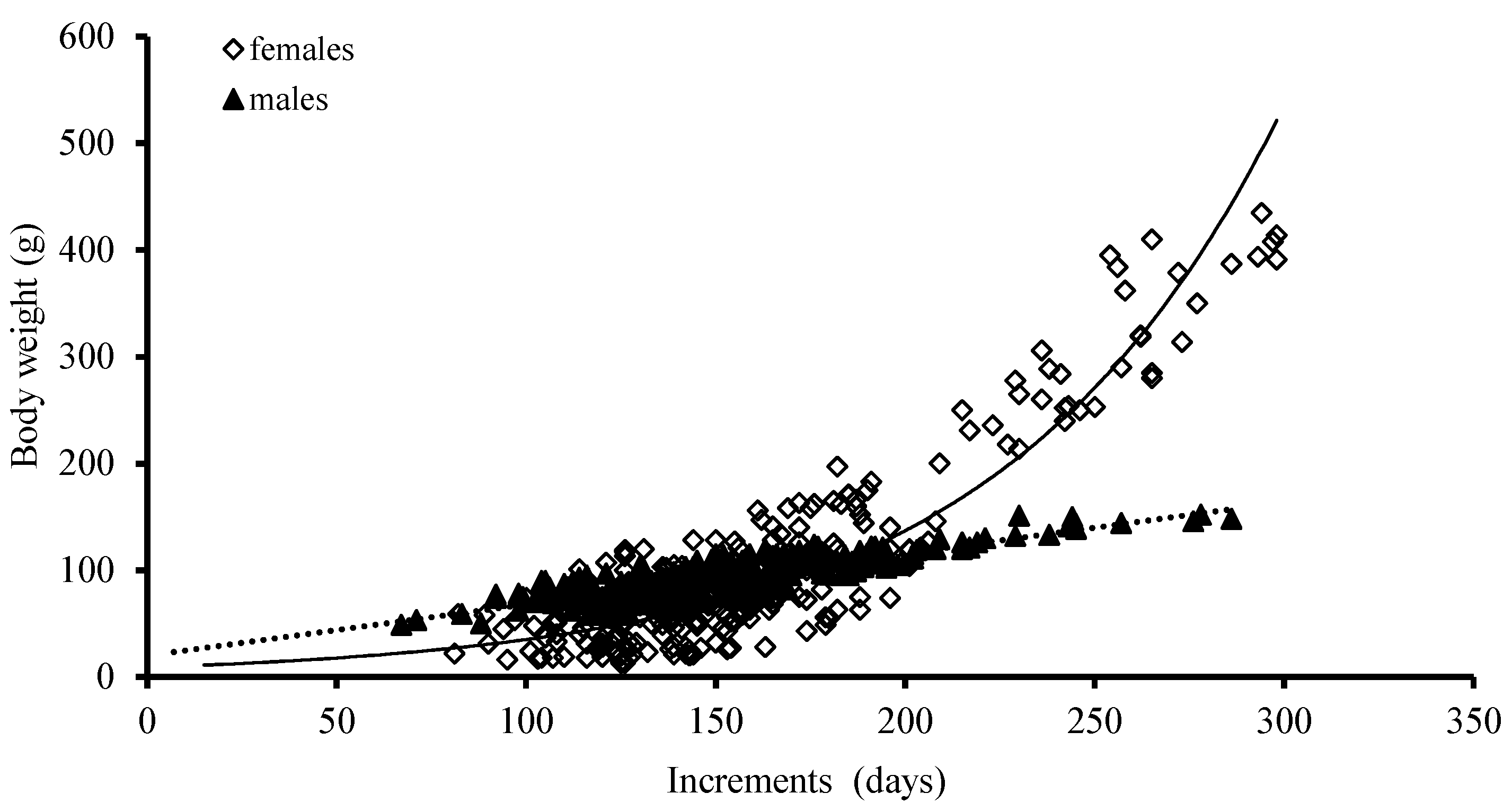
The best growth models for BW were an exponential function and a power function for females and males, based on the AIC model, respectively (Table 2; Figure 8).
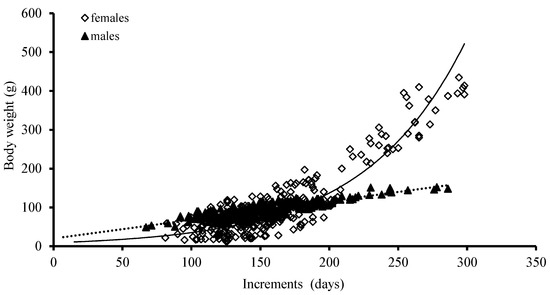
Figure 8.
Relationships between body weight (BW) and age for S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
3.4. Growth Patterns
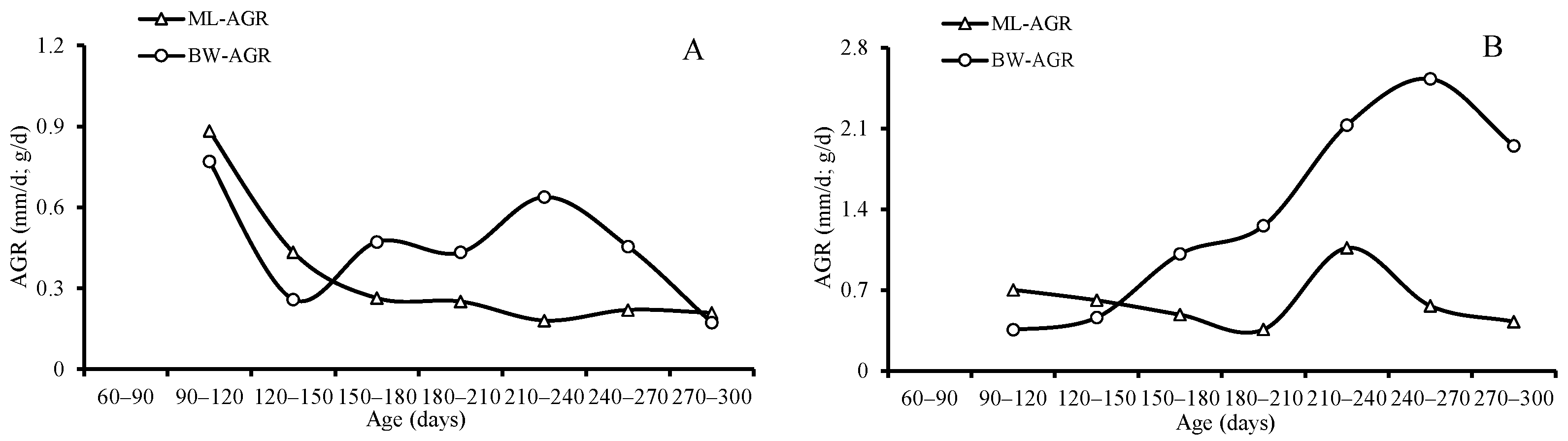
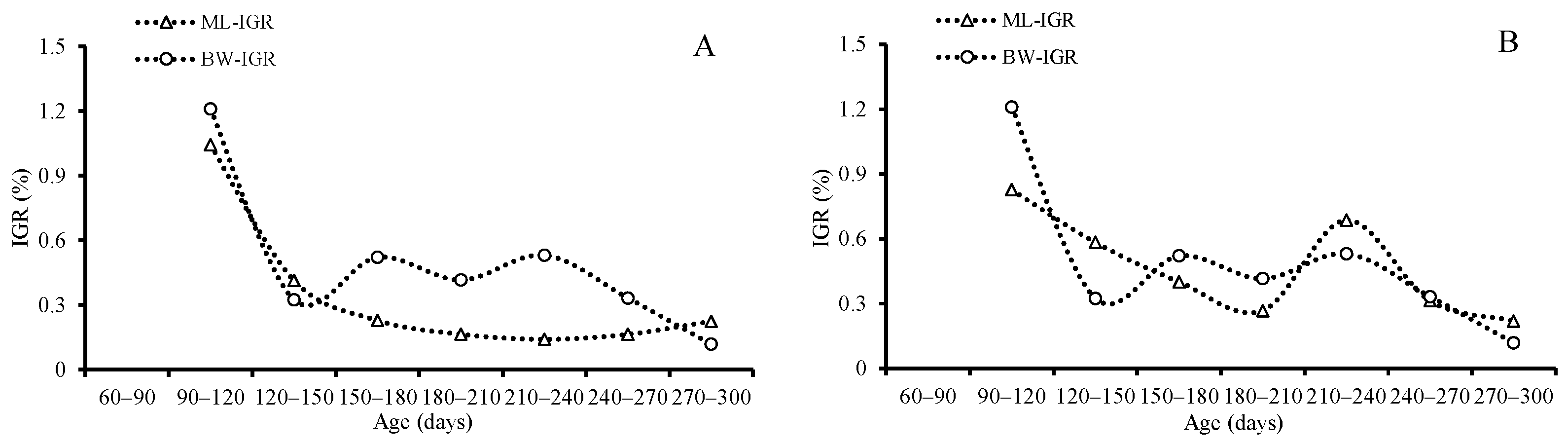
The ANCOVA results indicated that both the average of the IGRs for ML (p = 0.021) and BW (p = 0.035) and the AGRs for ML (p = 0.009) and BW (p = 0.041) differed significantly between sexes, and the females grew faster than the males. The average of AGRs and IGRs for ML was 0.60 mm/d and 0.47 for females and 0.35 mm/d and 0.34 for males, respectively; the average of AGRs and IGRs for BW was 1.39 g/d and 1.05 for females and 0.46 g/d and 0.49 for males, respectively (Table 3, Figure 9 and Figure 10).

Table 3.
Instantaneous relative growth rates (IGRs) and absolute growth rate (AGR) for mantle length (ML) and body weight (BW) for the winter–spring group of S. oualaniensis in the waters of the Xisha Islands of the South China Sea.
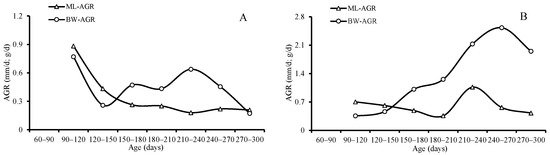
Figure 9.
Absolute growth rates (AGRs) of mantle length and body weight for S. oualaniensis: (A) males, and (B) females.
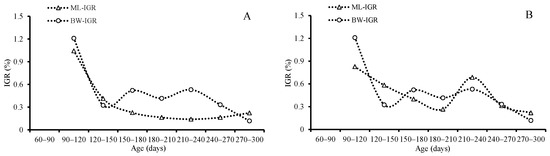
Figure 10.
Instantaneous growth rates (IGRs) of mantle length and body weight for S. oualaniensis: (A) males, and (B) females.
Both the averages of the AGRs and IGRs for ML and BW decreased with age until they reached the highest value (1.07 mm/d for ML, 2.11 g/d, and 2.53 for BW) at the age between 210 and 270 days, and then they decreased again for females; however, the average of the IGRs and AGRs for ML and BW reached the highest value (0.88 mm/d and 1.04 for ML and 0.770 g/d and 1.211 for BW) in the beginning at the age of 90–120 days, and then then decreased for males (Table 3, Figure 9 and Figure 10).
4. Discussion
4.1. Body Size
According to the range of ML for females and males and the absence of dorsal photophores on the backs of samples, we realized that our samples belonged to the dwarf form of the species in the waters of the Xisha Islands of the South China Sea. In previous studies, five forms were divided independently based on the presence of dorsal photophore and body size [1]. Characteristic features of S. oualaniensis are the very complicated morphological distinctions between different forms, and multiple forms vary both in size at maturity and in the possession of a distinctive, large dorsal photophore [20,44]. A middle-sized “typical” form with a photophore is found throughout the species range and co-occurs with D. gigas in the Eastern Tropical Pacific [1]. The equatorial waters of the Indian and Pacific Oceans (10–15° N to 10–15° S) are inhabited by an “early-maturing” dwarf form that lacks the dorsal photophore and may constitute a separate species [6,20,21,22,45,46,47,48]. A giant form of S. oualaniensis was documented in the Western Indian Ocean north of 15° to 17° N, such as in the Arabian Sea, and so on [1,4,20,26]. However, Zuyev et al. [49] proposed that the various forms of S. oualaniensis are indicative of active sympatric and allopatric speciation because of the high fecundity and rapid generational turnover common to all ommastrephid squid. Based on the above results, the samples in our study likely represent the dwarf, early-maturing, photophore-less form that others have suggested inhabit the waters of the Xisha Islands of the South China Sea [20,46,49,50].
4.2. Age
The age ranged from 81 to 298 days and from 67 to 286 days, both with a high concentration in the 90–210 d group, accounting for 92.0% of females and 81.6% of males, respectively. The life span of females was obviously longer than that of males. The results of this study were not similar to those of research performed off the Southwestern coast of India [50], the western Philippines of the South China Sea [51], and the Northwest Indian Ocean [4], where the life span was more than 10 months. The longevity of the samples in this study was larger than that of the squid from the Bashi Channel [45], the Eastern Tropical Pacific Ocean [12], the Northern Pacific Ocean [13], and the Bay of Bengal [15], where the estimated longevity of this type of squid was no greater than 6 months. The longevity of S. oualaniensis shows a geographic variation, and there are many factors responsible for this phenomenon, such as the temperature, the food availability, and so on [52]. Generally speaking, squid living in cold waters with lower temperatures and higher food availability have a longer life span than those which live in tropical waters [52]. This study found that females have longer life spans than males just as a common feature of squid growth.
4.3. Growth Models
There was a significant difference in the growth of ML to age; the linear and power functions were the best growth models for females and males in this study, respectively. The linear growth model for females was similar to that of squid from the Pacific Ocean [14], the Bay of Bengal [15], and the Eastern Tropical Pacific Ocean [12]; however, it was different from that of squid from the Bashi Channel [45]. Using the power function for males in this study led to results that were similar to those regarding squid from the Northern Pacific Ocean [13]. A previous study indicated that the growth of squid varied at different points in their life spans; a linear model might best fit a certain ontogenetic stage, and a different nonlinear model might best fit the entirety of the life cycle [43]. We found that females grow faster than males, just as the previous studies have found [13,15,50].
4.4. Population Structure
The hatching dates ranged from January to December, throughout the year, with a peak from November to March, which demonstrates a winter–spring group for the S. oualaniensis in this study. S. oualaniensis spawning occurs throughout the year, with different geographic peak-spawning groups [26]; different seasonal spawning groups have been identified based on statolith microstructure, such as the June-spawning, September-spawning, and February–March-spawning groups in Japanese and adjacent waters [26], the March–May-spawning group in the Arabian Sea [53], a spring-spawning and an autumn-spawning group in the Northwest Indian Ocean [54], a January–February-spawning group in the Eastern Tropical Pacific Ocean, and a December-spawning group in the Bashi Channel. However, the short duration of the sampling period may have had an influence on those results [12,45]. The hatching months in this study match those of S. oualaniensis in the Bashi Channel and the Eastern Tropical Pacific Ocean, suggesting a winter–spring group for S. oualaniensis in the waters of the Xisha Islands.
4.5. Growth Pattern
The average of AGRs and IGRs for ML was 0.60 mm/d and 0.47 for females and 0.35 mm/d and 0.34 for males. The average AGRs and IGRs for BW were 1.39 g/d and 1.05 for females and 0.46 g/d and 0.49 for males. The maximum AGRs and IGRs for ML were 1.07 mm/d and 0.83 for females and 0.88 mm/d and 1.04 for males. The maximum AGRs and IGRs for BW were 2.11 g/d and 2.53 for females and 0.770 g/d and 1.211 for males in this study. Previous studies have shown that S. oualaniensis is a very fast-growing species; the highest AGRs reached 3.8 mm/d [1], and the growth rate varied with different geographic distributions; the average growth rate in the ML range was 1.14 mm/d for females and 0.62 mm/d for males in the Bashi Channel [45], 1.32 mm/d for females in the Eastern Tropical Pacific Ocean [51], 1.35 mm/d for the spring-spawning group, and 1.62 mm/d for the autumn-spawning group in the Northwest Indian Ocean [54]. It seems that the squid in this study have a relatively lower growth rate. The growth rate might be influenced by environmental factors, such as the temperature and the availability of food during the earlier ontogenetic stage [33,55]. This study found that females grow faster than males, just as previous studies have reported [16,45]. The paralarval stage is suggested to last approximately 50–60 days (2 months), and the squid’s arms and fins grow rapidly during this period [56]. Because of the rapid development of their fins, their migratory ability improved in an observable manner. Juveniles were able to migrate from the edge of the continental shelf into oceanic waters for feeding after 50–60 days (2 months) [56]. High growth rates were displayed by males in our study from under 90–120 days (3–4 months); then, they decreased rapidly from under 120 to 150 days (4–5 months). Lower ML growth rates for females and males were found in our study from under 120 to 150 days. Compared to the lower growth rate of the ML and the faster growth rate of BW, squid in the subadult stage exhibited a significantly faster growth rate for the gonads, especially in males. The lower growth rates of the ML, BW, and muscle fibers were detected easily at the maturity stage for tropical species because most of the fuel from feeding was used for reproduction rather for than body growth [57]. Similar results for the age of first maturity suggested that S. oualaniensis reaches first maturity from under 85 to 136 days (3–4 months) in Eastern Tropical Pacific Ocean and the Bashi Channel [12,45]. These results show that the squid first reach maturity from under 120 to 150 days (4–5 months), which is the subadult stage. Because of this intermittent, multibatch spawning, females spawn continuously during a long spawning season (1–3 months) after their first spawning behavior [1,58]. In order to enhance their fecundity for the next spawning, female ML would continuously grow after the first spawning behavior from under 180 to 210 days (6–7 months). A slightly increasing fluctuation was also displayed in male BW, but a nearly unchangeable, lower level of fluctuation for ML was observed between 180 and 210 days (6–7 months). These phenomena suggest that the inflection point for male and female S. oualaniensis growth after first maturity and spawning may occur from 180 to 210 days (6–7 months).
5. Conclusions
Here, we have summarized all of the results of studies on the age and growth patterns of S. oualaniensis. Our results show that the samples belonged to the dwarf form and the winter–spring hatching group. The relationships between the ML and age were fitted into the linear and power functions for females and males; BW–Age were fitted into the exponential and power function for females and males. We confirmed that the subadult stage occurred at approximately 120–150 days (4–5 months) for both females and males. Additionally, the inflection points of growth occurred at approximately 180–210 days (6–7 months) for females and males in the waters of the Xisha Islands of the South China Sea. These results would be favorable and effective as parameters used in the assessment and management of this group of S. oualaniensis.
Author Contributions
Conceptualization, H.L.; methodology, H.L. and M.Z.; software, Z.C. and T.S.; formal analysis, Z.C. and K.L.; investigation, K.L., M.Z. and Y.O.; resources, H.L.; data curation, H.L.; writing—original draft preparation, H.L.; writing—review and editing, H.L. and Z.C.; supervision, H.L.; project administration, H.L.; funding acquisition, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2019YFD0901402) and the National Natural Science Funding of China (NSFC41506184).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Code of Ethics of the University Department of Marine Studies; all specimens obtained from the surveys were already dead.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Support for 4 scientific surveys made by “Qiong Sanya Yu 72106” and “Qiong Sanya Yu 72060” is gratefully acknowledged. We would like to thank the teachers and students from the laboratory of the Shanghai Ocean University for their work and help in sample collection and biological analysis, and for the valuable comments on the revision of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jereb, P.; Roper, C.F.E. Cephalopods of the World, Volume 2: Myopsid and Oegopsid Squids An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date; FAO: Rome, Italy, 2010; pp. 315–318. [Google Scholar]
- Chen, Z.Y.; Lu, H.J.; Liu, W.; Liu, K.; Chen, X.J. Beak microstructure estimates of the age, growth, and population structure of purpleback flying squid (Sthenoteuthis oualaniensis) in the Xisha Islands Waters of the South China Sea. Fishes 2022, 7, 187. [Google Scholar] [CrossRef]
- Wang, Y.G.; Chen, X.J. The Resource and Biology of Economic Oceanic Squid in the World; China Ocean Press: Beijing, China, 2005; pp. 190–194. (In Chinese) [Google Scholar]
- Chen, X.J.; Liu, B.L.; Tian, S.Q.; Qian, W.G.; Zhao, X.H. Fishery biology of purpleback squid, Sthenoteuthis oualaniensis, in the northwest Indian Ocean. Fish. Res. 2007, 83, 98–104. [Google Scholar]
- Zhang, P.; Yang, L.; Zhang, X.F.; Tan, Y.G. The present status and prospect on exploitation of tuna and squid fishery resources in South China Sea. South China Fish. Sci. 2010, 6, 68–74, (In Chinese with English abstract). [Google Scholar]
- Lu, H.J.; Tong, Y.H.; Liu, W.; Liu, K.; Dong, Z.X.; Chen, X.; Chen, X.J. Fisheries biological characteristics of Sthenoteuthis oualaniensis in the spring season in the El Nino year of 2016 in the Zhongsha Islands waters of South China Sea. J. Fish. China 2018, 36, 112–121, (In Chinese with English abstract). [Google Scholar]
- Chen, Z.Y.; Lu, H.J.; Tong, Y.H.; Tang, Y.; Liu, W.; Cheng, X.; Chen, X.J. Beak growth characteristic of Sthenoteuthis oualaniensis in the waters of Xisha Island in the South China Sea. J. Ocean Univ. Shanghai 2019, 28, 373–383, (In Chinese with English abstract). [Google Scholar]
- Lu, H.J.; Wang, H.H.; Liu, K.; Chen, X.Y.; He, J.R.; Chen, X.J. Growth characteristics of statolith of Sthenoteuthis oualaniensis in the Northwest Indian Ocean in spring and winter in the El Nino year. Chin. J. Ecol. 2020, 39, 3694–3703, (In Chinese with English abstract). [Google Scholar]
- Lu, H.J.; Zhang, X.; Tong, Y.H.; Tang, Y.; Liu, K.; Liu, W.; Chen, X.J. Statolith microstructure and growth characteristics of Sthenoeuthis oualaniensis in the Xisha Islands waters of the South China Sea. J. Fish. China 2020, 44, 767–776, (In Chinese with English abstract). [Google Scholar]
- He, J.R.; Lu, H.J.; Chen, X.Y.; Liu, K.; Wang, H.H.; Chen, X.J. Factors influencing beak morphology of Sthenoteuthis oualaniensis in the northwest Indian Ocean. Chin. J. Appl. Ecol. 2021, 32, 1881–1889, (In Chinese with English abstract). [Google Scholar]
- Bizikov, V.A. Growth of Sthenoteuthis oualaniensis, using a new method based on gladius microstructure. ICES J. Mar. Sci. 1995, 199, 445–458. [Google Scholar]
- Liu, B.L.; Chen, X.J.; Li, J.H.; Chen, Y. Age, growth and maturation of Sthenoteuthis oualaniensis in the eastern tropical Pacific Ocean by statolith analysis. Mar. Freshw. Res. 2016, 67, 1973–1981. [Google Scholar] [CrossRef]
- Takagi, K.; Yatsu, A. Age determination using Statolith microstructure of the purpleback flying squid, Sthenoteuthis oualaniensis, in the North Pacific Ocean. Nippon Suisan Gakkaishi 1996, 65, 98–113. [Google Scholar]
- Takagi, K.; Kitahara, T.; Suzuki, N.; Mori, J. The age and growth of Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae) in the Pacific Ocean. Bull. Mar. Sci. 2002, 71, 1105–1108. [Google Scholar]
- Sukramongkol, N.; Promjinda, S.; Prommas, R. Age and reproduction of Sthenoteuthis oualaniensis in the Bay of Bengal. In The Ecosystem-Based Fishery Management in the Bay of Bengal; Department of Fisheries, Ministry of Agriculture and Cooperatives: Phuket, Thailand, 2007; pp. 195–205. [Google Scholar]
- Dunning, M.; Brandt, S.B. Distribution and life history of deep-water squid of commercial interest from Australia. Aust. J. Mar. Freshw. Res. 1985, 36, 343–359. [Google Scholar] [CrossRef]
- Rancurel, P. Note pour servir á la connaissance de Symplectoteuthis oualaniensis (Lesson, 1830) (Cephalopoda, Oegopsida): Variations ontogéniques du bec supérieur. Cah. De L’indo Pac. 1980, 2, 217–232. [Google Scholar]
- Tung, I. On the Reproduction of Common Squid, Symplectoteuthis oualanrensrs (Lesson); Report of the Institute of Fishery Biology of Ministry of Economic Affairs and National Taiwan University: Taiwan, China, 1976; pp. 6–48. [Google Scholar]
- Young, R.E.; Hirota, J. Review of the ecology of Sthenoteuthis oualaniensis near the Hawaiian Archipelago. In Contributed Papers to International Symposium on Large Pelagic Squids; Okutani, T., Ed.; Japan Marine Fishery Resources Research Center: Tokyo, Japan, 1998; pp. 131–143. [Google Scholar]
- Nesis, K.N. Population structure of oceanic Ommastrephehids, with particular reference to Sthenoteuthis oualaniensis: A review. In Recent Advances in Fisheries Biology; Okutani, K., O’Dor, R.K., Kubodera, T., Eds.; Tokai University Press: Tokyo, Japan, 1993; pp. 375–383. [Google Scholar]
- Clarke, M. A review of the systematics and ecology of oceanic squids. Adv. Mar. Biol. 1966, 4, 91–300. [Google Scholar]
- Lu, H.J.; Wang, C.J.; Chen, X.J. Preliminary study on the biological characteristics of Sthenoteuthis oualaniensis in the high seas nearby the equator of eastern Pacific during April to June. J. Ocean Univ. China 2014, 23, 441–447, (In Chinese with English abstract). [Google Scholar]
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Tian, S.Q. Current exploitation and some scientific issues in the sustainable utilization of Ommastrephidae. J. Ocean Univ. China 2012, 21, 831–840, (In Chinese with English abstract). [Google Scholar]
- Zhang, J.; Chen, G.B.; Zhang, P.; Chen, Z.Z.; Fan, J.T. Estimation of purpleback flying squid (Sthenoteuthis oualaniensis) resource in the central and southern South China Sea based on fisheries acoustics and light-falling net. J. Fish. Sci. China 2014, 10, 822–831, (In Chinese with English abstract). [Google Scholar]
- Siriraksophon, S.; Sukramongkol, N.; Nakamura, Y. Exploration of oceanic squid, Sthenoteuthis oualaniensis resources in the South China Sea, Vietnamese waters. In Proceedings of the SEAFDEC Seminar on Fishery Resources in the South China Sea, Area IV: Vietnamese Waters, Bangkok, Thailand, 18–20 September 2001; pp. 181–197. [Google Scholar]
- Okutani, T.; Tung, I.H. Reviews of biology of commercially important squids in Japanese and adjacent waters, I. Symplectoteuthis oualaniensis (Lesson). Veliger 1978, 21, 87–94. [Google Scholar]
- Labe, L.L. Catch rate of oceanic squid by jigging method in the South China Sea area III: Western Philippines. In Proceedings of the SEAFDEC Seminar on Fishery Resources in the South China Sea: Area III, Bangkok, Thailand, 13–15 July 2000; pp. 19–31. [Google Scholar]
- Qiu., Y.S.; Lin, Z.J.; Wang., Y.Z. Responses of fish production to fishing and climate variability in the northern South China Sea. Prog. Oceanogr. 2010, 85, 197–212. [Google Scholar] [CrossRef]
- Shchetinnikov, A.S. Feeding spectrum of squid Sthenoteuthis oualaniensis (Oegopsida) in the Eastern Pacific. J. Mar. Biol. Assoc. UK 1992, 72, 849–860. [Google Scholar] [CrossRef]
- Young, R.E. A brief review of the biology of the oceanic squid, Symplecttoteuthis oualaniensis (Lesson). Comp. Biochem. Physiol. B 1975, 52, 141–143. [Google Scholar] [CrossRef]
- Wang, M.C.; Walker, W.A.; Shao, K.T.; Chou, L.S. Comparative analysis of the diets of pygmy sperm whales and dwarf sperm whales in Taiwanese waters. Acta Zool. Taiwanica 2002, 13, 53–62. [Google Scholar]
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Chen, Y. Age, growth and population structure of jumbo flying squid, Dosidicus gigas, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters. J. Mar. Biol. Assoc. UK 2011, 91, 229–235. [Google Scholar] [CrossRef]
- Keyl, F.; ArgÜelles, J.; Tafur, R. Interannual variability in size structure, age, and growth of jumbo squid (Dosidicus gigas) assessed by modal progression analysis. ICES J. Mar. Sci. 2011, 68, 507–518. [Google Scholar] [CrossRef]
- Yatsu, A.; Midorikawa, S.; Shimada, T.; Uozumi, Y. Age and growth of the neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fish. Res. 1997, 29, 257–270. [Google Scholar] [CrossRef]
- Bigelow, K. Age and Growth of Three Species of Squid Paralarvae from Hawaiian Waters, as Determined by Statolith Microstructures. Master’s Thesis, University of Hawaii, Hilo, HI, USA, 1991. [Google Scholar]
- Arkhipkin, A.I.; Bizikov, A.V.; Doubleday, Z.A.; Laptikhovsky, V.V.; Lishchenko, F.V.; Perales-Raya, C.; Hollyman, P.R. Techniques for estimating the age and growth of molluscs: Cephalopoda. J. Shellfish Res. 2018, 37, 783–792. [Google Scholar] [CrossRef]
- Rodhouse, P.G.; Hatfield, E.M.C. Age determination in squid using statolith growth increments. Fish. Res. 1990, 8, 323–334. [Google Scholar] [CrossRef]
- Jackson, G.D. Application and future potential of statolith increment analysis in squid and sepiolids. Can. J. Fish. Aquat. Sci. 1994, 51, 2612–2625. [Google Scholar] [CrossRef]
- Villanueva, R. Deep-sea cephalopods of the north-western Mediterranean: Indications of up-slope ontogenetic migration in to bathy benthic species. J. Zool. 1992, 227, 267–276. [Google Scholar] [CrossRef]
- Malcolm, H. Modeling and Quantitative Methods in Fisheries; Chapman and Hall/CRC: New York, NY, USA, 2001; pp. 227–232. [Google Scholar]
- Hiramatsu, K. Application of maximum likelihood method and AIC to fish population dynamics. In Fish Population Dynamics and Statistical Models; Matsumiya, Y., Ed.; Koseisha Koseikaku: Tokyo, Japan, 1993; pp. 9–21. (In Japanese) [Google Scholar]
- Imai, C.; Sakai, H.; Katsura, K. Growth model for the endangered cyprinid fish Tribolodon nakamurai based on otolith analyses. Fish. Sci. 2002, 68, 843–848. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Roa-Ureta, R. Identification of ontogenetic growth models for squid. Mar. Freshw. Res. 2005, 56, 371–386. [Google Scholar] [CrossRef]
- Dunning, M. A Review of the Systematics, Distribution and Biology of the Arrow Squid Genera Ommastrephes Orbigny, 1835, Sthenoteuthis Verrill, 1880, and Ornithoteuthis Okada, 1927 (Cephalopoda, Ommastrephidae); Voss, N.A., Vecchione, M., Toll, R.B., Sweeney, M.J., Eds.; Systematics and biogeography of cephalopods, Smithsonian Contributions to Zoology 586; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 425–433. [Google Scholar]
- Liu, B.L.; Lin, J.Y.; Feng, C.L.; Li, J.H.; Su, H. Estimation of age, growth and maturation of purpleback flying squid, Sthenoteuthis oualaniensis, in Bashi Channel, central Pacific Ocean. J. Ocean Univ. China 2017, 16, 525–531. [Google Scholar] [CrossRef]
- Clarke, M. Large light organs on the dorsal surfaces of the squids Ommastrephes pteropus, ‘Symplectoteuthis oualaniensis’ and ‘Dosidicus gigas’. J. Molluscan Stud. 1965, 36, 319–321. [Google Scholar] [CrossRef]
- Nigmatullin, C.M.; Tsygankov, V.Y.; Sabirov, R.M. On the taxonomic status of the early-maturing and late-maturing forms of the squid Sthenoteuthis oualaniensis (Lesson). In Taxonomy and Ecology of Cephalopods; Starobogatov, Y.I., Nesis, K.N., Eds.; Zoological Institute of Academy of Sciences USSR: Leningrad, Russia, 1983; pp. 94–96. (In Russian) [Google Scholar]
- Staaf, D.J.; Ruiz-Cooley, R.I.; Elliger, C.; Lebaric, Z.; Campos, B.; Markaida, U.; Gilly, W.F. Ommastrephid squids Sthenoteuthis oualaniensis and Dosidicus gigas in the eastern Pacific show convergent biogeographic breaks but contrasting population structures. Mar. Ecol. Prog. Ser. 2010, 418, 165–178. [Google Scholar] [CrossRef]
- Zuyev, G.; Nigmatullin, C.; Chesalin, M.; Nesis, K.N. Main results of long-term worldwide studies on tropical nektonic oceanic squid genus Sthenoteuthis: An overview of the Soviet investigations. Bull. Mar. Sci. 2002, 71, 1019–1060. [Google Scholar]
- Chembian, J.; Mathew, S. Growth and mortality of the oceanic squid Sthenoteuthis oualaniensis (Lesson, 1830) off south-west coast of India. Indian J. Fish. 2016, 6, 27–34. [Google Scholar] [CrossRef][Green Version]
- Zakaria, M.Z.B. Age and growth studies of oceanic squid, Sthenoteuthis oualaniensis using statoliths in the South China Sea, Area III, western Philippines. In Proceedings of the SEAFDEC Seminar on Fishery Resources in the South China Sea, Area III: Western Philippines, Bangkok, Thailand, 13–15 July 2000; pp. 118–134. [Google Scholar]
- Arkhipkin, A.I. Diversity in growth and longevity in short-lived animals: Squid of the suborder Oegopsina. Mar. Freshw. Res. 2004, 55, 341–355. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Mathew, J.; Alloycious, P.S. Population characteristics and some spects of the biology of oceanic squid Sthenoteuthis oualaniensis (Lesson, 1830). J. Mar. Ecol. Assoc. India 2006, 48, 256–259. [Google Scholar]
- Liu, B.L.; Chen, X.J.; Zhong, J.S. Age, growth and population structure of squid Sthenoteuthis oualaniensis in northwest Indian Ocean by statolith microstructure. J. Dalian Fish. Univ. 2009, 24, 206–212, (In Chinese with English abstract). [Google Scholar]
- Pecl, G.T.; Moltschaniwskyj, N.A.; Tracey, S.R.; Jordan, A.R. Interannual plasticity of squid life history and population structure: Ecological and management implications. Oecologia 2004, 139, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Sajikumar, K.K.; Ragesh, N.; Venkatesan, V.; Said-Koya, K.P.; Sasikumar, G.; Kripa, V.; Mohamed, K.S. Morphological development and distribution of paralarvae juveniles of purple back flying squid Sthenoteuthis oualaniensis (Ommastrephidae), in the south eastern Arabian Sea. Vie Et Milieu 2018, 68, 75–86. [Google Scholar]
- Jackson, G.D. Advances in defining the life histories of myopsid squid. Mar. Freshw. Res. 2004, 55, 357–365. [Google Scholar] [CrossRef]
- Harman, R.F.; Young, R.E.; Reid, S.B.; Mangold, K.M.; Hixon, R.F. Evidence for multiple spawning in the tropical oceanic squid Stenoteuthis oualaniensis. Mar. Biol. 1989, 101, 513–519. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).