Analysis of At-Haulback Mortality and Influencing Factors of Indian Ocean Swordfish (Xiphias gladius)
Abstract
:1. Introduction
2. Materials and Methods
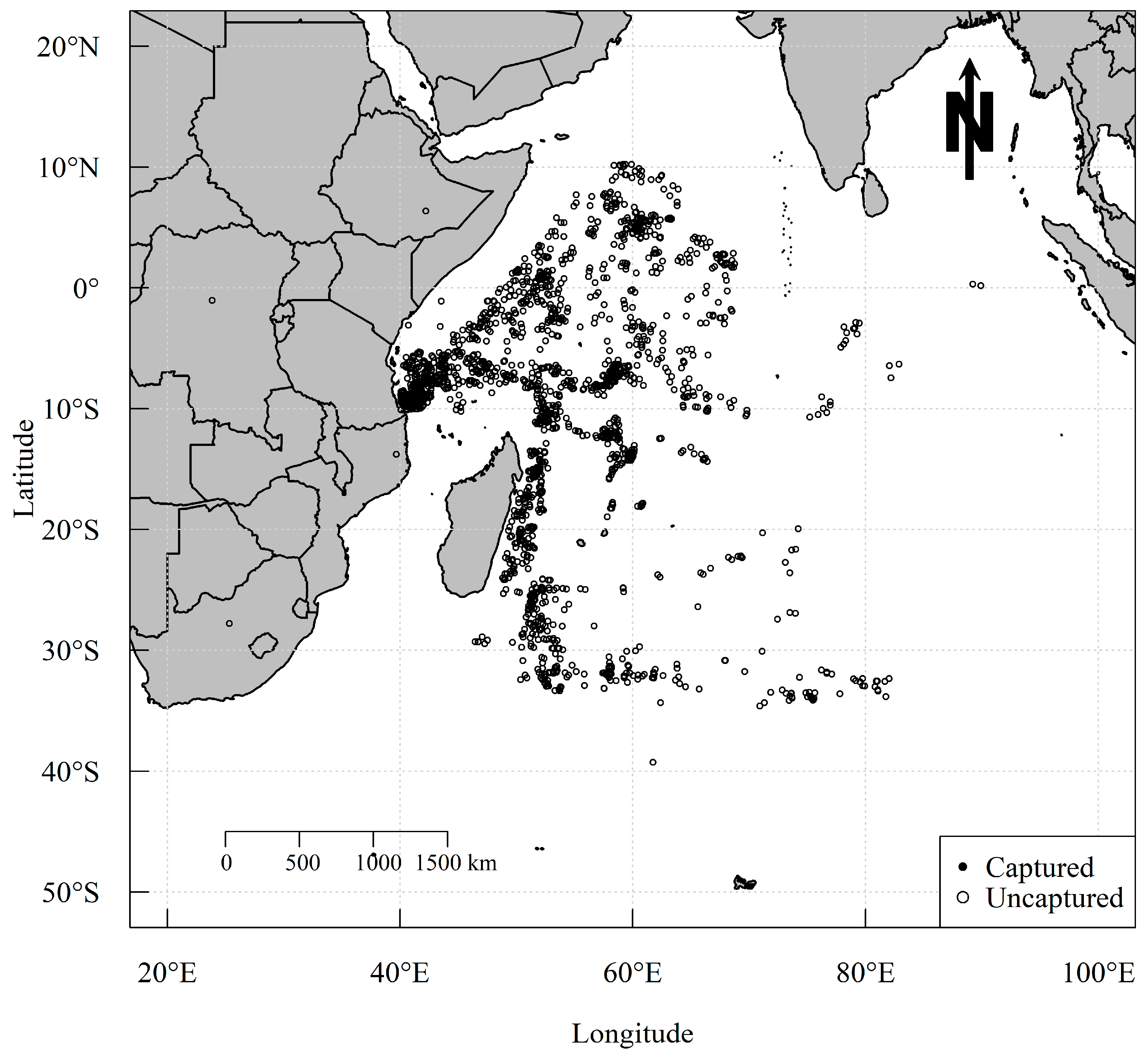
2.1. Data Collection
2.2. Data Analysis
2.3. Model Diagnosis and Goodness of Fit Test
3. Results
3.1. Analysis of Observed At-Haulback Mortality
3.2. The Establishment of the GLM Model
3.3. Predictions of the Probabilities of At-Haulback Mortality
4. Discussion
4.1. Observed At-Haulback Mortality
4.2. Factors Affecting the At-Haulback Mortality of Swordfish
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitchell, J.F.; Martell, S.J.D.; Walters, C.J.; Jensen, O.P.; Kaplan, I.C.; Watters, J.; Essington, T.E.; Boggs, C.H. Billfishes in an ecosystem context. Bull. Mar. Sci. 2006, 79, 669–682. [Google Scholar]
- Nakamura, I. Billfishes of the world. An annotated and illustrated catalogue of marlins, sailfishes, spearfishes and swordfishes known to Date. FAO Fish. Synop. 1985, 5, 125. [Google Scholar]
- Neilson, J.D.; Smith, S.; Royer, F.; Paul, S.D.; Porter, J.M.; Lutcavage, M. Investigations of horizontal movements of Atlantic swordfish using pop-up satellite archival tags. In Tagging and Tracking of Marine Animals with Electronic Devices; Springer: Dordrecht, The Netherlands, 2009; Volume 9, pp. 145–159. [Google Scholar]
- Takahashi, M.; Okamura, H.; Yokawa, K.; Okazaki, M. Swimming behaviour and migration of a swordfish recorded by an archival tag. Mar. Freshwater. Res. 2003, 54, 527–534. [Google Scholar] [CrossRef]
- Poisson, F.; Fauvel, C. Reproductive dynamics of swordfish (Xiphias gladius) in the southwestern Indian Ocean (Reunion Island). Part 1: Oocyte development, sexual maturity and spawning. Aquat. Living. Resour. 2009, 22, 45–58. [Google Scholar] [CrossRef]
- Wang, S.P.; Lin, C.H.; Chiang, W.C. Age and growth analysis of swordfish (Xiphias gladius) in the Indian Ocean based on the specimens collected by Taiwanese observer program. In Proceedings of the 8th Session of the IOTC Working Party on Billfish, Victoria, Seychelles, 12 July 2010. IOTC-2010-WPB-08-rev1. [Google Scholar]
- IOTC Secretariat. Nominal Catches by Fleet, Year, Gear, IOTC Area and Species. In Proceedings of the 19th Session of the IOTC Working Party on Billfish, Online/Virtual, 13–16 September 2021; IOTC-2021-WPB19-DATA03. Available online: https://iotc.org/WPB/19/Data/03-NC (accessed on 13 September 2022).
- IOTC Secretariat. Outcomes of the 23rd Scientific Committee meeting. In Proceedings of the 19th Session of the IOTC Working Party on Billfish, Online/Virtual, 13–16 September 2021; IOTC–2021–WPB19–03. Available online: https://iotc.org/documents/WPB/19/03 (accessed on 13 September 2022).
- Wang, S.P. Stock Assessment of Swordfish (Xiphias gladius) in the Indian Ocean Using A Stock Production Model Incorporating Covariates (ASPIC). In Proceedings of the 15th Session of the IOTC Working Party on Billfish, San Sebastián, Spain, 10–14 September 2017. IOTC–2017–WPB15–21. [Google Scholar]
- Coelho, R.; Fernandez-Carvalho, J.; Lino, P.G.; Santos, M.S. An overview of the hooking mortality of elasmobranchs caught in a swordfish pelagic longline fishery in the Atlantic Ocean. Aquat. Living. Resour. 2012, 25, 311–319. [Google Scholar] [CrossRef]
- Carruthers, E.H.; Neilson, J.D.; Smith, S.C. Overlooked bycatch mitigation opportunities in pelagic longline fisheries: Soak time and temperature effects on swordfish (Xiphias gladius) and blue shark (Prionace glauca) catch. Fish. Res. 2011, 108, 112–120. [Google Scholar] [CrossRef]
- Gilman, E.; Huang, H.W. Review of effects of pelagic longline hook and bait type on sea turtle catch rate, anatomical hooking position and at-vessel mortality rate. Rev. Fish. Biol. Fish. 2017, 27, 43–52. [Google Scholar] [CrossRef]
- Muoneke, M.I.; Childress, W.M. Hooking mortality: A review for recreational fisheries. Rev. Fish. Sci. 1994, 2, 123–156. [Google Scholar] [CrossRef]
- Coelho, R.; Lino, P.G.; Santos, M.N. At-Haulback Mortality of Elasmobranchs Caught on the Portuguese Longline Swordfish Fishery in the Indian Ocean. In Proceedings of the 9th Session of the IOTC Working Party on Billfish, Victoria, Seychelles, 4–7 July 2011. IOTC–2011–WPEB07–31 2011. [Google Scholar]
- Poisson, F.; Filmalter, J.D.; Vernet, A.L.; Laurent, D. Mortality rate of silky sharks (Carcharhinus falciformis) caught in the tropical tuna purse seine fishery in the Indian Ocean. Can. J. Fish. Aquat. Sci. 2014, 71, 795–798. [Google Scholar] [CrossRef]
- Coelho, R.; Muñoz-Lechuga, R. Hooking mortality of swordfish in pelagic longlines: Comments on the efficiency of minimum retention sizes. Rev. Fish. Biol. Fish. 2019, 29, 453–463. [Google Scholar] [CrossRef]
- Eddy, C.; Brill, R.; Bernal, D. Rates of at-vessel mortality and post-release survival of pelagic sharks captured with tuna purse seines around drifting fish aggregating devices (FADs) in the equatorial eastern Pacific Ocean. Fish. Res. 2016, 174, 109–117. [Google Scholar] [CrossRef]
- Butcher, P.A.; Peddemors, V.M.; Mandelman, J.W.; McGrath, S.P.; Cullis, B.R. At-vessel mortality and blood biochemical status of elasmobranchs caught in an Australian commercial longline fishery. Glob. Ecol. Conserv. 2015, 3, 878–889. [Google Scholar] [CrossRef]
- Cooke, S.J.; Suski, C.D. Are circle hooks effective tools for conserving freshwater and marine recreational catch-and-release fisheries? Aquat. Conserv. Mar. Freshwat. Ecosyst. 2004, 14, 299–326. [Google Scholar] [CrossRef]
- Reinhardt, J.F.; Weaver, J.; Latham, P.J.; Dell’Apa, A.; Serafy, J.E.; Browder, J.A.; Christman, M.; Foster, D.G.; Blankinship, D.R. Catch rate and at-vessel mortality of circle hooks versus J-hooks in pelagic longline fisheries: A global meta-analysis. Fish Fish. 2018, 19, 413–430. [Google Scholar] [CrossRef]
- Coelho, R.; Infante, P.; Santos, M.N. Application of generalized linear models and generalized estimation equations to model at-haulback mortality of blue sharks captured in a pelagic longline fishery in the Atlantic Ocean. Fish. Res. 2013, 145, 66–75. [Google Scholar] [CrossRef]
- Reeves, K.A.; Bruesewitz, R.E. Factors influencing the hooking mortality of walleyes caught by recreational anglers on Mille Lacs, Minnesota. N. Am. J. Fish. Manag. 2007, 27, 443–452. [Google Scholar] [CrossRef]
- Ghani, I.M.M.; Ahmad, S. Stepwise multiple regression method to forecast fish landing. Procedia-Soc. Behav. Sci. 2010, 8, 549–554. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. Bull. Int. Stat. Inst. 1973, 50, 267–281. [Google Scholar]
- Xue, Y.; Chen, L.P. Statistical Modeling and R Software, 1st ed.; Tsinghua University Press: Beijing, China, 2007. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Fushiki, T. Estimation of prediction error by using K-fold cross-validation. Stat. Comput. 2011, 21, 137–146. [Google Scholar] [CrossRef]
- Naimi, B. Usdm: Uncertainty Analysis for Species Distribution Models, R Package Version 1.1-18; Springer: New York, NY, USA, 25 June 2017. Available online: https://cran.r-project.org/web/packages/usdm/index.html (accessed on 13 September 2022).
- LeDell, E.; Petersen, M.; Laan, M.V.D. cvAUC: Cross-Validated Area Under the ROC Curve Confidence Intervals, R Package Version 1.1.4; Springer: New York, NY, USA, 2022. Available online: https://cran.r-project.org/web/packages/cvAUC/index.html (accessed on 13 September 2022).
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R. J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. Car: Companion to Applied Regression, R Package Version 3.1-0; Springer: New York, NY, USA, 2022. Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 13 September 2022).
- Brownrigg, M.R. Mapdata: Extra Map Databases, R Package Version 2.3.0; Springer: New York, NY, USA, 2018. Available online: https://cran.r-project.org/web/packages/mapdata/index.html (accessed on 13 September 2022).
- Bivand, R.; Lewin-Koh, N.J.; Pebesma, J.; Archer, E.; Bivand, R. maptools: Tools for Handling Spatial Objects, R Package Version 1.1-4; Springer: New York, NY, USA, 2022. Available online: https://cran.r-project.org/web/packages/maptools/index.html (accessed on 13 September 2022).
- Sun, C.L.; Wang, S.P.; Porch, C.E.; Yeh, S.Z. Sex-specific yield per recruit and spawning stock biomass per recruit for the swordfish, Xiphias gladius, in the waters around Taiwan. Fish. Res. 2005, 71, 61–69. [Google Scholar] [CrossRef]
- Punt, A.E.; Campbell, R.A.; Smith, A.D.M. Evaluating empirical indicators and reference points for fisheries management: Application to the broadbill swordfish fishery off eastern Australia. Mar. Freshw. Res. 2001, 52, 819–832. [Google Scholar] [CrossRef]
- Dewar, H.; Prince, E.D.; Musyl, M.K.; Brill, R.W.; Sepulveda, C.; Luo, J.G.; Foley, D.; Orbesen, E.S.; Domeier, M.L.; Nasby-lucas, N.; et al. Movements and behaviors of swordfish in the atlantic and pacific oceans examined using pop-up satellite archival tags. Fish. Oceanogr. 2011, 20, 219–241. [Google Scholar] [CrossRef]
- West, W.M.; Kerwath, S.E.; Silva, D.C.; Wilke, C.G.; Marsac, F. Horizontal and Vertical Movements of Swordfish Tagged with Pop Up-Satellite Transmitters in the South-West Indian Ocean, off South Africa. In Proceedings of the 10th Session of the IOTC Working Party on Billfish, Cape Town, South Africa, 11–15 September 2012. IOTC-2012-WPB10-16. [Google Scholar]
- Curran, D.; Bigelow, K. Effects of circle hooks on pelagic catches in the Hawaii-based tuna longline fishery. Fish. Res. 2011, 109, 265–275. [Google Scholar] [CrossRef]
- Epperly, S.P.; Watson, J.W.; Foster, D.G.; Shah, A.K. Anatomical hooking location and condition of animals captured with pelagic longlines: The grand banks experiments 2002–2003. Bull. Mar. Sci. 2012, 88, 513–527. [Google Scholar] [CrossRef]
- Huang, H.W.; Swimmer, Y.; Bigelow, K.; Bigelow, K.; Gutierrez, A.; Foster, D.G. Influence of hook type on catch of commercial and bycatch species in an Atlantic tuna fishery. Mar. Policy 2016, 65, 68–75. [Google Scholar] [CrossRef]
- Godin, A.C.; Carlson, J.K.; Burgener, V. The effect of circle hooks on shark catchability and at-vessel mortality rates in longlines fisheries. Bull. Mar. Sci. 2012, 88, 469–483. [Google Scholar] [CrossRef]
- Aneesh Kumar, K.V.; Paresh, S.K.; Pravin, P.; Madhu, V.R.; Meenakumari, B. Effect of hook design on longline catches in Lakshadweep Sea, India. Indian J. Fish. 2013, 60, 21–27. [Google Scholar]
- Morgan, A.; Carlson, J.K. Capture time, size and hooking mortality of bottom longline-caught sharks. Fish. Res. 2010, 101, 32–37. [Google Scholar] [CrossRef]
- Neilson, D.J.; Waiwood, G.; Smith, S.J. Survival of Atlantic Halibut (Hippoglossus hippoglossus) caught by longline and otter trawl gear. Can. J. Fish. Aquat. Sci. 1989, 46, 887–897. [Google Scholar] [CrossRef]
- Poisson, F.; Fauvel, C. Reproductive dynamics of swordfish (Xiphias gladius) in the southwestern Indian Ocean (Reunion Island). Part 2: Fecundity and spawning pattern. Aquat. Living Resour. 2009, 22, 59–68. [Google Scholar] [CrossRef]
- Muoneke, M.I. Seasonal hooking mortality of bluegills caught on natural baits. N. Am. J. Fish. Manag. 1992, 12, 645–649. [Google Scholar] [CrossRef]
- Braccini, J.M.; Waltrick, D. Species-specific at-vessel mortality of sharks and rays captured by demersal longlines. Mar. Policy 2019, 99, 94–98. [Google Scholar] [CrossRef]
- Dotson, T. Mortalities in trout caused by gear type and angler-induced stress. N. Am. J. Fish. Manag. 1982, 2, 60–65. [Google Scholar] [CrossRef]
- Nuhfer, A.J.; Alexander, G.R. Hooking mortality of trophy-sized wild brook trout caught on artificial lures. N. Am. J. Fish. Manag. 1992, 12, 634–644. [Google Scholar] [CrossRef]
- Veiga, P.; Gonçalves, J.M.S.; Erzin, K. Short-term hooking mortality of three marine fish species (Sparidae) caught by recreational angling in the south Portugal. Fish. Res. 2011, 108, 58–64. [Google Scholar] [CrossRef]
- Morgan, A.; Burgess, G.H. At-vessel fishing mortality for six species of sharks caught in the northwest Atlantic and Gulf of Mexico. Gulf. Caribb. Res. 2007, 19, 123–129. [Google Scholar] [CrossRef]
- Orbesen, E.S.; Brown, C.A.; Snodgrass, D.; Serafy, J.E.; Walter, J.F., III. At-vessel and postrelease mortality rates of bluefin tuna (Thunnus thynnus) associated with pelagic longline gear in the northern Gulf of Mexico. Fish. Bull. 2019, 117, 15–32. [Google Scholar]





| Explanatory Variable | Name | Type | Scope/Classification |
|---|---|---|---|
| Biological Characteristics | Sex | Categorical | Female/Male |
| Lower Jaw-fork Length | Continuous | 59–273 (cm) | |
| Environmental Factors | Sea Surface Temperature | Continuous | 13.88–30.37 (°C) |
| Dissolved Oxygen | Continuous | 196.87–247.93 | |
| Space-time Elements | Quarter | Categorical | First (Jan, Feb, Mar)/Second (Apr, May, Jun)/Third (Jul, Aug, Sept)/Forth (Oct, Nov, Dec) (Quarter) |
| Longitude | Continuous | 40° E–81° E | |
| Latitude | Continuous | 40° N–11° S | |
| Hook Type | Categorical | Japanese Tuna Hook/Circle Hook/Others | |
| Target Fish Species | Categorical | Bigeye Tuna/Albacore Tuna |
| df | Deviance | Resid.Df | Resid.Dev | |
|---|---|---|---|---|
| NULL | 1143 | 1495.2 | ||
| Quarter | 3 | 6.4178 | 1140 | 1488.8 |
| Hook.type | 2 | 22.1269 | 1138 | 1466.7 |
| LJFL | 1 | 12.6856 | 1137 | 1454.0 |
| Lon | 1 | 14.3614 | 1136 | 1439.6 |
| Hook.type:LJFL | 2 | 7.1935 | 1134 | 1432.4 |
| LJFL:Lon | 1 | 24.5354 | 1133 | 1407.9 |
| Estimate | Std. Error | z Value | Pr(>|z|) | |
|---|---|---|---|---|
| (Intercept) | 9.141 | 2.942 | 3.107 | <0.01 |
| Quarter2 | 0.050 | 0.294 | 0.171 | 0.864 |
| Quarter 3 | 0.588 | 0.284 | 2.068 | <0.05 |
| Quarter 4 | 0.020 | 0.260 | 0.075 | 0.940 |
| Hook type Japanese tuna hook | 6.607 | 1.843 | 3.585 | <0.01 |
| Hook type Others | 4.417 | 2.220 | 1.989 | <0.05 |
| LJFL | −0.050 | 0.018 | −2.779 | <0.01 |
| Lon | −0.247 | 0.045 | −5.439 | <0.01 |
| Hook type Japanese tuna hook: LJFL | −0.033 | 0.011 | −3.008 | <0.01 |
| Hook type Others: LJFL | −0.026 | 0.014 | −1.900 | 0.057 |
| LJFL: Lon | 0.001 | 0.001 | 4.770 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Wang, X.; Li, X.; Wu, F.; Zhu, J. Analysis of At-Haulback Mortality and Influencing Factors of Indian Ocean Swordfish (Xiphias gladius). Fishes 2022, 7, 256. https://doi.org/10.3390/fishes7050256
Guo Y, Wang X, Li X, Wu F, Zhu J. Analysis of At-Haulback Mortality and Influencing Factors of Indian Ocean Swordfish (Xiphias gladius). Fishes. 2022; 7(5):256. https://doi.org/10.3390/fishes7050256
Chicago/Turabian StyleGuo, Yingcong, Xuefang Wang, Xiuzhen Li, Feng Wu, and Jiangfeng Zhu. 2022. "Analysis of At-Haulback Mortality and Influencing Factors of Indian Ocean Swordfish (Xiphias gladius)" Fishes 7, no. 5: 256. https://doi.org/10.3390/fishes7050256






