The Effect of Knocked-Down Anti-Müllerian Hormone mRNA on Reproductive Characters of Male Nile Tilapia (Oreochromis niloticus) through Inhibition of the TGF-Beta Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Construction of Antisense RNA Knocked-Down Model
2.2.1. Experimental Fish
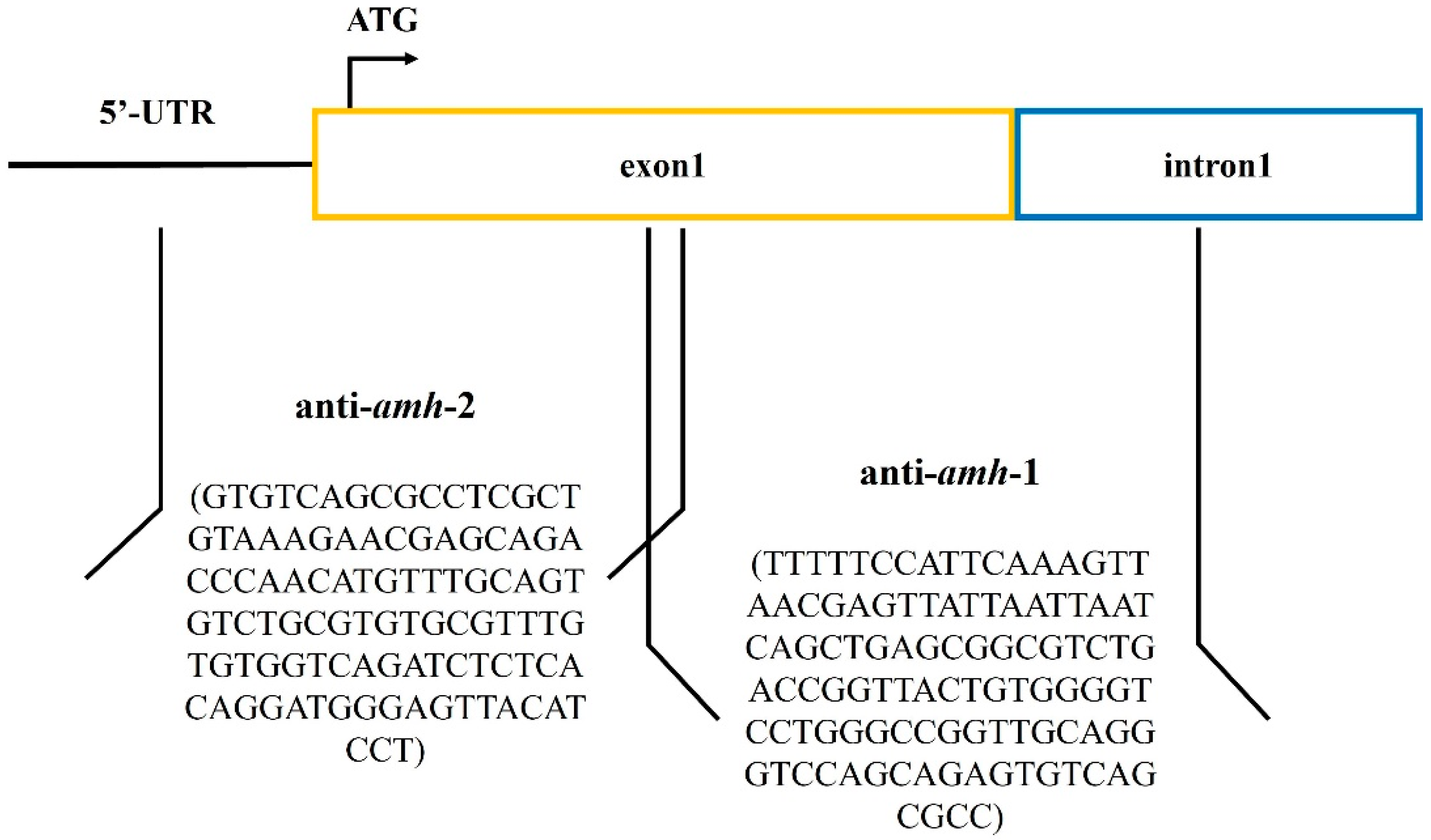
2.2.2. Design Antisense RNA Sequences
2.2.3. PCR Amplification
2.2.4. Preparation of Transfection Reagent
2.2.5. Artificial Insemination and Incubation
2.2.6. Experimental Fish Management
2.2.7. Detection of Positive Rate of Transfected Experimental Fish
2.3. Sampling
2.4. Index Determination and Calculation
2.4.1. Determining Growth Performance
2.4.2. Determination of Serum Hormones
2.5. Hematoxylin-Eosin Staining
2.6. Western Blot Analysis
2.7. RNA Extraction and Reverse Transcription
2.8. qRT-PCR
2.9. Absolute Quantitative PCR
2.9.1. Amplification of Amh Fragment
2.9.2. Amh Fragment Cloning and Verification
2.9.3. Plasmid DNA Extraction
2.10. RNA Sequencing
2.10.1. Library Construction and Sequencing
2.10.2. Assemble and Annotate Transcripts
2.10.3. Identification of Differentially Expressed Genes
2.10.4. qRT-PCR Validation
2.11. Statistical Analysis
3. Results
3.1. Determination of Positive Transfection Rate
3.2. Antisense RNA Inhibits Expression of amh mRNA and Protein in Testis
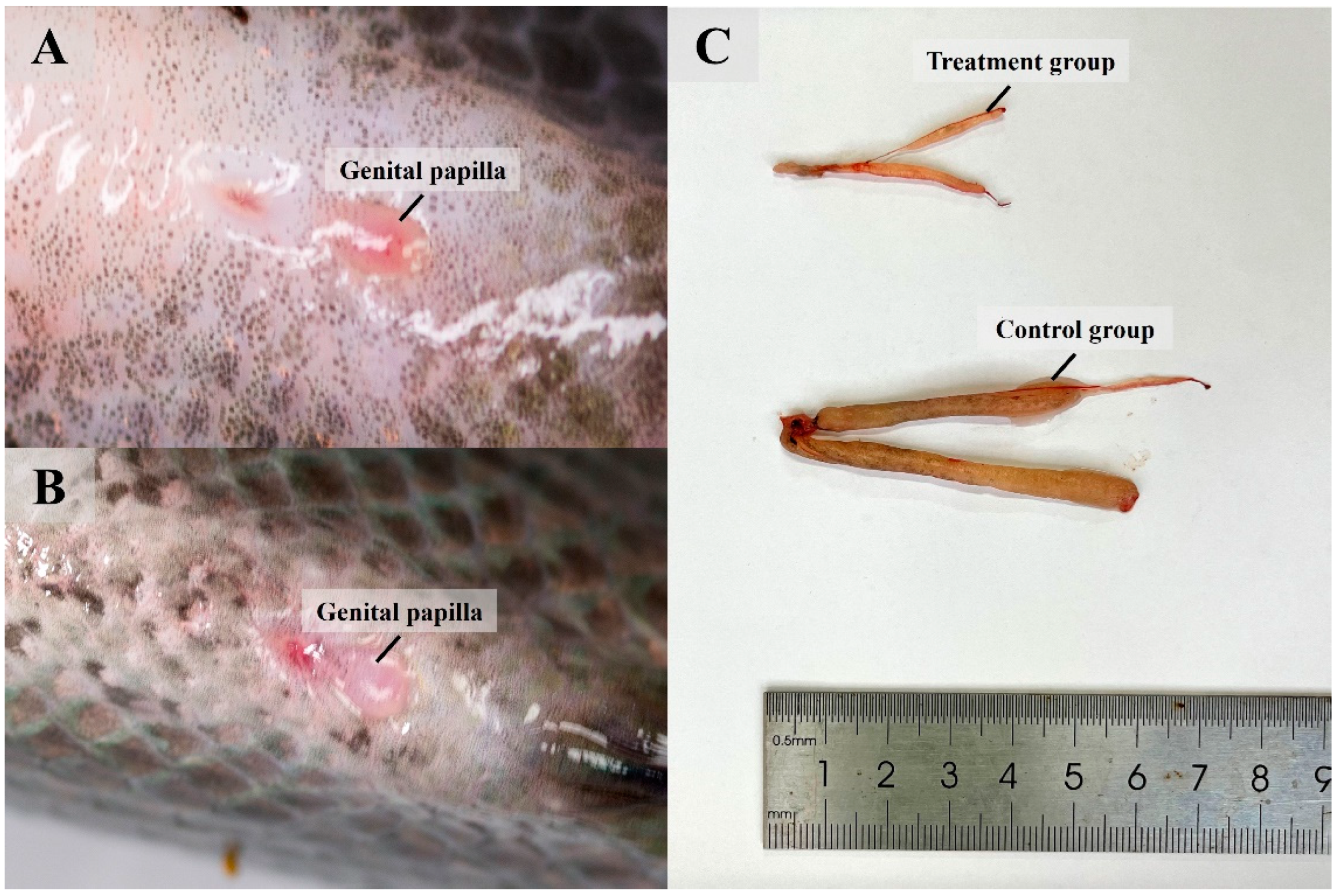
3.3. Knocked-Down Amh Affects Formation of Male Secondary Sexual Characteristics
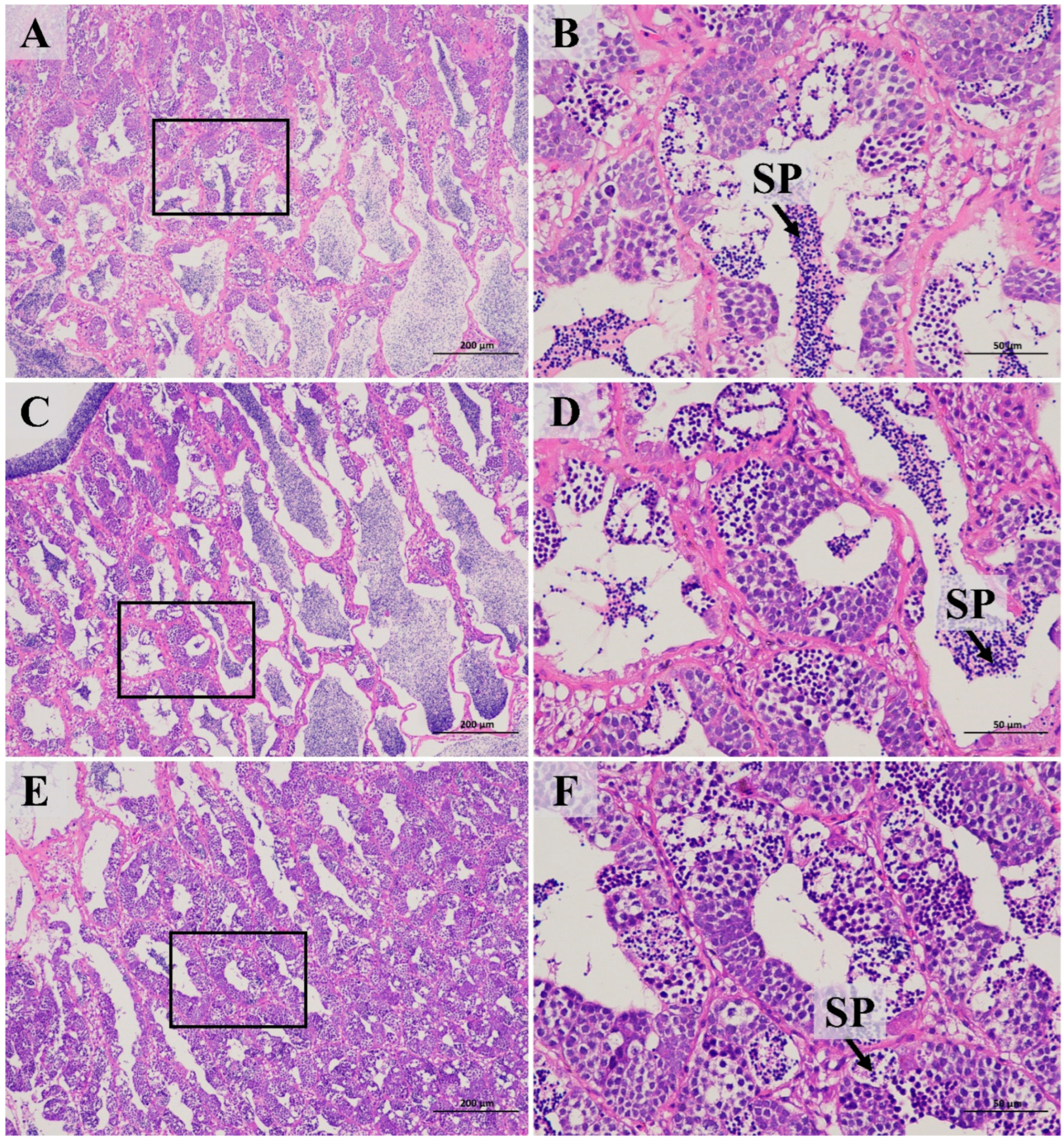
3.4. Knocked-Down Amh Inhibits Testis Development and Maturation
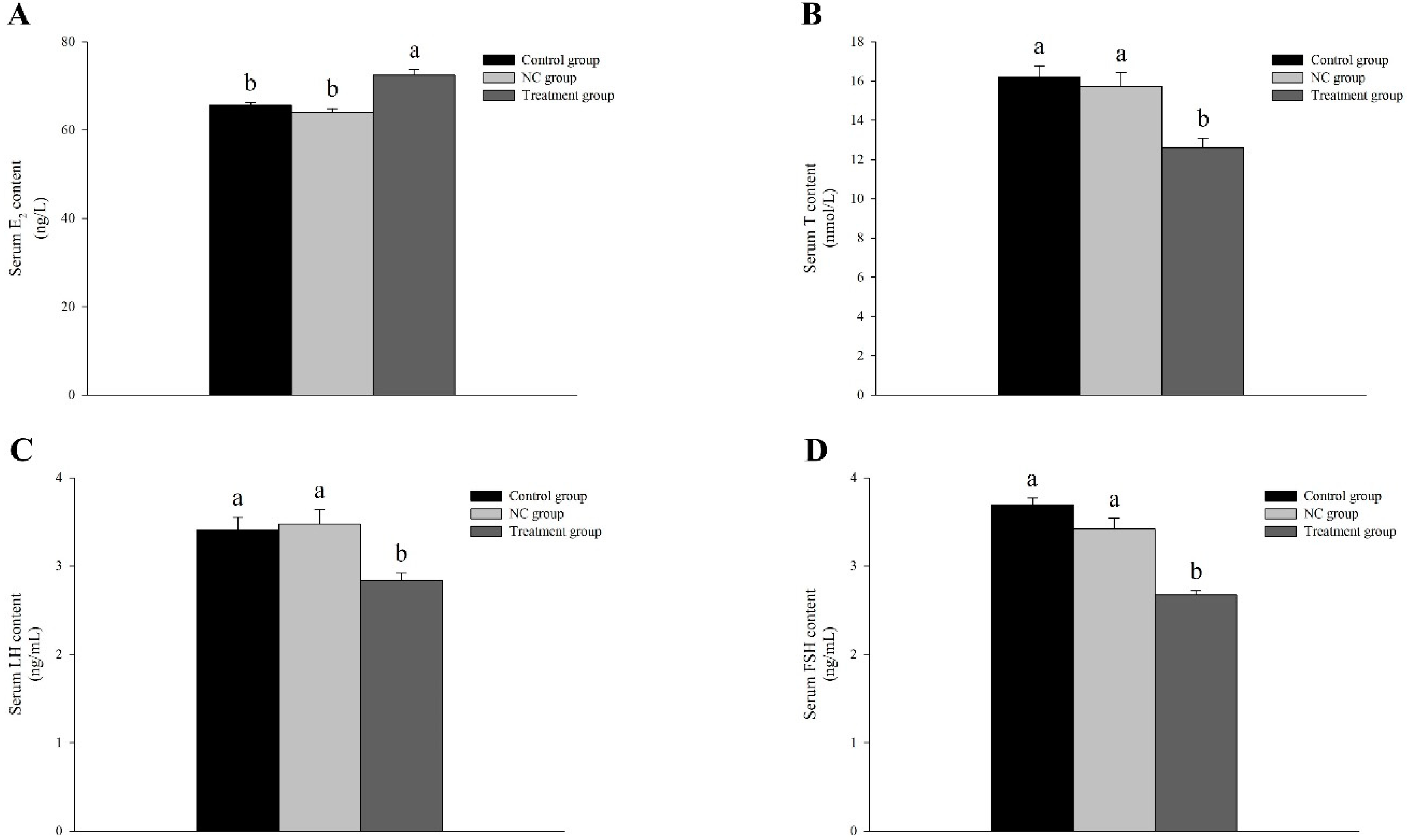
3.5. Knocked-Down Amh Affects Gonadal Regulation-Related Hormone Levels in Male
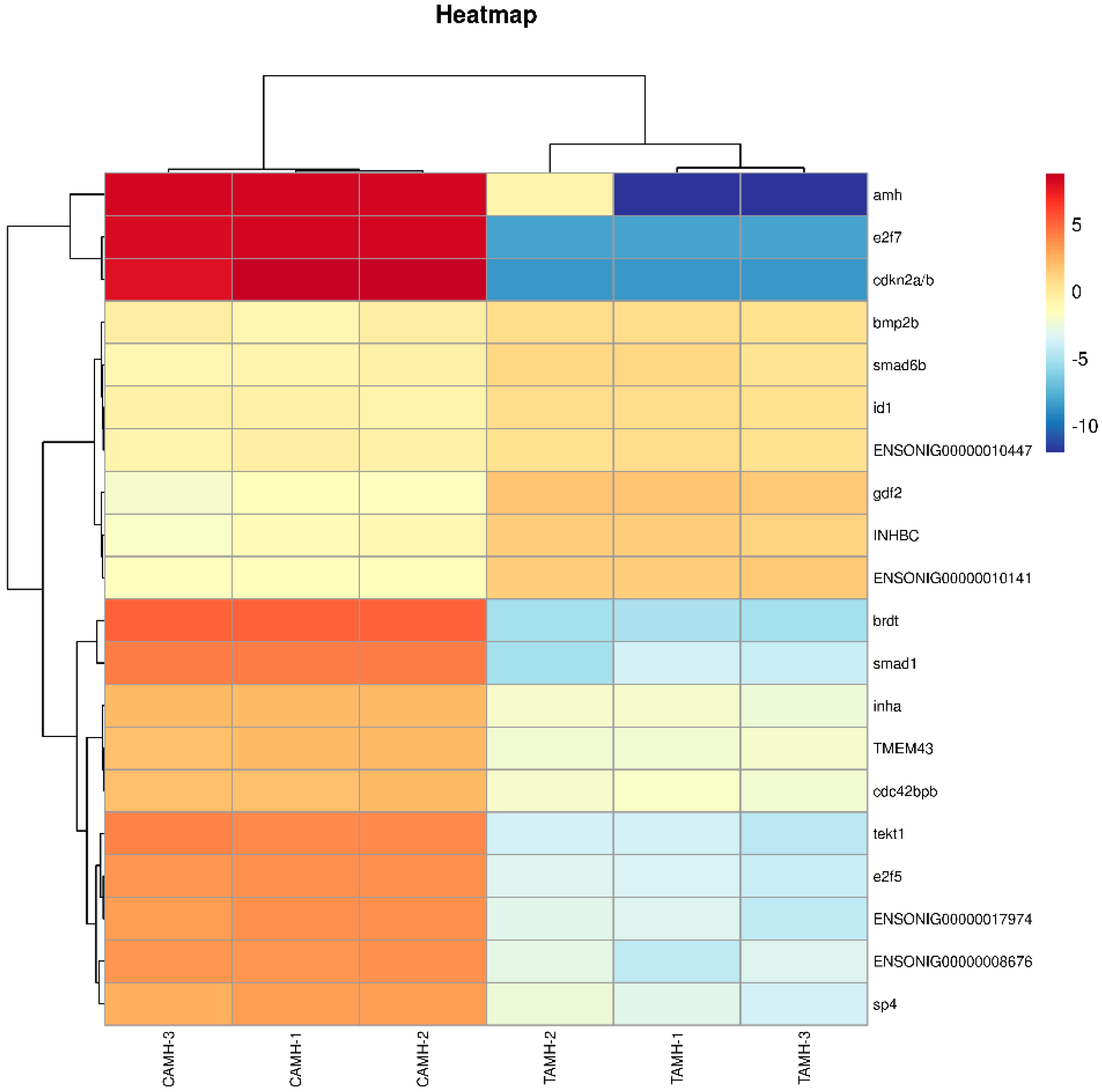
3.6. Transcriptome Analysis Reveals the Effect of Knocked-Down Amh on Testis Development
3.6.1. Functional Annotation by GO and KEGG Analysis
3.6.2. TGF-Beta Signaling Pathway
3.6.3. RNA-Sequencing Data Validation
4. Discussion
4.1. Knocked-Down Amh Leads to Increase in Body Weight and Decrease in Male GSI
4.2. Knocked-Down Amh Inhibits Testis Development
4.3. Knocked-Down Amh Affects Sex Maintenance in Males
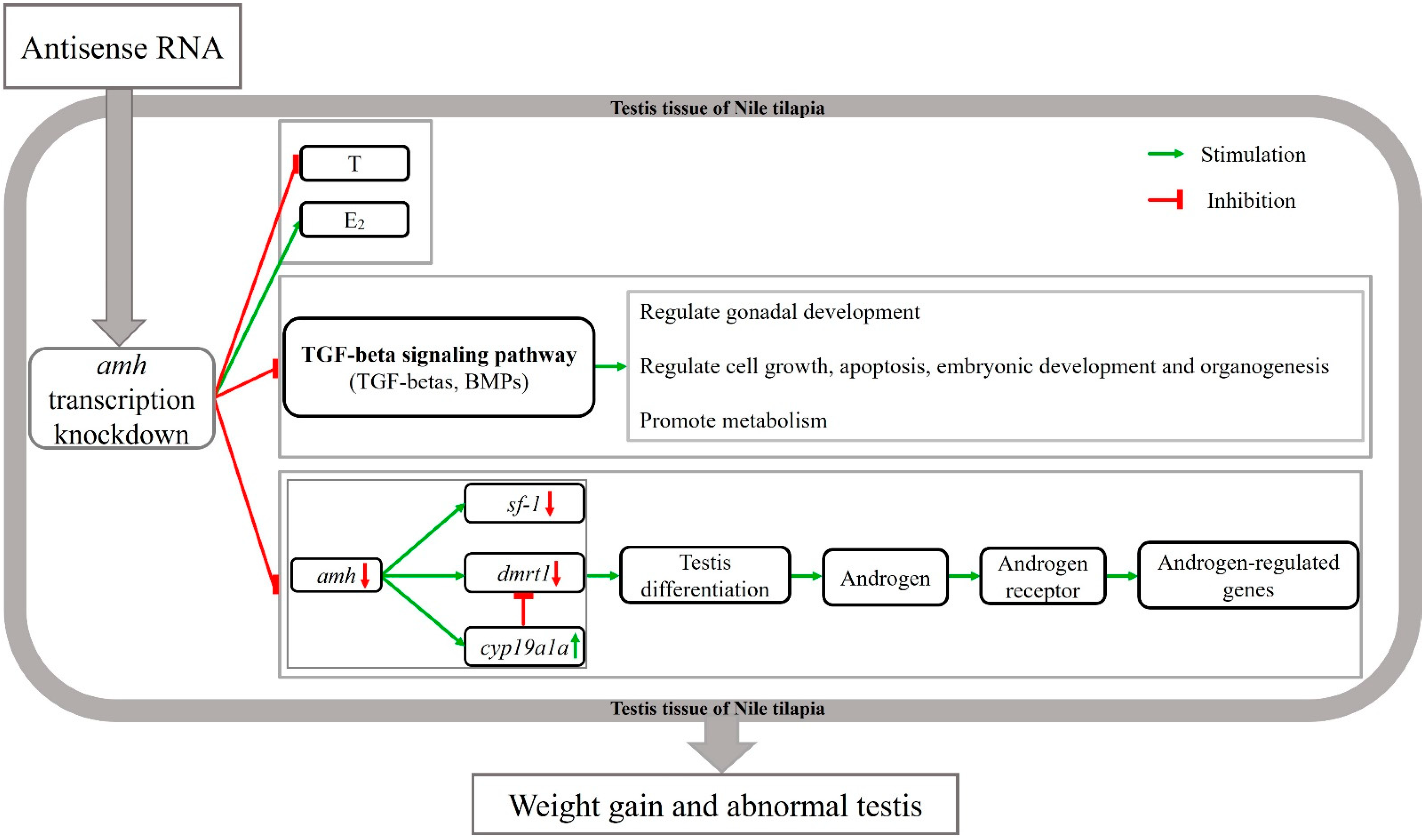
4.4. Molecular Mechanism of Knocked-Down Amh on Male Gonad Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Camblong, J.; Iglesias, N.; Fickentscher, C.; Dieppois, G.; Stutz, F. Antisense RNA Stabilization Induces Transcriptional Gene Silencing via Histone Deacetylation in S. cerevisiae. Cell 2007, 131, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Boonanuntanasarn, S. Gene Knockdown: A Powerful Tool for Gene Function Study in Fish. J. World Aquac. Soc. 2008, 39, 311–323. [Google Scholar] [CrossRef]
- Tomizawa, J.-I.; Itoh, T.; Selzer, G.; Som, T. Inhibition of ColEI RNA primer formation by a plasmid-specified small RNA. Proc. Natl. Acad. Sci. USA 1981, 78, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Örvar, B.; Ellis, B. Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. Plant J. 2002, 11, 1297–1305. [Google Scholar] [CrossRef]
- Fish, J.E.; Matouk, C.C.; Yeboah, E.; Bevan, S.C.; Khan, M.; Patil, K.; Ohh, M.; Marsden, P.A. Hypoxia-inducible Expression of a Natural cis-Antisense Transcript Inhibits Endothelial Nitric-oxide Synthase. J. Biol. Chem. 2007, 282, 15652–15666. [Google Scholar] [CrossRef] [PubMed]
- Harshe, R.; Xie, A.; Vuerich, M.; Frank, L.; Gromova, B.; Zhang, H.; Robles, R.; Mukherjee, S.; Csizmadia, E.; Kokkotou, E.; et al. Endogenous antisense RNA curbs CD39 expression in Crohn’s disease. Nat. Commun. 2020, 11, 5894. [Google Scholar] [CrossRef] [PubMed]
- Uzbekova, S.; Chyb, J.; Ferriere, F.; Bailhache, T.; Prunet, P.; Alestrom, P.; Breton, B. Transgenic rainbow trout expressed sGnRH-antisense RNA under the control of sGnRH promoter of Atlantic salmon. J. Mol. Endocrinol. 2001, 25, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-M.; Qiang, J.; Zhu, J.-H.; Li, H.-X.; Tao, Y.-F.; He, J.; Xu, P.; Dong, Z.-J. Transcriptional inhibition of steroidogenic factor 1 in vivo in Oreochromis niloticus increased weight and suppressed gonad development. Gene 2022, 809, 146023. [Google Scholar] [CrossRef]
- Qiang, J.; Cao, Z.-M.; Zhu, H.-J.; Tao, Y.-F.; He, J.; Xu, P. Knock-down of amh transcription by antisense RNA reduces FSH and increases follicular atresia in female Oreochromis niloticus. Gene 2022, 842, 146792. [Google Scholar] [CrossRef]
- Abernathy, J.; Overturf, K. Expression of Antisense Long Noncoding RNAs as Potential Regulators in Rainbow Trout with Different Tolerance to Plant-Based Diets. Anim. Biotechnol. 2018, 30, 87–94. [Google Scholar] [CrossRef]
- Josso, N.; di Clemente, N.; Gouédard, L. Anti-Müllerian hormone and its receptors. Mol. Cell. Endocrinol. 2001, 179, 25–32. [Google Scholar] [CrossRef]
- Monsivais, D.; Matzuk, M.; Pangas, S. The TGF-β Family in the Reproductive Tract. Cold Spring Harb. Perspect. Biol. 2017, 9, a022251. [Google Scholar] [CrossRef]
- Rey, R.; Lukas-Croisier, C.; Lasala, C.; Bedecarrás, P. AMH/MIS: What we know already about the gene, the protein and its regulation. Mol. Cell. Endocrinol. 2003, 211, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish—Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Tripurani, S.; Burton, J.; Clementi, C.; Larina, I.; Pangas, S. SMAD Signaling Is Required for Structural Integrity of the Female Reproductive Tract and Uterine Function During Early Pregnancy in Mice. Biol. Reprod. 2016, 95, 44. [Google Scholar] [CrossRef] [PubMed]
- Lochab, A.K.; Extavour, C.G. Bone Morphogenetic Protein (BMP) signaling in animal reproductive system development and function. Dev. Biol. 2017, 427, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Spiller, C.; Burnet, G.; Bowles, J. Regulation of fetal male germ cell development by members of the TGFβ superfamily. Stem Cell Res. 2017, 24, 174–180. [Google Scholar] [CrossRef]
- Kamiya, T.; Kai, W.; Tasumi, S.; Oka, A.; Matsunaga, T.; Mizuno, N.; Fujita, M.; Suetake, H.; Suzuki, S.; Hosoya, S.; et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 2012, 8, e1002798. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.-N.; Zhou, L.; Sun, L.; Tao, W.; et al. A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia, Oreochromis niloticus. PLOS Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strüssmann, C.A. A Y-linked anti-Müllerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.H.; Derynck, R. Specificity and versatility in TGF-beta signaling through SMADS. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Jiang, D.; Huang, Y.; Li, B.; Chen, C.; Lin, H.; Xia, J. Identifying a Major QTL Associated with Salinity Tolerance in Nile Tilapia Using QTL-Seq. Mar. Biotechnol. 2018, 20, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lind, C.E.; Safari, A.; Agyakwah, S.K.; Attipoe, F.Y.K.; El-Naggar, G.O.; Hamzah, A.; Hulata, G.; Ibrahim, N.A.; Khaw, H.L.; Nguyen, N.H.; et al. Differences in sexual size dimorphism among farmed tilapia species and strains undergoing genetic improvement for body weight. Aquac. Rep. 2015, 1, 20–27. [Google Scholar] [CrossRef]
- Desprez, D.; Géraz, E.; Hoareau, M.C.; Mélard, C.; Bosc, P.; Baroiller, J.F. Production of a high percentage of male offspring with a natural androgen, 11β-hydroxyandrostenedione (11βOHA4), in Florida red tilapia. Aquaculture 2003, 216, 55–65. [Google Scholar] [CrossRef]
- Mañanós, E.L.; Swanson, P.; Stubblefield, J.; Zohar, Y. Purification of Gonadotropin II from a Teleost Fish, the Hybrid Striped Bass, and Development of a Specific Enzyme-Linked Immunosorbent Assay. Gen. Comp. Endocrinol. 1997, 108, 209–222. [Google Scholar] [CrossRef]
- Aizen, J.; Kasuto, H.; Levavi-Sivan, B. Development of specific enzyme-linked immunosorbent assay for determining LH and FSH levels in tilapia, using recombinant gonadotropins. Gen. Comp. Endocrinol. 2007, 153, 323–332. [Google Scholar] [CrossRef]
- Alvarenga, É.; França, L. Effects of Different Temperatures on Testis Structure and Function, with Emphasis on Somatic Cells, in Sexually Mature Nile Tilapias (Oreochromis niloticus). Biol. Reprod. 2008, 80, 537–544. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.; Pertea, G.; Mortazavi, A.; Kwan, G.; Baren, M.; Salzberg, S.; Wold, B.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Jiang, J.-L.; Xu, J.; Ye, L.; Sun, M.-L.; Jiang, Z.-Q.; Mao, M.-G. Identification of differentially expressed genes in gills of tiger puffer (Takifugu rubripes) in response to low-salinity stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 243–244, 110437. [Google Scholar] [CrossRef]
- Li, H.; Qiang, J.; Song, C.; Xu, P. Transcriptome profiling reveal Acanthopanax senticosus improves growth performance, immunity and antioxidant capacity by regulating lipid metabolism in GIFT (Oreochromis niloticus). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 37, 100784. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Nain, V. TALENs—An indispensable tool in the era of CRISPR: A mini review. J. Genet. Eng. Biotechnol. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Wu, J.; Zhang, X.; Luo, J.; Wang, H.; Ming, D. The Advance of CRISPR-Cas9-Based and NIR/CRISPR-Cas9-Based Imaging System. Front. Chem. 2021, 9, 786354. [Google Scholar] [CrossRef]
- Hruscha, A.; Krawitz, P.; Rechenberg, A.; Heinrich, V.; Hecht, J.; Haass, C.; Schmid, B. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Dev. Camb. Engl. 2013, 140, 4982–4987. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Kinoshita, M.; Ng, T.H.; Chang, Y.-H.; Maekawa, S.; Chiang, Y.-A.; Aoki, T.; Wang, H.-C. Using CRISPR/Cas9-mediated gene editing to further explore growth and trade-off effects in myostatin-mutated F4 medaka (Oryzias latipes). Sci. Rep. 2017, 7, 11435. [Google Scholar] [CrossRef]
- Ouyang, J.; Songlei, X.; Zhou, Q.; Cui, H. Research progress and applications of gene editing technology CRISPR/Cas in zebrafish. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Næve, I.; Mommens, M.; Arukwe, A.; Kjørsvik, E. Ultrasound as a noninvasive tool for monitoring reproductive physiology in female Atlantic salmon (Salmo salar). Physiol. Rep. 2018, 6, e13640. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, B.; Chen, W.; Ge, W. Anti-Müllerian hormone (Amh/amh) plays dual roles in maintaining gonadal homeostasis and gametogenesis in zebrafish. Mol. Cell. Endocrinol. 2020, 517, 110963. [Google Scholar] [CrossRef]
- Milnes, M.R.; Bermudez, D.S.; Bryan, T.A.; Edwards, T.M.; Gunderson, M.P.; Larkin, I.L.V.; Moore, B.C.; Guillette, L.J. Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ. Res. 2006, 100, 3–17. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Martinez, E.R.M.; Butzge, A.J.; Doretto, L.B.; Ricci, J.M.B.; Rodrigues, M.S.; Vigoya, A.A.A.; Gómez-González, N.E.; Stewart, A.B.; Nóbrega, R.H. Molecular characterization and expression analysis of anti-Müllerian hormone in common carp (Cyprinus carpio) adult testes. Gene Expr. Patterns 2021, 40, 119169. [Google Scholar] [CrossRef]
- Skaar, K.; Nóbrega, R.; Magaraki, A.; Olsen, L.; Schulz, R.; Male, R. Proteolytically Activated, Recombinant Anti-Mullerian Hormone Inhibits Androgen Secretion, Proliferation, and Differentiation of Spermatogonia in Adult Zebrafish Testis Organ Cultures. Endocrinology 2011, 152, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.; Wang, R.; Chen, S. Transcriptomics analysis revealing candidate networks and genes for the body size sexual dimorphism of Chinese tongue sole (Cynoglossus semilaevis). Funct. Integr. Genom. 2018, 18, 327–339. [Google Scholar] [CrossRef]
- Liu, X.; Dai, S.; Wu, J.; Wei, X.; Zhou, X.; Chen, M.; Tan, D.; Pu, D.; Li, M.; Wang, D. Roles of Anti-Müllerian hormone (Amh) and its duplicates in sex determination and germ cell proliferation of Nile tilapia. Genetics 2021, 220, iyab237. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J. Neuroendocrinology of sexual plasticity in teleost fishes. Front. Neuroendocrinol. 2010, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Zhao, J.; Tang, S.; Zhao, Y. Effects of Estradiol and Testosterone on the Expression of Growth-related Genes in Female and Male Nile Tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2017, 49, 216–228. [Google Scholar] [CrossRef]
- Linderoth, M.; Hansson, T.; Liewenborg, B.; Sundberg, H.; Noaksson, E.; Hanson, M.; Zebühr, Y.; Balk, L. Basic physiological biomarkers in adult female perch (Perca fluviatilis) in a chronically polluted gradient in the Stockholm recipient (Sweden). Mar. Pollut. Bull. 2006, 53, 437–450. [Google Scholar] [CrossRef]
- Marchand, M.; Wagenaar, G.; Barnhoorn, I. Preliminary results on sperm motility and testicular histology of two feral fish species, Oreochromis mossambicus and Clarias gariepinus, from a currently DDT-sprayed area, South Africa. J. Appl. Ichthyol. 2008, 24, 423–429. [Google Scholar] [CrossRef]
- Louiz, I.; Ben-Attia, M.; Ben-Hassine, O.K. Gonadosomatic index and gonad histopathology of Gobius niger (Gobiidea, Teleost) from Bizerta lagoon (Tunisia): Evidence of reproduction disturbance. Fish. Res. 2009, 100, 266–273. [Google Scholar] [CrossRef]
- Paul-Prasanth, B.; Shibata, Y.; Horiguchi, R.; Nagahama, Y. Exposure to Diethylstilbestrol During Embryonic and Larval Stages of Medaka Fish (Oryzias latipes) Leads to Sex Reversal in Genetic Males and Reduced Gonad Weight in Genetic Females. Endocrinology 2011, 152, 707–717. [Google Scholar] [CrossRef][Green Version]
- Song, W.; Wang, Z.; Liu, H. Effects of individual and binary mixtures of estrogens on male goldfish (Carassius auratus). Fish Physiol. Biochem. 2014, 40, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Weltzien, F.-A.; Taranger, G.; Karlsen, Ø.; Norberg, B. Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002, 132, 567–575. [Google Scholar] [CrossRef]
- Barbotin, A.-L.; Peigné, M.; Malone, S.; Giacobini, P. Emerging Roles of Anti-Müllerian Hormone in Hypothalamic-Pituitary Function. Neuroendocrinology 2019, 109, 218–229. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Liu, S.; Zhao, C.; Miao, Y.; Jin, L.; Wang, D.; Zhou, L. Cyp17a1 is Required for Female Sex Determination and Male Fertility by Regulating Sex Steroid Biosynthesis in Fish. Endocrinology 2021, 162, bqab205. [Google Scholar] [CrossRef] [PubMed]
- Walker, W. Androgen Actions in the Testis and the Regulation of Spermatogenesis. Adv. Exp. Med. Biol. 2021, 1288, 175–203. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Tsai, M.-Y.; Xu, Q.; Mu, X.-M.; Lardy, H.; Huang, K.; Lin, H.; Yeh, S.-D.; Altuwaijri, S.; Zhou, X.; et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 13498–13503. [Google Scholar] [CrossRef] [PubMed]
- Zohar, Y.; Muñoz-Cueto, J.; Elizur, A.; Kah, O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2009, 165, 438–455. [Google Scholar] [CrossRef]
- Liu, H.; Lamm, M.S.; Rutherford, K.; Black, M.A.; Godwin, J.R.; Gemmell, N.J. Large-scale transcriptome sequencing reveals novel expression patterns for key sex-related genes in a sex-changing fish. Biol. Sex Differ. 2015, 6, 26. [Google Scholar] [CrossRef]
- Hollander Cohen, L.; Golan, M.; Levavi-Sivan, B. Differential Regulation of Gonadotropins as Revealed by Transcriptomes of Distinct LH and FSH Cells of Fish Pituitary. Int. J. Mol. Sci. 2021, 22, 6478. [Google Scholar] [CrossRef]
- Oduwole, O.; Huhtaniemi, I.; Misrahi, M. The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited. Int. J. Mol. Sci. 2021, 22, 12735. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Piferrer, F.; Guiguen, Y. Fish Gonadogenesis. Part II: Molecular Biology and Genomics of Sex Differentiation. Rev. Fish. Sci. 2008, 16, 35–55. [Google Scholar] [CrossRef]
- Wu, G.-C.; Tomy, S.; Chang, C.-F. The Expression of nr0b1 and nr5a4 During Gonad Development and Sex Change in Protandrous Black Porgy Fish, Acanthopagrus schlegeli1. Biol. Reprod. 2008, 78, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Guo, W.; Gao, Y.; Tang, R.; Li, D. Molecular cloning and characterization of amh and dax1 genes and their expression during sex inversion in rice-field eel Monopterus albus. Sci. Rep. 2015, 5, 16667. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shinomiya, A.; Kinoshita, M.; Suzuki, A.; Kobayashi, T.; Paul-Prasanth, B.; Lau, E.-L.; Hamaguchi, S.; Sakaizumi, M.; Nagahama, Y. DMY gene induces male development in genetically female (XX) medaka fish. Proc. Natl. Acad. Sci. USA 2007, 104, 3865–3870. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, M.; Wang, L.; Zeshu, Y.; Wang, J.; Yu, Q.; Xiao, L.; Lu, M.; Shuisheng, L.; Zhang, Y.; et al. Overexpression of Anti-müllerian Hormone Gene in vivo Affects Gonad Sex Differentiation in Undifferentiated Orange-Spotted Groupers (Epinephelus coioides). Front. Endocrinol. 2019, 10, 210. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [Google Scholar] [CrossRef]
- Wang, C.; Ruan, L.; Shi, H.; Xu, X. Wnt5b regulates apoptosis in Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish Immunol. 2018, 74, 318–324. [Google Scholar] [CrossRef]
- Wei, M.; Xu, W.; Li, H.; Wang, L.; Xiu, Y.; Yang, Y.; Li, Y.; Zhao, F.; Chen, S. Molecular characterization and expression analysis of a novel r-spondin member (rspo2l) in Chinese tongue sole (Cynoglossus semilaevis). Fish Shellfish Immunol. 2018, 72, 436–442. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Cao, M.; Wang, X.; Wang, G.; Li, J. Identification of wnt2 in the pearl mussel Hyriopsis cumingii and its role in innate immunity and gonadal development. Fish Shellfish Immunol. 2021, 118, 85–93. [Google Scholar] [CrossRef]
- Font de Mora, J.; Esteban, L.; Burks, D.; Núñez Alonso, A.; Garcés, C.; García-Barrado, M.; Iglesias-Osma, M.C.; Moratinos, J.; Ward, J.; Santos, E. Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO J. 2003, 22, 3039–3049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, Y.; Xu, X.; Dai, Y.; Jiale, L.J. Transcriptome Analysis of the Liver and Muscle Tissues of Black Carp (Mylopharyngodon piceus) of Different Growth Rates. Mar. Biotechnol. 2020, 22, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Harashima, H.; Dissmeyer, N.; Schnittger, A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol. 2013, 23, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Sasaoka, T.; Wada, T.; Tsuneki, H. Lipid phosphatases as a possible therapeutic target in cases of type 2 diabetes and obesity. Pharmacol. Ther. 2006, 112, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Triay, C.; Courcelle, M.; Caminade, P.; Bezault, E.; Baroiller, J.-F.; Kocher, T.; D’Cotta, H. Polymorphism of Sex Determination amongst Wild Populations Suggests its Rapid Turnover within the Nile Tilapia Species Helena D’Cotta. Front. Genet. 2022, 13, 820772. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.-H. The regulation of TGFβ signal transduction. Development 2009, 136, 3699–3714. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, S.; Hall, B. Signal transduction and TGF-β superfamily receptors. Biochem. Cell Biol. 2011, 74, 299–314. [Google Scholar] [CrossRef]
- Gahr, A.; Weber, G. Identification and expression of Smads associated with TGF-β/activin/nodal signaling pathways in the rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2012, 38, 123–1244. [Google Scholar] [CrossRef]
- Liu, T.; Feng, X.-H. Regulation of TGF-beta signalling by protein phosphatases. Biochem. J. 2010, 430, 191–198. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Y.-G. Regulation of TGF-β Signal Transduction. Scientifica 2014, 2014, 874065. [Google Scholar] [CrossRef]
- Schmierer, B.; Hill, C. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2008, 8, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.O.; Sieber, C.; Bhushan, R.; Börgermann, J.H.; Graf, D.; Knaus, P. BMPs: From bone to body morphogenetic proteins. Sci. Signal. 2010, 3, mr1. [Google Scholar] [CrossRef] [PubMed]
- Sawatari, E.; Shikina, S.; Takeuchi, T.; Yoshizaki, G. A novel transforming growth factor-β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss). Dev. Biol. 2007, 301, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, G.; Li, M.; Zhu, F.; Liu, Q.; Naruse, K.; Herpin, A.; Nagahama, Y.; Li, J.; Hong, Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 2016, 6, 19738. [Google Scholar] [CrossRef]



| Gene Name | Gene Description | Primer Sequence (5′–3′) |
|---|---|---|
| cyp19a1a | cytochrome P450 family 19 subfamily A polypeptide 1a | F: 5′-GCTACAGGATCTCGAAGGGC-3′ R: 5′-ACCGAACGGCTGAAAGGTAG-3′ |
| amh | Anti-Müllerian hormone | F: 5′-GCTTATCCTCCAGCGAGACC-3′ R: 5′-TTGGCTCCCAGTGAAACCTC-3′ |
| sf-1 | steroidogenic factor 1 | F: 5′-TTTGTCCTTCGGCTCAGTCC-3′ R: 5′-CGTGTACCTCGGTGTGTTGA-3′ |
| smad3a | smad family member 3a | F: 5′-TGGCTGGACAAGGTGCTTAC-3′ R: 5′-TTGTGTAGCCGTTCTCGTCC-3′ |
| smad5 | smad family member 5 | F: 5′-GGCTGAATACGATGACTCCCC-3′ R: 5′-GCCTCACTGGTGCAAGTCT-3′ |
| gsdf | gonadal somatic cell derived factor | F: 5′-GAGCAGTGGAACCGAACCTT-3′ R: 5′-GAACAACACTTCAGGCTCGC-3′ |
| tgfb2 | transforming growth factor beta 2 | F: 5′-TGCTGTGTCTCCCAAGACCT-3′ R: 5′-CGGCACTTTGACGGTACGTT-3′ |
| tgfbr1b | transforming growth factor beta receptor 1b | F: 5′-GACTTGATCCCACGAGACCG-3′ R: 5′-GGCCACCGGGTCTTTGTT-3′ |
| dmrt1 | double sex and mab-3-related transcription factor 1 | F: 5′-CGCAGTACCAGATGCCTCAT-3′ R: 5′-CAGGCTAAAGAAGGGTGGCA-3′ |
| β-actin | F: 5′-CCACACAGTGCCCATCTACGA-3′ R: 5′-CCACGCTCTGTCAGGATCTTCA-3′ |
| Measurement | Control Group (n = 12) | Negative Control (NC) Group (n = 12) | Treatment Group (n = 12) |
|---|---|---|---|
| Final body weight (g) | 249.41 b ± 18.33 | 242.57 b ± 22.14 | 316.76 a ± 24.48 |
| Gonadal weight (g) | 2.38 b ± 0.63 | 2.41 b ± 0.53 | 0.32 a ± 0.09 |
| Gonadosomatic index (GSI) | 0.96 b ± 0.11 | 1.00 b ± 0.13 | 0.10 a ± 0.04 |
| Sample | Raw Reads | Valid Reads | Valid Bases (G) | Valid Ratio (Reads) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|
| CAMH 1 | 51,507,620 | 49,543,150 | 7.43 | 96.19 | 99.90 | 97.71 | 48.0 |
| CAMH 2 | 43,773,786 | 42,042,262 | 6.31 | 96.04 | 99.91 | 97.71 | 48.0 |
| CAMH 3 | 41,260,474 | 39,431,276 | 5.91 | 95.57 | 99.97 | 98.20 | 48.0 |
| TAMH 1 | 49,581,146 | 43,477,524 | 6.52 | 87.69 | 99.91 | 97.75 | 52.5 |
| TAMH 2 | 47,641,762 | 44,245,380 | 6.64 | 92.87 | 99.92 | 97.82 | 51.0 |
| TAMH 3 | 42,748,522 | 37,271,364 | 5.59 | 87.19 | 99.97 | 98.01 | 46.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Tao, Y.; Cao, Z.; Lu, S.; Xu, P.; Qiang, J. The Effect of Knocked-Down Anti-Müllerian Hormone mRNA on Reproductive Characters of Male Nile Tilapia (Oreochromis niloticus) through Inhibition of the TGF-Beta Signaling Pathway. Fishes 2022, 7, 299. https://doi.org/10.3390/fishes7050299
Yan Y, Tao Y, Cao Z, Lu S, Xu P, Qiang J. The Effect of Knocked-Down Anti-Müllerian Hormone mRNA on Reproductive Characters of Male Nile Tilapia (Oreochromis niloticus) through Inhibition of the TGF-Beta Signaling Pathway. Fishes. 2022; 7(5):299. https://doi.org/10.3390/fishes7050299
Chicago/Turabian StyleYan, Yue, Yifan Tao, Zheming Cao, Siqi Lu, Pao Xu, and Jun Qiang. 2022. "The Effect of Knocked-Down Anti-Müllerian Hormone mRNA on Reproductive Characters of Male Nile Tilapia (Oreochromis niloticus) through Inhibition of the TGF-Beta Signaling Pathway" Fishes 7, no. 5: 299. https://doi.org/10.3390/fishes7050299
APA StyleYan, Y., Tao, Y., Cao, Z., Lu, S., Xu, P., & Qiang, J. (2022). The Effect of Knocked-Down Anti-Müllerian Hormone mRNA on Reproductive Characters of Male Nile Tilapia (Oreochromis niloticus) through Inhibition of the TGF-Beta Signaling Pathway. Fishes, 7(5), 299. https://doi.org/10.3390/fishes7050299






