Abstract
The excessive worldwide production of plastic materials results in omnipresent microplastic pollution. Scientific studies dealing with the impacts of microplastics on aquatic ecosystems focus mainly on the marine environment, documenting the effect on the functional traits of various organisms. Polystyrene, one of the most commonly used plastics, has become a widely used model in this respect. In our study, freshwater shrimps (Neocardina heteropoda) were exposed to virgin polystyrene particles (size 0.5 mm; nominal concentration 8 mgL−1), and their behavioral and physiological responses were compared to control shrimp. The exposed shrimps exhibited modified activity patterns (greater speeds, accelerations and distances moved), accompanied by a lowered standard metabolic rate (SMR). The observed effects differed in their progression from the 7th to 14th day of exposure, from undetectable changes (distance, SMR) to significant differences (speed, acceleration). Significant differences were also detected in the behavioral syndromes expressed by the exposed and controlled shrimps, indicating that the microplastics influence not only the particular traits, but also their functional relationships. As such, our study contributes to the integration of behavioral ecotoxicology in risk assessment, documenting the adverse performance of freshwater invertebrates exposed to microplastics with the potential to transpose the problem to higher levels of the food web.
1. Introduction
Plastics, lightweight and long-living materials, are globally recognized as a serious environmental hazard, endangering all biota, including humans [1,2]. The annual global plastics production increased from 0.35 million tons in the 1950s to approx. 350 million tons in the 2010s and is growing still [3]. They are widely used in daily life, including in all industry sectors, because of their first-class physicochemical properties [4]. Almost half of the plastic produced is destined for short-term use (less than one year), which results in huge amounts of plastic waste [5,6]. Most of the plastic waste (ca. 79%) is accumulated in landfills or the natural environment, and, if the current trends continue, about 12,000 million tons of plastic waste will be released there by 2050 [6]. The main concern related to plastic waste in the environment is that large plastic breaks down, due to weathering (degradation), UV radiation and fragmentation—mechanical abrasion and biological degradation, to small particles (micro or nano plastics) that are harmful to biota [4,7]. However, microplastics, defined as synthetic polymers with a size between 1 μm and 5 mm, can also be produced as primary particles, as found in textiles, medicine, personal care products and other commodities [8,9].
In the aquatic environment, where there is a continuous discharge and presence of microplastics, special attention has been dedicated to management and mitigation strategies [10]. The plastics in marine ecosystems are more visible to human society, as they accumulate on beaches or in the gigantic North Pacific Garbage Patch [11]. Accordingly, ecotoxicological studies of microplastics have been conducted using predominantly marine (77%) as opposed to freshwater (23%) organisms [12]. There is also a deficiency of ecotoxicological studies on groups of organisms other than fish in freshwater environments, emphasizing that the effects of microplastics on invertebrates in freshwater ecosystems have been under-studied and deserve further attention [12].
Awareness that contaminants might be affecting wildlife and human behavior is still, despite decades of research, broadly lacking [13]. Thus, the authors recommend integrating behavioral ecotoxicology [14,15] in risk assessment more thoroughly. Such integration requires data about which traits are affected by specific pollutants and how. Detailed metanalyses [16] and reviews [17] summarizing current knowledge about the impact of microplastics on various behavioral as well as physiological traits of fishes were published recently. Microplastics were proved to have a generally negative effect on the functional traits of fishes, including behavior, by the body accumulation of contaminants [18], with interspecies differences varying from negative to non-significant or even positive effects [18]. Activity is one of five main personality-related behavioral axes [17], reflecting many ecologically important behaviors [19], and as such is widely used in behavioral ecotoxicology. Exposure to microplastics was proved to decrease activity in larval zebrafish [20], as well as to immobilize the invertebrate Daphnia magna [21] and the whiteleg shrimp Litopenaeus vannamei [22]. Behavioral features are generally linked to metabolic costs on an individual level [18,23,24]. Metabolism was also shown to be altered by microplastics in fish [25] as well as invertebrates [26]. However, the disclosure of whether microplastics influence the functional relationship between activity measures and metabolism, i.e., behavioral syndrome [27], is missing.
The aim of the present study was to verify the effect of microplastics on the activity, metabolism and context-specific expression of their behavioral syndrome in freshwater invertebrates. Three main hypotheses were formulated: microplastics would (i) induce a decrease of activity measures [21], expressed as distance moved, speed and acceleration; (ii) reduce metabolism [26], expressed as standard metabolic rate; and (iii) cause a context-specific expression of the behavioral syndrome between activity measures and metabolism [28]. The freshwater shrimp Neocardina heteropoda, suggested as a useful model species in ecotoxicology [29,30], was used in the present study. Shrimps are generally vulnerable to microplastic pollution [31], influencing their oxidative stress as well as survival [32]. Shrimps were experimentally exposed to waterborne virgin polystyrene particles (size 0.2-0.5 mm; nominal concentration 8 mg L−1), as polystyrene, one of the most used plastics [8], has become a widely used model in this respect [33,34].
2. Materials and Methods
2.1. Experimental Animals
The shrimp Neocardina heteropoda live mainly in the freshwaters of Taiwan [35]. Adult shrimp can reach a maximum body length of 40 mm (mean length 25 mm female; 20 mm male). The male is usually less colored, and its tail, which is not required for carrying eggs, is narrower. Shrimps reach sexual maturity when they are around 4–6 months old. Neocardina heteropoda are primarily biofilm eaters, and, in nature, they can live up to 2 years, depending on conditions. The natural color of the shrimp is green brown, but there is a wide range of colors. Neocardina heteropoda prefer clean water with a pH of 6.5–8 and a temperature of 14–29 °C (56–84 °F). These shrimps are also classified as Neocardina denticulata sinensis, also currently known as Neocardina davidi [36]. Juvenile freshwater shrimps Neocardinia heteropoda (240 individuals; mean length 8.8 mm; range 6.8–11.9 mm) were obtained from the local supplier (Krevetky.cz, Prague, Czech Republic) in two equal batches that were treated one after another. Each batch included 120 randomized and equally distributed individuals in 6 glass tanks (i.e., 20 individuals per tank—footprint 30 × 15 cm, depth 20 cm). Shrimps were kept in tanks for 14 days prior to the start of the trial to habituate them to experimental conditions. The water temperature was controlled automatically using external air conditioning and held at an average of 20 °C. The light was controlled under a 12-h day/12-h night regime. The shrimps were fed ad libitum with commercial flakes (Tetra Werke, Melle, Germany) once a day, and two-thirds of the water volume was renewed with treated dechlorinated municipal tap water every third day to habituate the shrimps in the experimental conditions. No shrimp mortality during the experiment was recorded.
2.2. Experimental Design
Three tanks were randomly assigned as “Exposed” and three as “Control” at the beginning of the experiment. Polystyrene particles (Sigma Alldrich, Saint-Louis, MO, USA) (size 0.2–0.5 mm) were distributed in the concentration of 8 mg L−1 in exposed tanks with dechlorinated tap water (the temperature of the water was 20 °C). Dechlorinated tap water was also used in a similar study by [25]. Treated tap drinking water in the Czech Republic is expected to be polystyrene MP free, despite the fact that it may contain traces of other MP particles like PET, PP or PE [37]. A heterogeneous mixture of water/microplastic was made, and the shrimps were exposed to the contaminated water. We estimated the concentration of 8 mg L−1 as the environmentally relevant concentration for heavily polluted freshwater habitats based on the mass of plastic particles [38] and the particle concentration reported for Three Gorges Reservoir [39]. Rearing (temperature, photoperiod, feeding and water renewal) was conducted in the same manner as during the acclimatization. Behavioral assay and respirometry were conducted using 10 exposed and 10 control shrimps (3 or 4 individuals per tank), randomly selected, during the 7th and 14th day, resulting in the use of 40 individuals during 1 completed trial. All individuals were subjected to behavioral assay first and then to respirometry (see the detailed description below). After the first trial was finished, the second trial was conducted in the same manner, resulting in the overall use of 40 control and 40 exposed individuals. No significant size differences were detected between the two groups (p = 0.76, n = 80).
2.3. Behavioural Assay
Every specimen was inserted in the circular Petri dish (90 × 25 mm) separately and after one minute of habituation had its behavior recorded for 5 min using a GoPro HERO 4 digital camera (GoPro, Inc.; San Mateo, CA, USA) placed above the arena. Four individuals placed in four experimental arenas were treated simultaneously. After behavioral observation, the shrimps were individually placed in labeled bottles to enable the individual to be tracked before the metabolic rates assay.
2.4. Metabolic Rates
The metabolic rates of individual shrimps were estimated indirectly by measuring oxygen consumption rates using a microplate respirometry system with 24 wells (1600 µL each; Loligo Systems, Viborg, Denmark). Measurements were performed in an air-conditioned darkened chamber. All wells, fitted with individual oxygen sensor spots, were filled with aged, filtered tap water treated with UV light to minimize microbial respiration. The metabolic rates of twenty individual shrimps (ten control and ten exposed) were measured at the same time, leaving four wells empty for the control of potential microbial respiration. Measurements lasted for 120 min and were automatically processed using AutoResp1TM software (Loligo Systems, Viborg, Denmark). Observations made immediately before and after the measurements showed that the shrimp remained mostly motionless during the experiments, so the measured oxygen consumption rates were used as a proxy for the standard metabolic rate (SMR) of Neocardina heteropoda. Shrimps were dried for 48 h at 65 °C and weighed (mean 3.18 g; range 1.19–5.92 g) after the metabolic rates assay.
2.5. Presence of Polystyrene in Shrimps
To verify that polystyrene particles were present in the exposed shrimps, we performed a complementary microscopic assay. Confocal imaging was performed using the FV10-ASW 4.2 software. Images were acquired using a 20× objective in a channel scan mode with one active channel FITC. Settings for the laser power and the photomultiplier tube detector, including high voltage (HV), gain and offset, were set according to the manufacturer’s instructions. Optical zoom, pixels per frame and pixel scan speed were set to match with the Nyquist theorem. Scanning was performed in unidirectional mode using Kalman filtering (average of 4 frames) to augment signal-to-noise. The same confocal settings were maintained for all specimens.
2.6. Data Analysis
Behavioral assays were analyzed using the video tracking software LoliTrack (Loligo Systems, Viborg, Denmark). Every individual shrimp was assigned its average ‘acceleration’, ‘speed’ and overall ‘distance’ moved.
Metabolic rates data were analyzed using the AutoResp software (Loligo Systems, Viborg, Denmark). The oxygen consumption rate (MO2) was derived from the decrease in the chamber oxygen partial pressure (pO2) during the measuring period. All MO2 measurements were corrected for the microbial respiration recorded in empty wells. SMR estimated from the slope of oxygen decrease was corrected for individual dry mass and expressed in mg O2 g−1 h−1.
Several class variables were defined for arrangement the data. The sign ‘ID’ identified individual shrimps; ‘treatment’ distinguished between shrimps ‘exposed’ to microplastics and ‘control’; ‘date’ acquired four values to differentiate among particular test days, and ‘time sequence’ was used to define the progression of the effects from the ‘7th day’ to the ‘14th day’ of exposition.
2.7. Statistical Analysis
Statistical analyses were performed using the SAS software package (SAS Institute Inc., version 9.4, www.sas.com (accessed on 22 November 2021), and a linear mixed model (LMM) with random factors (PROC MIXED) was proposed. The data were transformed for normality prior to the LMM analyses when necessary. The random factors were used to account for the effect of the individual shrimp ‘ID’ and the ‘day’ of the experiment. For every single dependent variable, i.e., ‘acceleration’, ‘speed’, ‘distance’ and ‘SMR’, a separate model was fitted, using ‘treatment’ and the interaction of ‘treatment’ and ‘time sequence’ as explanatory variables. Separate models were also fitted in order to determine the significance of relationships (i.e., behavioral syndromes) among particular behavioral and physiological traits for particular treatments. In other words, one model was fitted for the relationship between the ‘SMR’ and the ‘acceleration’ of the exposed shrimps, the second model was fitted for the relationship between the ‘SMR’ and the ‘acceleration’ of the control shrimps, etc. The significance of explanatory variables was assessed using F-tests. Least-squares means (LSM), henceforth referred to as ‘adjusted means’, were computed for each significant class exploratory variable. The differences between classes were tested with t-tests, and a Tukey–Kramer adjustment was used for multiple comparisons. Associations between the dependent variables and other continuous variables were estimated by fitting a random coefficient model using PROC MIXED, as described by Tao et al. [40]. With this random coefficient model, we calculated the predicted values for the dependent variables and plotted them against the continuous variables by using the predicted regression lines. The degrees of freedom were calculated using the Kenward–Roger method [41].
3. Results
3.1. Overall Effects
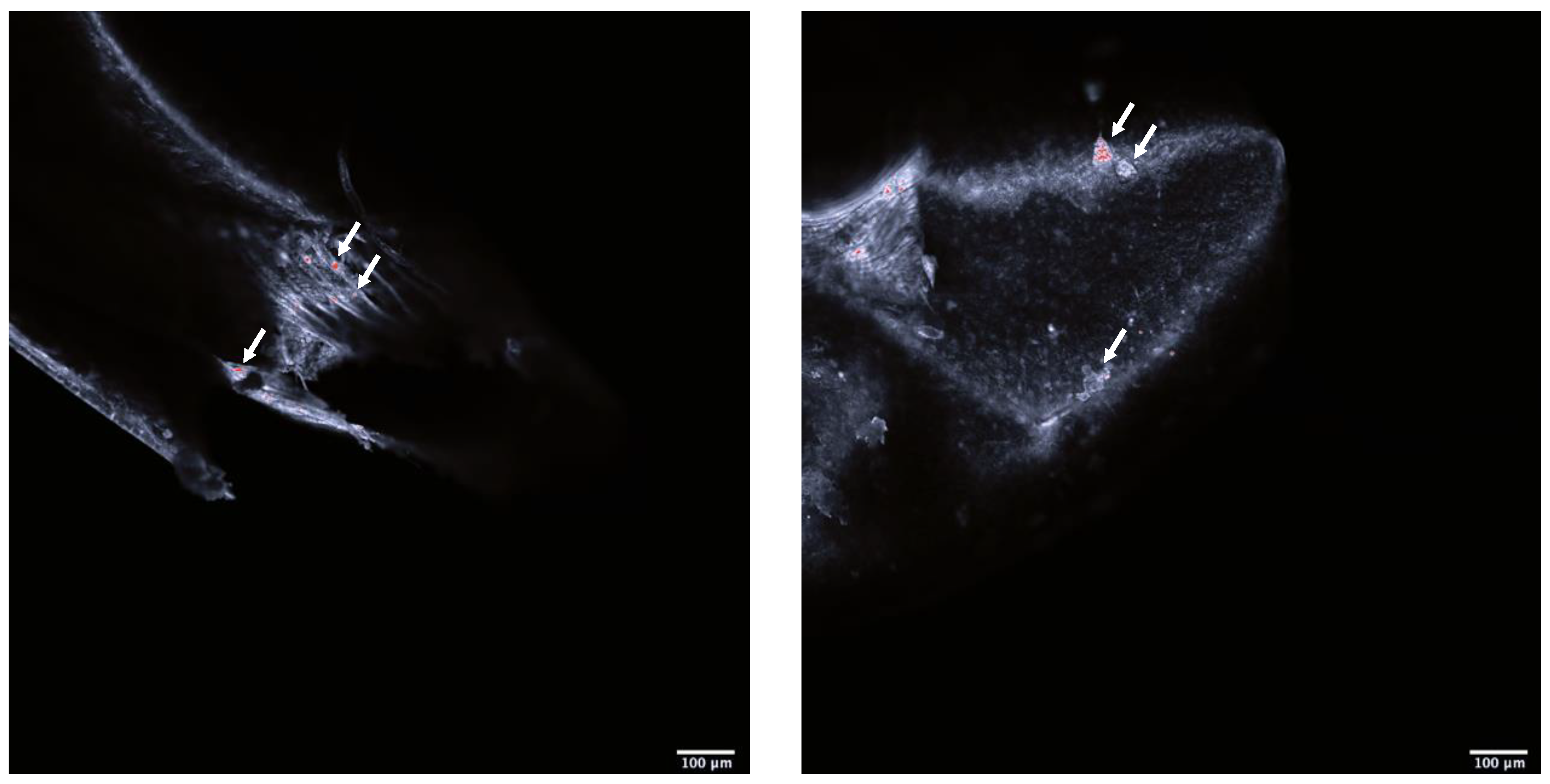
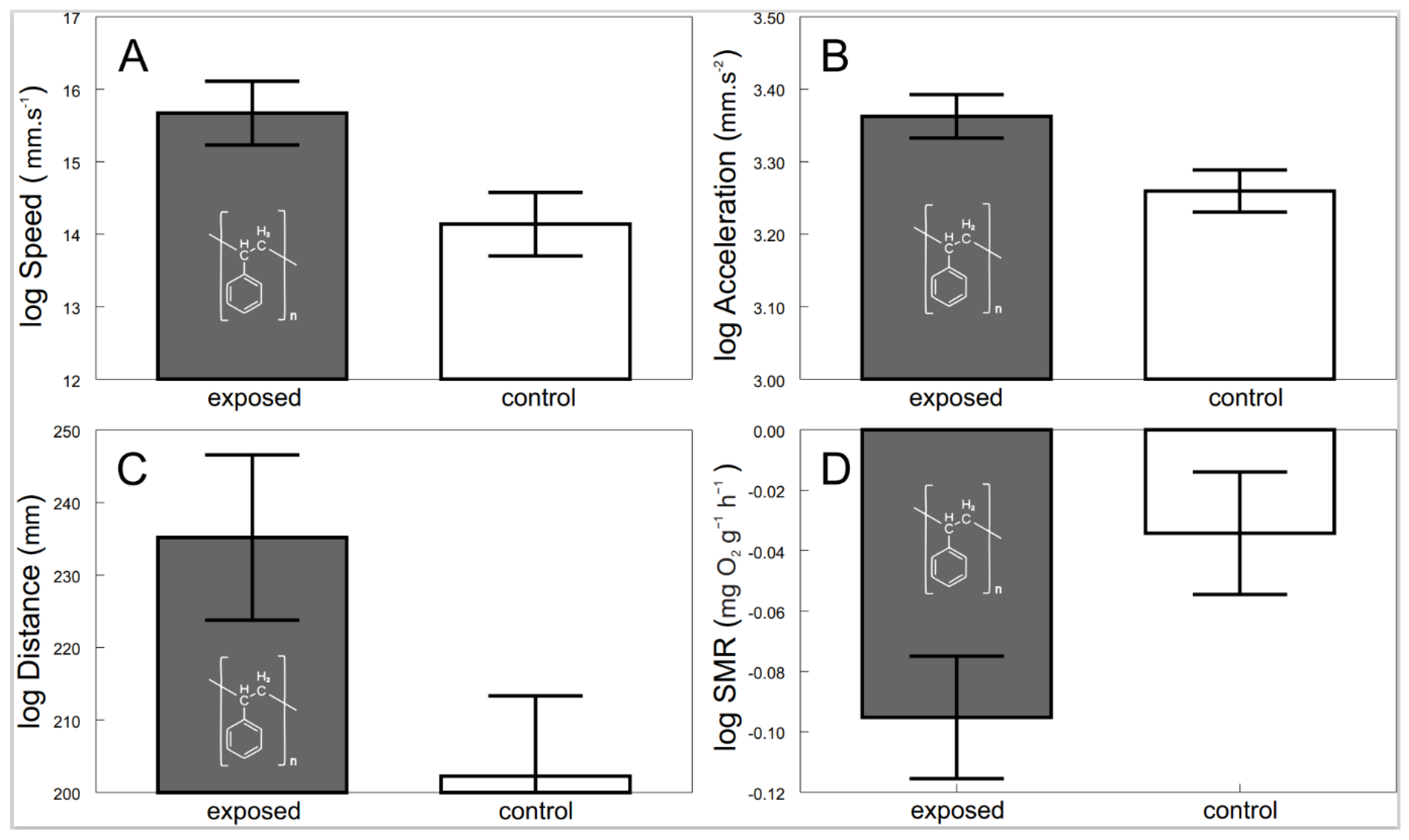
Confocal imaging indicated that the exposed shrimps contained polystyrene particles (Figure 1). There were significant behavioral as well as physiological differences between the shrimps exposed to microplastics and the control. The exposed shrimps generally moved with greater speed (F1, 54.2 = 6.21, p < 0.0158; Figure 2A) and acceleration (F1, 49.1 = 6.06, p < 0.0173; Figure 2B) over larger distances (F1, 31.4 = 4.29, p < 0.0467; Figure 2C). Furthermore, physiological screening indicated that the exposed shrimps had a lowered standard metabolic rate when compared to the control (F1, 64.6 = 4.52, p < 0.0373; Figure 2D).
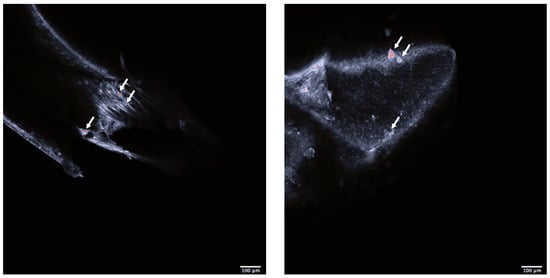
Figure 1.
Head (left) and body (right) of Neocardina heteropoda. White arrows are pointing to overexposed structures considered to be polystyrene nanoparticles. Scale bar 1000 μm. Red dots are overexposed structure of Neocardina heteropoda.
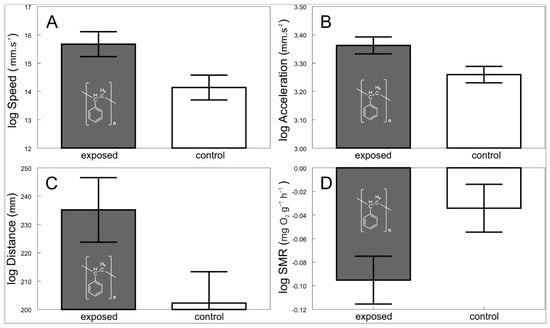
Figure 2.
Speed (A), acceleration (B), distance (C) and standard metabolic rate (D) of exposed (grey bars with polystyrene formula) and control (white bars) shrimps. Values are adjusted means (+/−S.E.).
3.2. Progression of the Effects
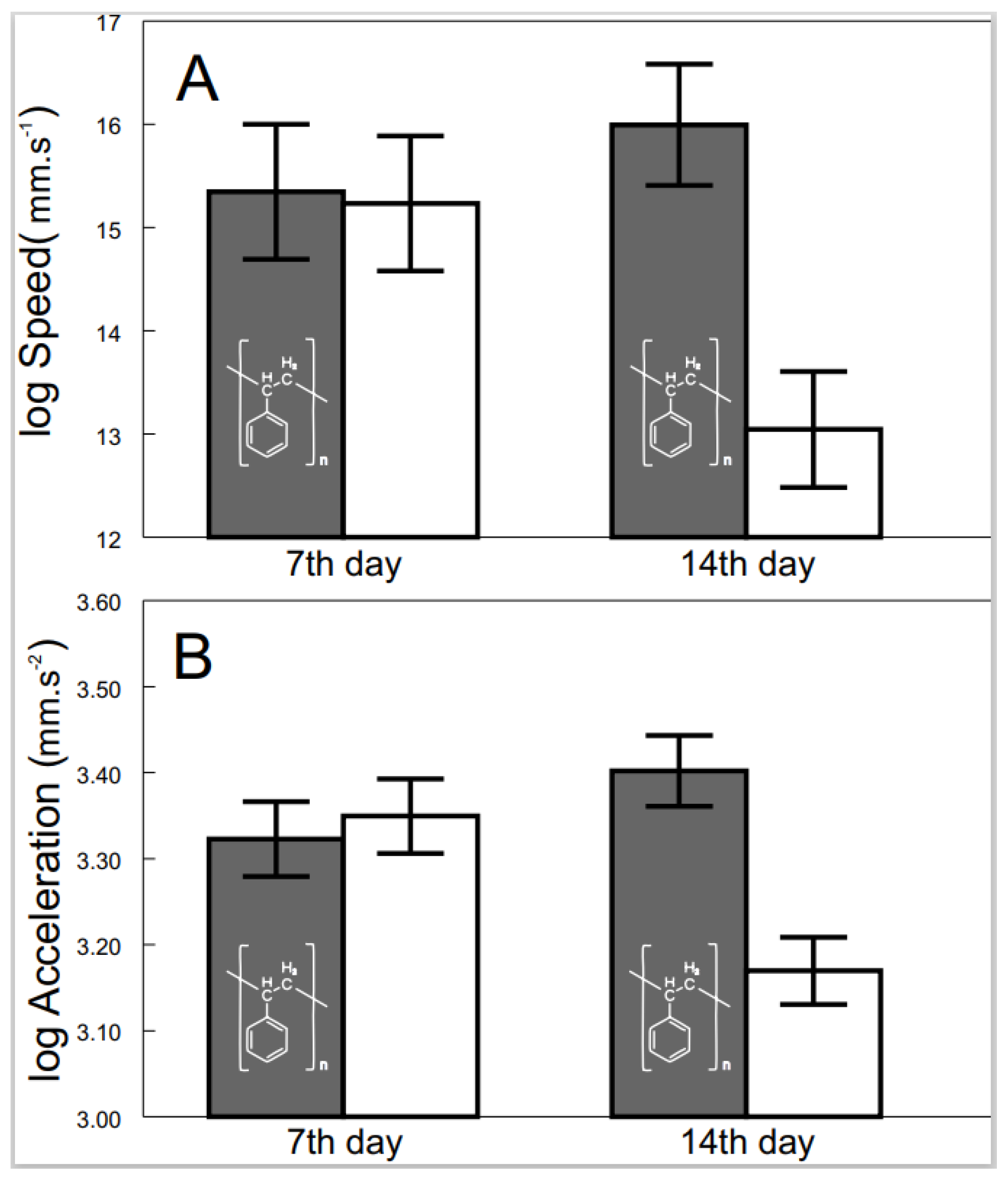
The effects of exposure to microplastics were observed after 7 and 14 days to delineate their progression. Progression from non-detectable effects after 7 days to significant differences after 14 days of exposition was detected for speed (F2, 54.1 = 3.50, p < 0.0371; Figure 3A) and acceleration (F2, 49.1 = 5.63, p < 0.0063; Figure 3B). In contrast, the progression of the effects on the distance and standard metabolic rate was not significant at all.
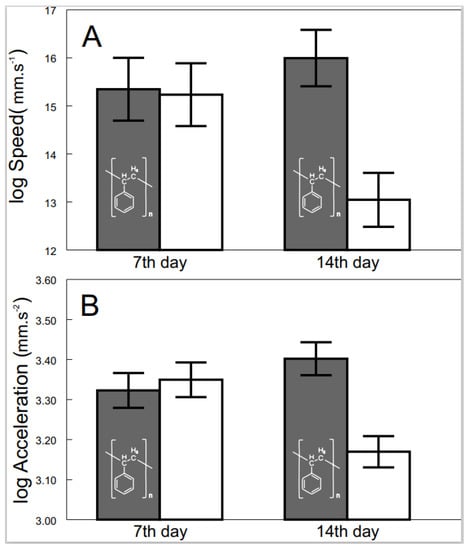
Figure 3.
Speed (A) and acceleration (B) of exposed (grey bars with polystyrene formula) and control (white bars) shrimp across time. Values are adjusted means (+/−S.E.).
3.3. Behavioural Syndromes
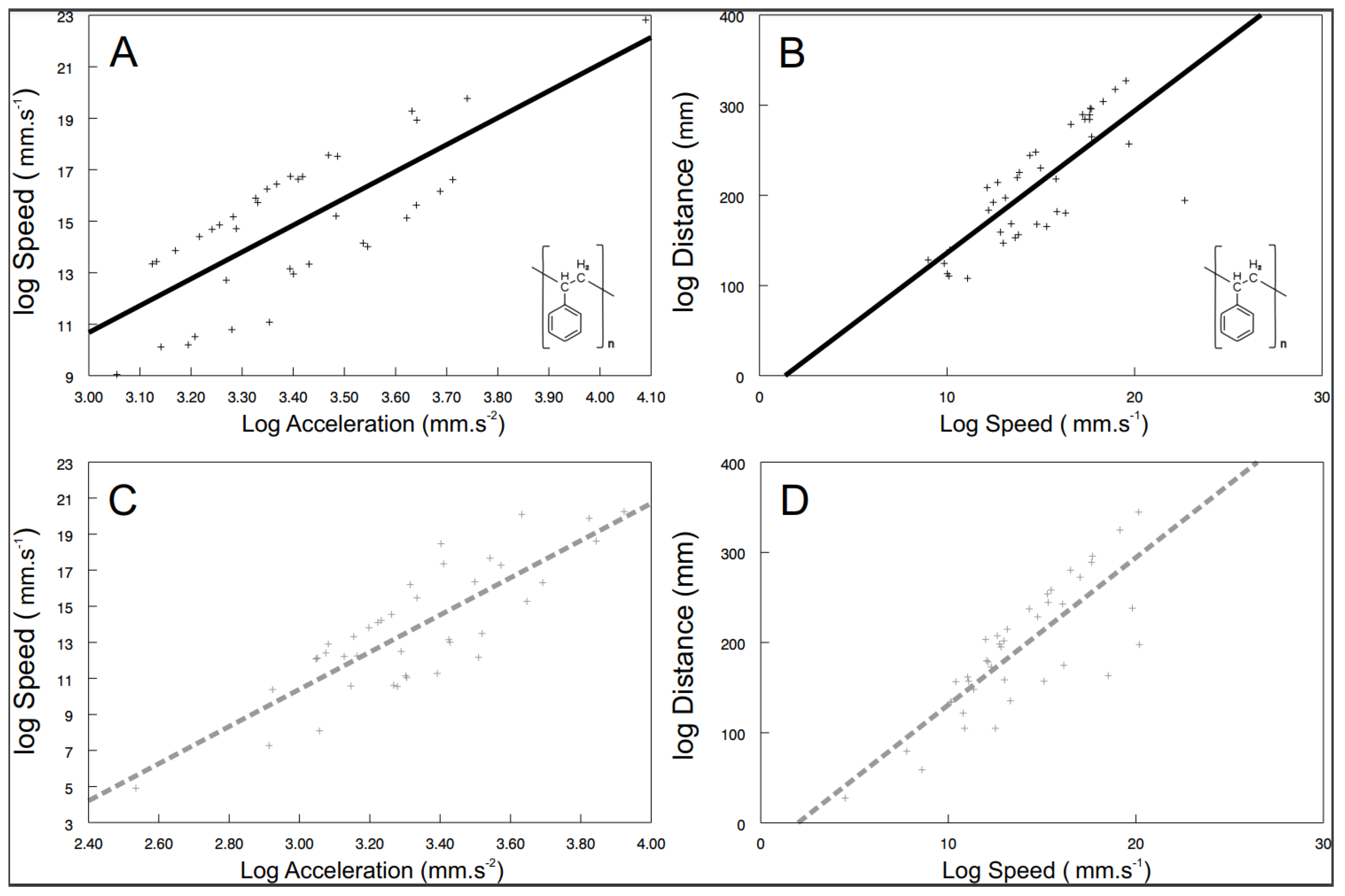
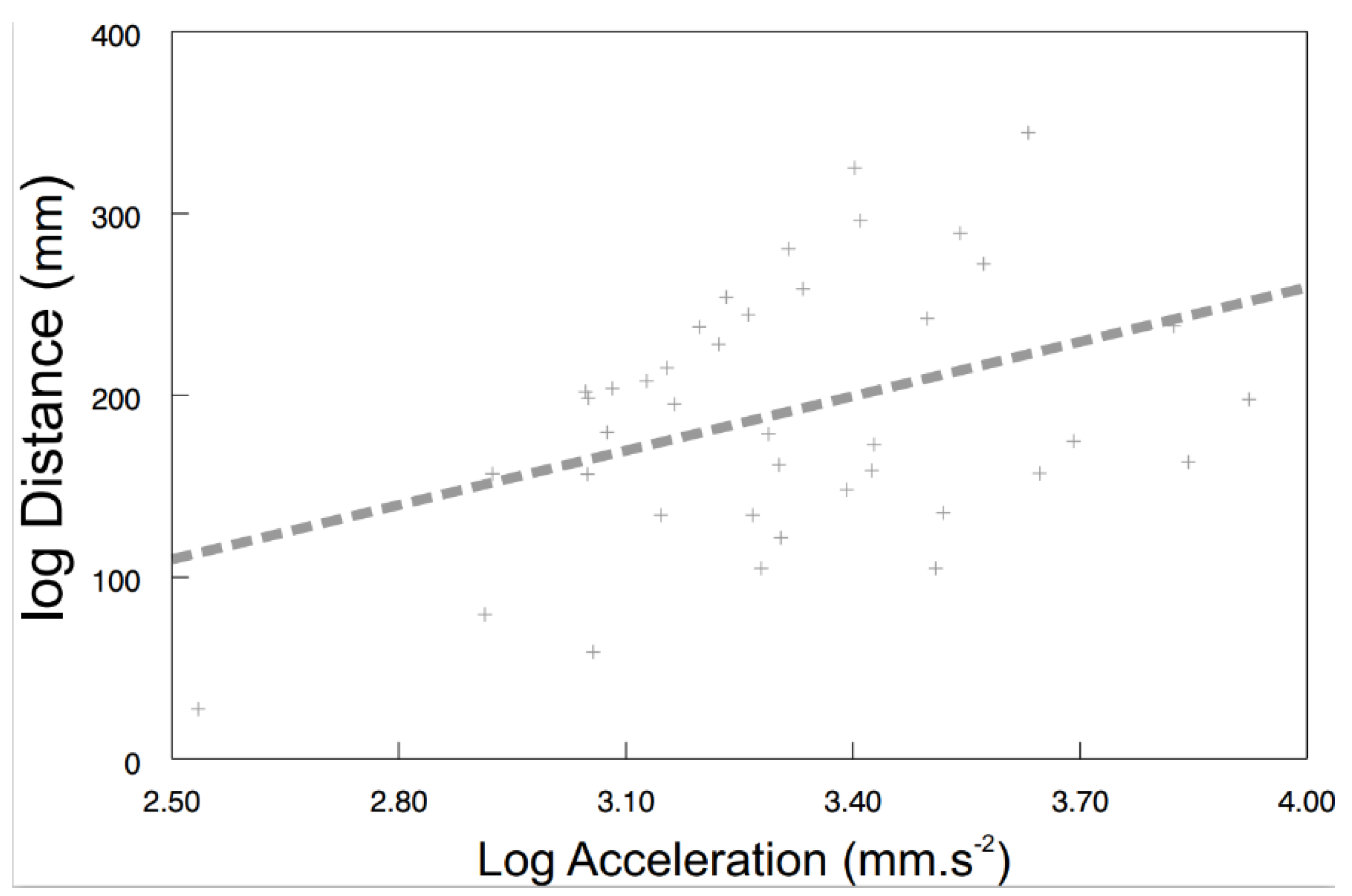
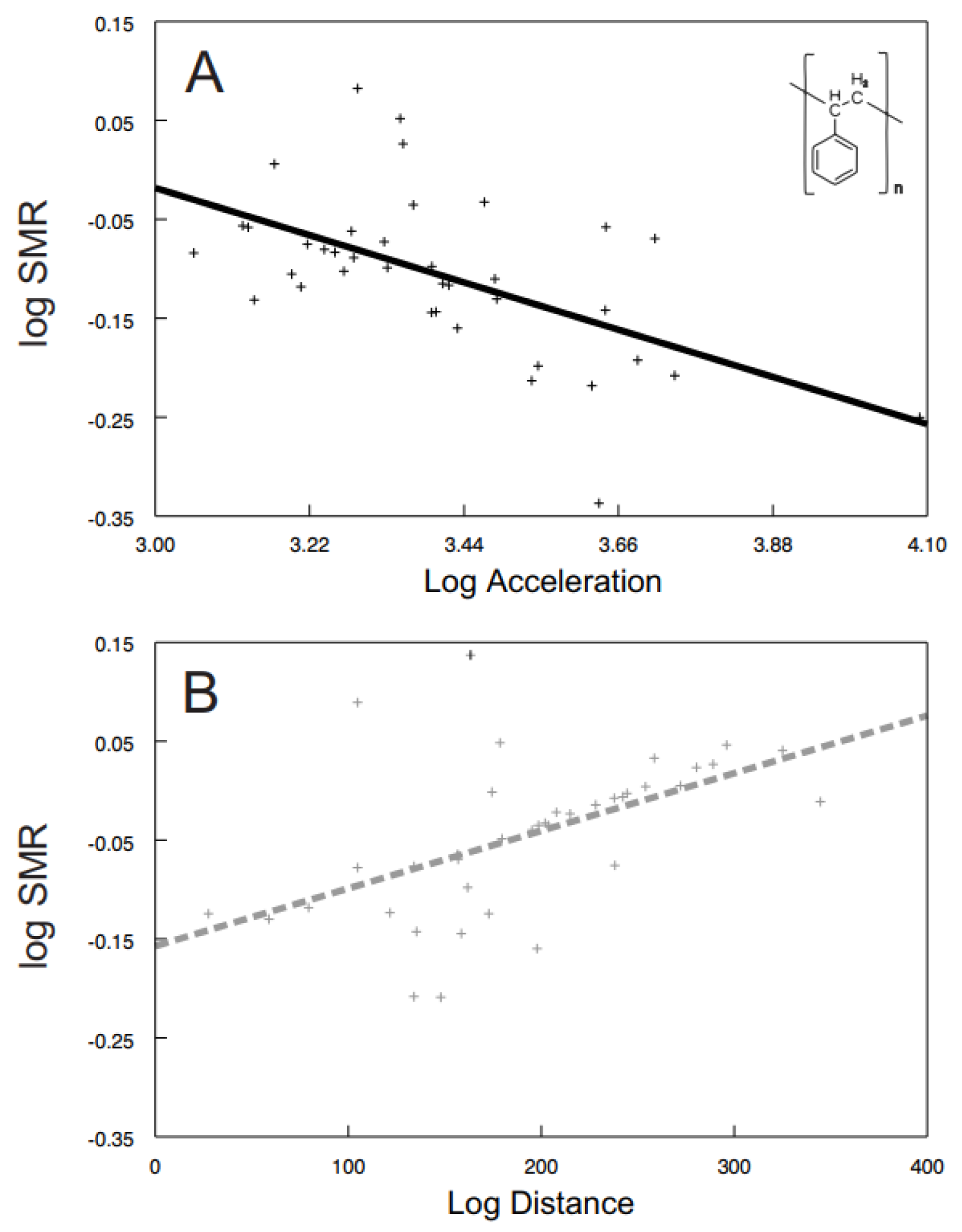
Some of the behavioral syndromes, like the relationship between speed and acceleration or distance and speed, were identical for the exposed (speed vs. acceleration: F1, 26.6 = 38.57, p < 0.0001; Figure 4A; distance vs. speed: F1, 24.9 = 185.24, p < 0.0001; Figure 4B) as well as the control (speed vs. acceleration: F1, 30 = 96.42, p < 0.0001; Figure 4C; distance vs. speed: F1, 20.9 = 507.04, p < 0.0001; Figure 4D) shrimps. Nevertheless, significant differences were also detected in behavioral syndromes, indicating that the microplastics influence not only the particular behavioral traits, but also their functional relationships. While the control shrimps showed a significant positive relationship between distance and acceleration (F1, 40 = 4.47, p < 0.0407; Figure 5), the exposed ones did not (F1, 9.26 = 0.72, p > 0.4173). Differences were also observed in the interconnection of physiological and behavioral variables. The standard metabolic rate of the exposed shrimps was significantly related to acceleration only (acceleration: F1, 28.3 = 4.76, p < 0.0377; Figure 6A; distance: F1, 34.6 = 1.60, p < 0.2146; speed: F1, 32.4 = 0.01, p < 0.9078), though the standard metabolic rate of the control shrimps showed a significant relationship exclusively with distance (distance: F1, 31.6 = 6.62, p < 0.015; Figure 6B; acceleration: F1, 29.4 = 0.26, p < 0.6159; speed: F1, 34.1 = 3.83, p < 0.0585).
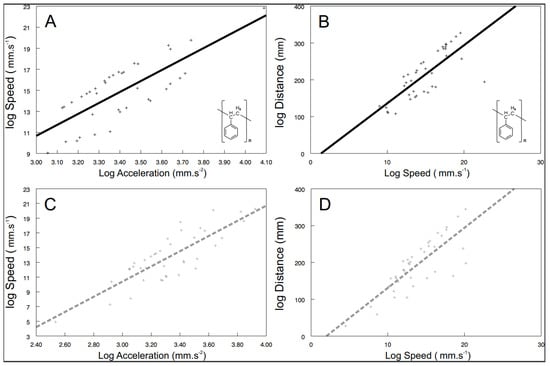
Figure 4.
The relationship between speed and acceleration as well as distance and speed of exposed ((A,B); black full line; polystyrene formula showed) and control ((C,D); grey dashed line)) shrimps. Predicted values are from particular LMM models. The curves were fitted by: (A) y = 10.4147x − 20.5596 (r2 = 0.58); (B) y = 15.7616x − 21.5961 (r2 = 0.60); (C) y = 10.3123x − 20.5471 (r2 = 0.68); (D) y=16.33x − 32.591 (r2 = 0.67).
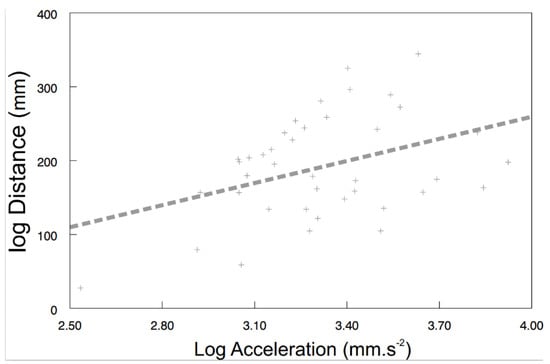
Figure 5.
The relationship between distance and acceleration of control shrimps. Predicted values are from a particular LMM model. The curve was fitted by: y = 99.6953x − 139.4662 (r2 = 0.16).
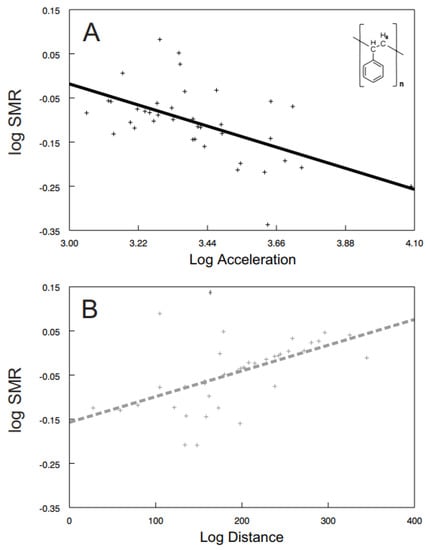
Figure 6.
The relationship between standard metabolic rate and (i) acceleration of exposed shrimps (A); black full line; polystyrene formula showed) and (ii) distance of control shrimps (B); grey dashed line). Predicted values are from particular LMM models. The curves were fitted by: (A) y = −0.2171x + 0.6331 (r2 = 0.32); (B) y = 0.0006x − 0.1571 (r2 = 0.28).
4. Discussion
Generally, polystyrene microparticles are expected to induce a decrease in activity measures in various aquatic organisms [21,33]. However, we found an increase in the swimming distance, speed and acceleration of exposed shrimps. As shown by Salerno et al. [16], the effect of microplastics on functional traits is species-specific, varying from positive to negative. Our study contributes to this suggestion of species-specific phenomena, confirming the need to study the effects of microplastics using various species to reveal the full structure of their potential impacts [17]. Different species face distinct microplastic encounter rates governed by foraging habitats [42] as well as physiological specificities [43]. Shrimps are generally vulnerable to microplastic pollution due to their scavenging mode of feeding and multipart intestine that retains microplastics for a long time, depending on the external conditions or the type of ingested food [31]. Body size is also an important factor in plastic ingestion, as it explains 42% of the variability in plastics consumed [44]. Jâms et al. [44] defined the maximum ingestible plastic-to-body size ratio as approximately 1:20, which roughly corresponds to the present study, with a mean 1:18 plastic-to-body-size ratio. Thus, shrimps in the present study were presumably able to ingest the whole polystyrene particles as well as their fragments, as a result of the shrimps’ scavenging mode of feeding [31]. However, exposure time and concentration issues also play an important role in detecting the adverse effects of microplastics on movement activity [21,45]. Swimming activity in marine crustaceans was generally altered after 48 h of exposure to polystyrene particle concentrations higher than 1 mg L−1 [45]. It is interesting to note that the effect changed from lowered (after 24 h of exposure) to accelerated (after 48 h of exposure) swimming speed in Artemia franciscana larvae [45]. In the present study, a progression of adverse effects on speed and acceleration was also observed. There was no effect on speed or acceleration after 7 days of exposure (i.e., 168 h), but the values of both variables were accelerated after 14 days of exposure (i.e., 336 h). Our results suggest that there is a need to conduct longer experiments to estimate the effects of microplastics during chronic exposure in the natural environment [16]. The other important point in this respect is to use the environmentally relevant concentration of microplastics. In the present study, we estimated the concentration of 8 mg L−1 to be roughly equal to the concentration reported in highly polluted freshwaters (Three Gorges Reservoir, China) [39]. Thus, all the adverse effects reported here can be suggested to occur in polluted natural ecosystems.
Microplastics are known to alter the physiology of aquatic organisms in various ways, from direct physical effects, like blocking the gastrointestinal tract, through the leaching of harmful chemicals, like plasticizers, from themselves to the desorption of harmful pollutants, like heavy metals, for which microplastics act as vectors [46]. The most common physiological alterations related to microplastics exposure include various histological damage, altered immune and protein activity, changes to blood biochemistry and interference with the endocrine and central nervous system [47,48,49,50,51]. Relatively wide attention is paid to the analyses of oxidative stress, which has been documented as a consequence of microplastic exposure in a wide range of aquatic organisms, from diatoms [52] through crustaceans to fishes [45]. Oxidative stress can be defined as a disturbance in the balance between the production of reactive oxygen species (free radicals) and antioxidant defenses which may result in damage in a variety of tissues [53]. Oxidative stress is related to the development of various diseases and plays a significant role in the development of metabolic disorders [52]. We found that shrimps exposed to microplastics had an altered standard metabolic rate in the present study, which is comparable to the increase of oxidative stress in brine shrimp larvae after 14 days of exposure to polystyrene microparticles [34]. Therefore, it seems plausible that there is a link between oxidative stress and altered metabolism in shrimps exposed to polystyrene microplastics, which may result in a range of adverse effects. Polystyrene chains have an affinity to lipids and affect the biological membranes [54], which may be how they alter shrimp metabolism. Accordingly, [26] documented the adverse effects of polystyrene microplastics on the lipid metabolism of another crustacean, the red claw crayfish (Cherax quadricarinatus). Altered metabolism was also observed in fish exposed to polystyrene microplastics not only through waterborne concentrations [25], but even through the food chain [33], suggesting that the adverse effects observed in shrimps can be expressed in apex predators after trophic transfer [55]. The aquaculture industry may also have an impact, especially when organisms live mainly in plastic containers, or most of the plastic products present are used in the aquaculture industry [56]. Animal personality traits are considered important contributors to the performance and fitness of individuals [57], as well as whole populations [58]. Therefore, extensive attention is paid to them [59]. Personality traits can be interconnected in various ways, creating mutual relationships called behavioral syndromes [18] that can be context specific [58]. Our data demonstrate that microplastic pollution alters some of a trait’s functional relationships. In other words, polystyrene microparticles modified the expression of a behavioral syndrome in shrimp personality traits. Individual stress is one of the most significant factors affecting the manifestation of behavioral traits [60], suggesting that the observed changes can be related to the oxidative stress discussed above. An analogous influence was found in another freshwater pollutant, analgesic tramadol, that modified the expression of behavioral syndromes in the European chub (Squalius cephalus); [61], suggesting that the pollution could influence these meaningful, evolutionary important relationships [62,63] in general.
To conclude, our study shows freshwater crustaceans’ altered behavioral and physiological responses to polystyrene microparticles, documenting that such pollution influences particular traits and their functional relationships. As shown in the example of another group of micropollutants (i.e., pharmaceuticals), such changes may disrupt ecological interactions (e.g., predator–prey interactions), with implications for ecosystem processes, population and community dynamics [64]. Almost 40 % of plastic production is represented by packaging destined for short-term use (less than one year) [5], and polystyrene plays a significant role in this respect [65,66,67]. The problem has been accelerated further due to the COVID-19-related increase in takeaway food consumption, which creates over 80 % of the plastic litter in the oceans [68]. Thus, there is a need to trigger public concern over this issue, as every member of society can help improve the current situation by changing their feeding habits.
5. Conclusions
The impact of microplastics on freshwaters, which are less studied than marine ecosystems, was evaluated. Our study focused on the effect of polystyrene microparticles (size 0.2–0.5 mm) on the freshwater shrimps Neocardina heteropoda. This study points to the impact of polystyrene microparticles on metabolic rates after 7 and 14 days of exposition in this freshwater ecosystem. The exposed shrimp Neocardina heteropoda exhibited modified activity patterns, accompanied by lowered a standard metabolic rate (SMR). This study points out that microplastics influence not only the traits but also their functional relationships, expressed as behavioral syndromes.
Author Contributions
Conceptualization, N.P.B., J.K. and P.H.; methodology, O.S. and T.M.; software, K.D. and J.F.E.C.; validation, J.R., M.T. and J.K.; formal analysis, N.P.B., P.H., T.M. and J.R.; investigation, O.S. and T.M.; resources, N.P.B. and J.K.; data curation, P.H., D.B. and N.P.B.; writing—original draft preparation, N.P.B.; writing—review and editing, N.P.B., P.H., J.K., J.R. and T.M; visualization, M.T.; supervision, O.S.; project administration; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the European Regional Development Fund–Project “Centre for the investigation of synthesis and transformation of nutritional substances in the food chain in interaction with potentially harmful substances of anthropogenic origin: comprehensive assessment of soil contamination risks for the quality of agricultural products” (No. CZ.02.1.01/0.0/0.0/16_019/0000845) and APVV19-0250.
Institutional Review Board Statement
Design of the experiment was in accordance with the valid legislation of the Czech Republic (law no. 246/1992, §19, art. 1, letter c) and was supervised by the Departmental Expert Committee for protection of animals against cruelty. As there is no obligation to have an approval for scientific work with invertebrates, this work did not receive a specific certificate.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Orb. Invisible-the Particles Inside Us; Tyree, C., Morrison, D., Eds.; 2017; Available online: https://orbmedia.org/the-invisibles (accessed on 17 July 2022).
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, W.; Chen, X.; He, Y.; Zhang, X.; Gong, H. A preliminary study of the association between colonization of microorganism on microplastics and intestinal microbiota in shrimp under natural conditions. J. Hazard Mater. 2021, 408, 124882. [Google Scholar] [CrossRef]
- Farrelly, T.; Shaw, I. Polystyrene as Hazardous Household Waste. In Household Hazardous Waste Management; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, occurrence, and degradation of microplastics in the aqueous environment. In Freshwater Microplastics; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Raza, A. Microplastics in Freshwater Systems: A Review on Its Accumulation and Effects on Fishes. Int. J. Res. Anal. Rev. -IJRAR 2018, 5. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef]
- Kwon, B.G.; Saido, K.; Koizumi, K.; Sato, H.; Ogawa, N.; Chung, S.Y.; Kusui, T.; Kodera, Y.; Kogure, K. Regional distribution of styrene analogues generated from polystyrene degradation along the coastlines of the North-East Pacific Ocean and Hawaii. Environ. Pollut. 2014, 188, 45–49. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, H.J.; Bertram, M.G.; Brodin, T.; Dang, Z.C.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The role of behavioral ecotoxicology in environmental protection. Environ. Sci. Technol. 2021, 55, 5620–5628. [Google Scholar] [CrossRef]
- Peterson, E.K.; Buchwalter, D.B.; Kerby, J.L.; LeFauve, M.K.; Varian-Ramos, C.W.; Swaddle, J.P. Integrative behavioral ecotoxicology: Bringing together fields to establish new insight to behavioural ecology, toxicology, and conservation. Curr. Zool. 2017, 63, 185–194. [Google Scholar] [CrossRef]
- Parrino, V.; Costa, G.; Giannetto, A.; De Marco, G.; Cammilleri, G.; Acar, Ü.; Piccione, G.; Fazio, F. Trace elements (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in Mytilus galloprovincialis and Tapes decussatus from Faro and Ganzirri Lakes (Sicily, Italy): Flow cytometry applied for hemocytes analysis. J. Trace Elem. Med. Biol. 2021, 68, 126870. [Google Scholar] [CrossRef]
- Salerno, M.; Berlino, M.; Mangano, M.C.; Sara, G. Microplastics and the functional traits of fishes: A global meta-analysis. Glob. Change Biol. 2021, 27, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Andreou, D.; Green, I.D.; Britton, J.R. Microplastics in freshwater fishes: Occurrence, impacts and future perspectives. Fish Fish. 2021, 22, 467–488. [Google Scholar] [CrossRef]
- Saborowski, R.; Korez, Š.; Riesbeck, S.; Weidung, M.; Bickmeyer, U.; Gutow, L. Shrimp and microplastics: A case study with the Atlantic ditch shrimp Palaemon varians. Ecotoxicol. Environ. Saf. 2022, 234, 113394. [Google Scholar] [CrossRef]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish. Immunol. 2019, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Castañeda, G.; Ruiz-Fernández, A.C.; Frías-Espericueta, M.G.; Rivera-Hernández, J.R.; Green-Ruiz, C.R.; Páez-Osuna, F. Microplastics in the tissues of commercial semi-intensive shrimp pond-farmed Litopenaeus vannamei from the Gulf of California ecoregion. Chemosphere 2022, 297, 13419. [Google Scholar] [CrossRef]
- Biro, P.A.; Stamps, J.A. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 2008, 23, 361–368. [Google Scholar] [CrossRef]
- Herrera, M.; Castanheira, M.F.; Conceição, L.E.C.; Martins, C.I. Linking risk taking and the behavioral and metabolic responses to confinementstress in gilthead seabream Sparus aurata. Appl. Anim. Behav. Sci. 2014, 155, 101–108. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Y.; Liu, S.; Zhang, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless? J. Hazard. Mater. 2020, 396, 122693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.L.; Weinersmith, K.L.; Brodin, T.; Saltz, J.B.; Sih, A. Behavioural syndromes in fishes: A review with implications for ecology and fisheries management. J. Fish Biol. 2011, 78, 395–435. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.J.; Feeney, W.E.; Marshall, H.H.; Cowlishaw, G.; Heinsohn, R. Animal personality: What are behavioural ecologists measuring? Biol. Rev. 2013, 88, 465–475. [Google Scholar] [CrossRef]
- FAO. Current Bibliography for Aquatic Science and Fisheries; Taylor & Francis Ltd.: London, UK, 1961. [Google Scholar]
- Li, R.; Weng, J.; Ren, L.; Wang, X.; Meng, Q.; Wang, L.; Sun, J. A novel microRNA and its PFK target control growth length in the freshwater shrimp (Neocaridina heteropoda). J. Exp. Biol. 2020, 223, jeb.223529. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala, India. Sci. Total Environ. 2020, 737, 139839. [Google Scholar] [CrossRef]
- Eom, H.-J.; Nam, S.-E.; Rhee, J.-S. Polystyrene microplastics induce mortality through acute cell stress and inhibition of cholinergic activity in a brine shrimp. Mol. Cell. Toxicol. 2020, 16, 233–243. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.-A.; Linse, S.; Malmendal, A.; Cedervall, A.T. Altered Behavior, Physiology, and Metabolism in Fish Exposed to Polystyrene Nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Suman, T.Y.; Jia, P.-P.; Li, W.-G.; Junaid, M.; Xin, G.-Y.; Wang, Y.; Pei, D.-S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef]
- Han, C.-C.; Hsu, K.-C.; Fang, L.-S.; Cheng, I.-M.; Lin, H.-D. Geographical and temporal origins of Neocaridina species (Decapoda: Caridea: Atyidae) in Taiwan. BMC Genet 2019, 20, 86. [Google Scholar] [CrossRef]
- Tropea, C.; Stumpf, L.; Greco, L.S.L. Effect of Temperature on Biochemical Composition, Growth and Reproduction of the Ornamental Red Cherry Shrimp Neocaridina heteropoda (Decapoda, Caridea). PLoS ONE. 2015, 10, e0119468. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.; Reisser, J.; Slat, B.; Ferrari, F.F.; Schmid, M.S.; Cunsolo, S.; Brambini, R.; Noble, K.; Sirks, L.-A.; Linders, T.E.W.; et al. The effect of particle properties on the depth profile of buoyant plastics in the ocean. Sci. Rep. 2016, 6, 33882. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef]
- Tao, J.; Tamis, R.; Fink, K.; Williams, B.; Nelson-White, T.; Craig, R. The neglected morula/compact stage embryo transfer. Hum. Reprod. 2002, 17, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Kenward, M.G.; Roger, J.H. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Bondelind, M.; Sokolova, E.; Nguyen, A.; Karlsson, D.; Karlsson, A.; Björklund, K. Hydrodynamic modelling of traffic-related microplastics discharged with stormwater into the Göta River in Sweden. Environ. Sci. Pollut. Res. 2020, 27, 24218–24230. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Eppe, G.; Roos, L.; Compère, P.; Das, K.; Parmentier, E. Morphology of the filtration apparatus of three planktivorous fishes and relation with ingested anthropogenic particles. Mar. Pollut. Bull. 2017, 116, 182–191. [Google Scholar] [CrossRef]
- Jâms, I.B.; Windsor, F.M.; Poudevigne-Durance, T.; Ormerod, S.J.; Durance, I. Estimating the size distribution of plastics ingested by animals. Nat. Commun. 2020, 11, 1594. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef]
- Strungaru, S.A.; Jijie, R.; Nicoara, M.; Plavan, G.; Faggio, C. Micro-(nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. TrAC–Trends Anal. Chem. 2019, 110, 116–128. [Google Scholar] [CrossRef]
- Rochman, C.M.; TKurobe IFlores, S.J. The Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Romano, N.; Ashikin, M.; Teh, J.C.; Syukri, F.; Karami, A. Effects of pristine polyvinyl chloride fragments on whole body histology and protease activity in silver barb Barbodes gonionotus fry. Environ. Pollut. 2018, 237, 1106–1111. [Google Scholar] [CrossRef]
- Athey, S.N.; Albotra, S.D.; Gordon, C.A.; Monteleone, B.; Seaton, P.; Andrady, A.L.; Taylor, A.R.; Brander, S.M. Trophic transfer of microplastics in an estuarine food chain and the effects of a sorbed legacy pollutant. Limnol. Oceanogr. Lett. 2020, 5, 154–162. [Google Scholar] [CrossRef]
- Roda, J.F.B.; Lauer, M.M.; Risso, W.E.; dos Reis, B.; Martinez, C. Microplastics and copper effects on the neotropical teleost Prochilodus lineatus: Is there any interaction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110659. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, R.; Qu, H.; Zuo, Y.; Yu, Z.; Hu, G.; Li, Z.; Chen, H.; Lin, B.; Wang, B.; et al. Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish. Chemosphere 2020, 248, 126067. [Google Scholar] [CrossRef]
- Lay, S.L.; Simard, G.; Martinez, M.C.; Andriantsitohaina, R. Oxidative Stress and Metabolic Pathologies:From an Adipocentric Point of View. Oxidative Med. Cell. Longev. 2014, 2014, 908539. [Google Scholar]
- Betteridge, D.J. What Is Oxidative Stress? Metabolism 2000, 49 (Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Rossi, G.; Bamoud, J.; Monticelli, L. Polystyrene nanoparticles perturb lipid membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef]
- Hurt, R.; O’Reilly, C.M.; Perry, W.L. Microplastic prevalence in two fish species in two U.S. reservoirs. Limnol. Oceanogr. Lett. 2020, 5, 147–153. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef] [PubMed]
- Ariyomo, T.O.; Watt, P.J. The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- Sih, A.; Chang, A.T.; Wey, T.W. Effects of behavioural type, social skill and the social environment on male mating success in water striders. Anim. Behav. 2014, 94, 9–17. [Google Scholar] [CrossRef]
- Roche, D.G.; Careau, V.; Binning, S.A. Demystifying animal ‘personality’(or not): Why individual variation matters to experimental biologists. J. Exp. Biol. 2016, 219, 3832–3843. [Google Scholar] [CrossRef]
- Moscicki, M.K.; Hurd, P.L. Sex, boldness and stress experience affect convict cichlid, Amatitlania nigrofasciata, open field behaviour. Anim. Behav. 2015, 107, 105–114. [Google Scholar] [CrossRef]
- Sancho-Santos, M.E.; Horký, P.; Grabicová, K.; Hubená, P.; Slavík, O.; Grabic, R.; Douda, K.; Randák, T. Traces of tramadol impact behaviour in a native european fish. Ecotoxicol. Environ. Saf. 2021, 212, 111999. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef]
- Wolf, M.; Weissing, F.J. Animal personalities: Consequences for ecology and evolution. Trends Ecol. Evol. 2012, 27, 452–461. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems-impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastic the Facts 2015-an Analysis of European Plastics Production, Demand and Waste Data. 2015. Available online: http://www.plasticseurope.org/Document/plastics—the-facts-2015.aspx?FolID¼2 (accessed on 17 July 2022).
- Morales-Caselles, C.; Viejo, J.; Martí, E.; González-Fernández, D.; Pragnell-Raasch, H.; González-Gordillo, J.I.; Montero, E.; Arroyo, G.M.; Hanke, G.; Salvo, V.S.; et al. An inshore–offshore sorting system revealed from global classification of ocean litter. Nat. Sustain. 2021, 4, 484–493. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).