Movement and Activity Patterns of Non-Native Wels Catfish (Silurus glanis Linnaeus, 1758) at the Confluence of a Large River and Its Colder Tributary
Abstract
:1. Introduction
2. Materials and Methods
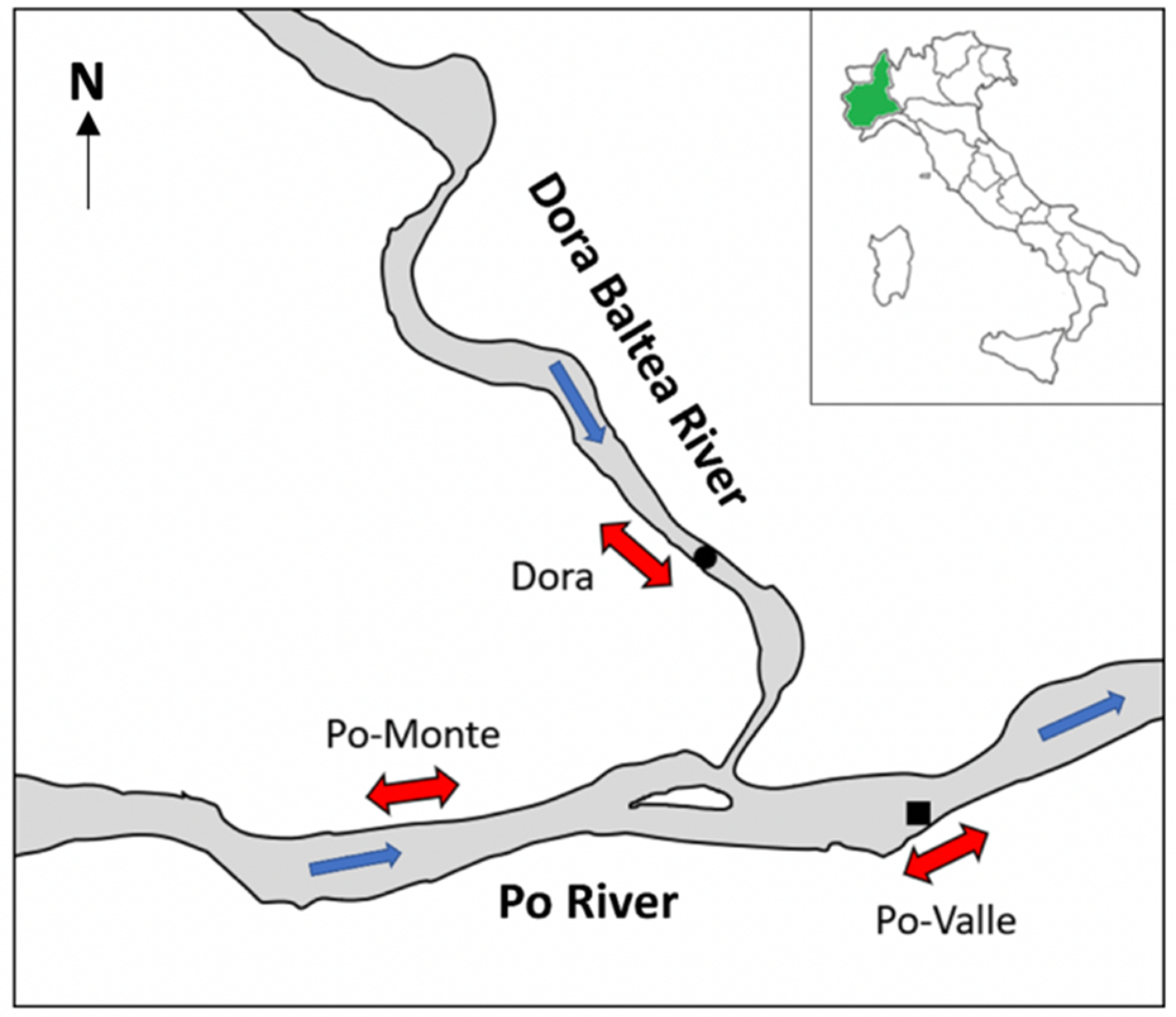
2.1. Study Area
2.2. Tagging Protocol
2.3. Data Interpretation
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cucherousset, J.; Olden, J.D. Ecological Impacts of Nonnative Freshwater Fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Gherardi, F.; Bertolino, S.; Bodon, M.; Casellato, S.; Cianfanelli, S.; Ferraguti, M.; Lori, E.; Mura, G.; Nocita, A.; Riccardi, N. Animal xenodiversity in Italian inland waters: Distribution, modes of arrival, and pathways. Biol. Invasions 2008, 10, 435–454. [Google Scholar] [CrossRef]
- Su, G.; Logez, M.; Xu, J.; Tao, S.; Villéger, S.; Brosse, S. Human impacts on global freshwater fish biodiversity. Science 2021, 371, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Boulêtreau, S.; Santoul, F. The end of the mythical giant catfish. Ecosphere 2016, 7, e01606. [Google Scholar] [CrossRef] [Green Version]
- Copp, G.H.; Robert Britton, J.; Cucherousset, J.; García-Berthou, E.; Kirk, R.; Peeler, E.; Stakėnas, S. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009, 10, 252–282. [Google Scholar] [CrossRef]
- Cucherousset, J.; Horky, P.; Slavík, O.; Ovidio, M.; Arlinghaus, R.; Boulêtreau, S.; Britton, R.; García-Berthou, E.; Santoul, F. Ecology, behaviour and management of the European catfish. Rev. Fish Biol. Fish. 2018, 28, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Cunico, A.M.; Vitule, J.R.S. First records of the European catfish, Silurus glanis Linnaeus, 1758 in the Americas (Brazil). BioInvasions Rec. 2014, 3, 117–122. [Google Scholar] [CrossRef]
- Vejřík, L.; Vejříková, I.; Blabolil, P.; Eloranta, A.P.; Kočvara, L.; Peterka, J.; Sajdlová, Z.; Chung, S.H.T.; Šmejkal, M.; Kiljunen, M.; et al. European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci. Rep. 2017, 7, 15970. [Google Scholar] [CrossRef] [Green Version]
- Wysujack, K.; Mehner, T. Can feeding of European catfish prevent cyprinids from reaching a size refuge? Ecol. Freshw. Fish 2005, 14, 87–95. [Google Scholar] [CrossRef]
- De Santis, V.; Volta, P. Spoiled for Choice during Cold Season? Habitat Use and Potential Impacts of the Invasive Silurus glanis L. In a Deep, Large, and Oligotrophic Lake (Lake Maggiore, North Italy). Water 2021, 13, 2549. [Google Scholar] [CrossRef]
- Cucherousset, J.; Boulêtreau, S.; Azémar, F.; Compin, A.; Guillaume, M.; Santoul, F. “Freshwater killer whales”: Beaching behavior of an alien fish to hunt land birds. PLoS ONE 2012, 7, e50840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulêtreau, S.; Fauvel, T.; Laventure, M.; Delacour, R.; Bouyssonnié, W.; Azémar, F.; Santoul, F. “The giants’ feast”: Predation of the large introduced European catfish on spawning migrating allis shads. Aquat. Ecol. 2021, 55, 75–83. [Google Scholar] [CrossRef]
- Boulêtreau, S.; Carry, L.; Meyer, E.; Filloux, D.; Menchi, O.; Mataix, V.; Santoul, F. High predation of native sea lamprey during spawning migration. Sci. Rep. 2020, 10, 6122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulêtreau, S.; Gaillagot, A.; Carry, L.; Tétard, S.; Oliveira, E.D.; Santoul, F. Adult Atlantic salmon have a new freshwater predator. PLoS ONE 2018, 13, e0196046. [Google Scholar] [CrossRef] [Green Version]
- Tamburello, N.; Côté, I. Movement ecology of Indo-Pacific lionfish on Caribbean coral reefs and its implications for invasion dynamics. Biol. Invasions 2015, 17, 1639–1653. [Google Scholar] [CrossRef]
- Brevé, N.W.P.; Verspui, R.; de Laak, G.A.J.; Bendall, B.; Breukelaar, A.W.; Spierts, I.L.Y. Explicit site fidelity of European catfish (Silurus glanis, L., 1758) to man-made habitat in the River Meuse, Netherlands. J. Appl. Ichthyol. 2014, 30, 472–478. [Google Scholar] [CrossRef]
- Carol, J.; Zamora, L.; García-Berthou, E. Preliminary telemetry data on the movement patterns and habitat use of European catfish (Silurus glanis) in a reservoir of the River Ebro, Spain. Ecol. Freshw. Fish 2007, 16, 450–456. [Google Scholar] [CrossRef]
- Daněk, T.; Horký, P.; Kalous, L.; Filinger, K.; Břicháček, V.; Slavík, O. Seasonal changes in diel activity of juvenile European catfish Silurus glanis (Linnaeus, 1758) in Byšická Lake, Central Bohemia. J. Appl. Ichthyol. 2016, 32, 1093–1098. [Google Scholar] [CrossRef]
- Slavík, O.; Horký, P.; Maciak, M.; Wackermannová, M. Familiarity, prior residency, resource availability and body mass as predictors of the movement activity of the European catfish. J. Ethol. 2016, 34, 23–30. [Google Scholar] [CrossRef]
- Boujard, T. Diel rhythms of feeding activity in the European catfish, Silurus glanis. Physiol. Behav. 1995, 58, 641–645. [Google Scholar] [CrossRef]
- Ferreira, M.A.M.F. European Catfish (Silurus glanis) Movements and Diet Ecology in a Newly Established Population in the Tagus drainage. Ph.D Thesis, Lisbon University, Lisbon, Portugal, 2019. [Google Scholar]
- Regione Piemonte. Piano Regionale per la Tutela E la Conservazione Degli Ambienti E Della Fauna Acquatica E L’Esercizio Della Pesca; Regione Piemonte: Torino, Italy, 2015; p. 97. [Google Scholar]
- Regione Piemonte. Rapporto Sullo Stato Dell’Ittiofauna in Piemonte; Regione Piemonte: Torino, Italy, 2021; p. 20. [Google Scholar]
- Comoglio, C.; Forneris, G.; Pascale, M.; Spairani, M.; Calles, O.; Barzan, M.; Balestrieri, A.; Vezza, P. Studio Sugli Spostamenti (Migrazioni) Delle Principali Specie Ittiche Del Bacino Della Bassa Dora Baltea; Regione Piemonte: Torino, Italy, 2012. [Google Scholar]
- Bianco, P.; Caputo, V.; Ferrito, V.; Lorenzoni, M.; Marzano, N.; Stefani, F.; Sabatini, A.; Tancioni, L. Pesci D’Acqua Dolce. In Lista Rossa IUCN dei Vertebrati Italiani; Rondinini, C., Battistoni, A., Peronace, V., Teofili, C., Eds.; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2013; p. 54. [Google Scholar]
- Autorità di Bacino del Fiume Po. Progetto Di Piano Stralcio per L’Assetto Idrogeologico (PAI); Autorità di Bacino del Fiume Po: Parma, Italy, 2001; p. 352. [Google Scholar]
- Regione Piemonte. Piano di Tutela delle Acque. Monografie Aree Idrografiche; Regione Piemonte: Torino, Italy, 2007; p. 752. [Google Scholar]
- Brown, R.S.; Cooke, S.J.; Anderson, W.G.; McKinley, R.S. Evidence to challenge the “2% rule” for biotelemetry. N. Am. J. Fish. Manag. 1999, 19, 867–871. [Google Scholar] [CrossRef]
- Winter, J. Underwater Biotelemetry. In Fisheries Techniques; American Fisheries Society: Bethesda, MD, USA, 1983; pp. 371–395. [Google Scholar]
- Pagès, J.F.; Bartumeus, F.; Hereu, B.; López-Sanz, À.; Romero, J.; Alcoverro, T. Evaluating a key herbivorous fish as a mobile link: A Brownian bridge approach. Mar. Ecol. Prog. Ser. 2013, 492, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Staveley, T.A.; Jacoby, D.M.; Perry, D.; van der Meijs, F.; Lagenfelt, I.; Cremle, M.; Gullström, M. Sea surface temperature dictates movement and habitat connectivity of Atlantic cod in a coastal fjord system. Ecol. Evol. 2019, 9, 9076–9086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownscombe, J.W.; Lédée, J.I.; Raby, G.D.; Struthers, D.P.; Gutowsky, L.F.G.; Nguyen, V.M.; Young, N.; Stokesbury, M.J.W.; Holbrook, C.M.; Brenden, T.O.; et al. Conducting and Interpreting Fish Telemetry Studies: Considerations for Researchers and Resource Managers. Rev. Fish Biol. Fish. 2019, 29, 369–400. [Google Scholar] [CrossRef]
- Kay, W.R. Movements and home ranges of radio-tracked Crocodylus porosus in the Cambridge Gulf region of Western Australia. Wildl. Res. 2004, 31, 495. [Google Scholar] [CrossRef] [Green Version]
- Nyqvist, D.; McCormick, S.D.; Greenberg, L.; Ardren, W.R.; Bergman, E.; Calles, O.; Castro-Santos, T. Downstream Migration and Multiple Dam Passage by Atlantic Salmon Smolts. N. Am. J. Fish. Manag. 2017, 37, 816–828. [Google Scholar] [CrossRef]
- Harbicht, A.B.; Castro-Santos, T.; Ardren, W.R.; Gorsky, D.; Fraser, D.J. Novel, Continuous Monitoring of Fine-Scale Movement Using Fixed-Position Radiotelemetry Arrays and Random Forest Location Fingerprinting. Methods Ecol. Evol. 2017, 8, 850–859. [Google Scholar] [CrossRef] [Green Version]
- Nyqvist, D.; Elghagen, J.; Heiss, M.; Calles, O. An Angled Rack with a Bypass and a Nature-Like Fishway Pass Atlantic Salmon Smolts Downstream at a Hydropower Dam. Mar. Freshw. Res. 2018, 69, 1894–1904. [Google Scholar] [CrossRef]
- Adams, N.S.; Beeman, J.W.; Eiler, J.H. Telemetry Techniques: A User Guide for Fisheries Research; American Fisheries Society: Bethesda, MD, USA, 2012. [Google Scholar]
- Sefick, S.A., Jr. Stream Metabolism—A Package for Calculating Single Station. 2009. Available online: http://www2.uaem.mx/r-mirror/web/packages/StreamMetabolism/StreamMetabolism.pdf (accessed on 21 September 2022).
- Pohlert, T. The pairwise multiple comparison of mean ranks package (PMCMR). R Package 2014, 27, 9. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sievert, C.; Parmer, C.; Hocking, T.; Chamberlain, S.; Ram, K.; Corvellec, M.; Despouy, P. Plotly: Create Interactive Web Graphics via plotly, Js. R Package Version 4.6.0. 2017. Available online: https://rdrr.io/cran/plotly/ (accessed on 21 September 2022).
- Wickham, H.; Francois, R. Dplyr: A Grammar of Data Manipulation, R Package Version 0.4, 1, 20. 2015. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 21 September 2022).
- Wickham, H.; Wickham, M.H. Package ‘plyr’. 2017. Available online: https://cran.r-project.org/web/packages/plyr/plyr.pdf (accessed on 21 September 2022).
- Grothendieck, G.; Grothendieck, M.G. Package ‘sqldf’. 2017. Available online: https://cran.r-project.org/web/packages/sqldf/sqldf.pdf (accessed on 21 September 2022).
- Rubenson, E.S.; Olden, J.D. Dynamism in the upstream invasion edge of a freshwater fish exposes range boundary constraints. Oecologia 2017, 184, 453–467. [Google Scholar] [CrossRef]
- David, J.A. Water quality and accelerated winter growth of European catfish using an enclosed recirculating system. Water Environ. J. 2006, 20, 233–239. [Google Scholar] [CrossRef]
- Slavík, O.; Horký, P.; Bartoš, L.; Kolářová, J.; Randák, T. Diurnal and seasonal behaviour of adult and juvenile European catfish as determined by radio-telemetry in the River Berounka, Czech Republic. J. Fish Biol. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Slavík, O.; Horký, P. Diel dualism in the energy consumption of the European catfish Silurus glanis. J. Fish Biol. 2012, 81, 2223–2234. [Google Scholar] [CrossRef]
- Fraser, D.F.; Gilliam, J.F.; Daley, M.J.; Le, A.N.; Skalski, G.T. Explaining leptokurtic movement distributions: Intrapopulation variation in boldness and exploration. Am. Nat. 2001, 158, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, F. Electroreceptive properties of Silurus glanis (L.). Experientia 1974, 30, 1035. [Google Scholar] [CrossRef]
- Pohlmann, K.; Grasso, F.W.; Breithaupt, T. Tracking wakes: The nocturnal predatory strategy of piscivorous catfish. Proc. Natl. Acad. Sci. USA 2001, 98, 7371–7374. [Google Scholar] [CrossRef] [Green Version]
- Lo Conte, P.; Città Metropolitana di Torino, Torino, Italy. Personal communication, 2022.
- Candiotto, A.; Bovero, S. Relazione Ittiologica Relativa al Contenimento Della Specie Silurus glanis nel Tratto di Fiume Po a Casale Monferrato; Regione Piemonte: Predosa, Italy, 2020; p. 20. [Google Scholar]
- Candiotto, A.; Private freshwater ichthyologist, Predosa, Italy; Bovero, S.; Private freshwater ichthyologist, Torino, Italy; Spairani, M.; Private freshwater ichthyologist, Gignod, Italy; Lo Conte, P.; Città Metropolitana di Torino, Torino, Italy. Personal communications, 2022.
- Crossin, G.T.; Heupel, M.R.; Holbrook, C.M.; Hussey, N.E.; Lowerre-Barbieri, S.K.; Nguyen, V.M.; Raby, G.D.; Cooke, S.J. Acoustic telemetry and fisheries management. Ecol. Appl. 2017, 27, 1031–1049. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyqvist, D.; Calles, O.; Forneris, G.; Comoglio, C. Movement and Activity Patterns of Non-Native Wels Catfish (Silurus glanis Linnaeus, 1758) at the Confluence of a Large River and Its Colder Tributary. Fishes 2022, 7, 325. https://doi.org/10.3390/fishes7060325
Nyqvist D, Calles O, Forneris G, Comoglio C. Movement and Activity Patterns of Non-Native Wels Catfish (Silurus glanis Linnaeus, 1758) at the Confluence of a Large River and Its Colder Tributary. Fishes. 2022; 7(6):325. https://doi.org/10.3390/fishes7060325
Chicago/Turabian StyleNyqvist, Daniel, Olle Calles, Gilberto Forneris, and Claudio Comoglio. 2022. "Movement and Activity Patterns of Non-Native Wels Catfish (Silurus glanis Linnaeus, 1758) at the Confluence of a Large River and Its Colder Tributary" Fishes 7, no. 6: 325. https://doi.org/10.3390/fishes7060325
APA StyleNyqvist, D., Calles, O., Forneris, G., & Comoglio, C. (2022). Movement and Activity Patterns of Non-Native Wels Catfish (Silurus glanis Linnaeus, 1758) at the Confluence of a Large River and Its Colder Tributary. Fishes, 7(6), 325. https://doi.org/10.3390/fishes7060325






