Individual Characteristics and Abiotic Factors Influence Out-Migration Dynamics of Juvenile Bull Trout
Abstract
:1. Introduction
2. Materials and Methods
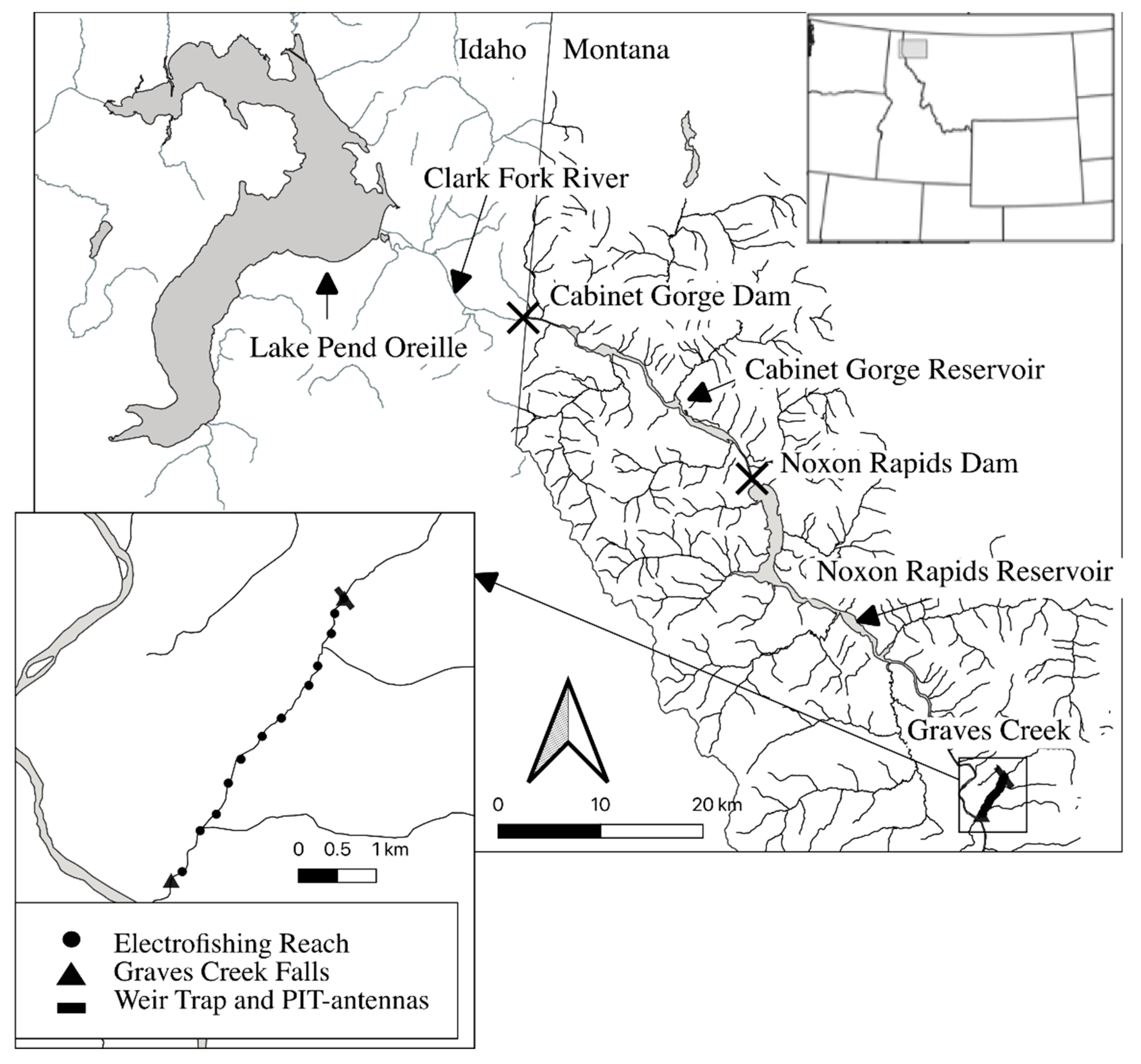
2.1. Study Area
2.2. Sampling and Abundance Estimates
2.3. Monitoring Out-Migration
2.4. Abiotic Factors
2.5. Individual Characteristics Influencing Age and Timing of Out-Migration
2.6. Inter-Annual Variation in Density and Magnitude of Out-Migration by Age Class
2.7. Abiotic Cues to Out-Migration
3. Results
3.1. Biological Characteristics Influencing Age and Timing of Out-Migration
3.2. Inter-Annual Variation in Density and Magnitude of Out-Migration by Age Class
3.3. Abiotic Cues to Out-Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapman, B.B.; Brönmark, C.; Nilsson, J.-Å.; Hansson, L.-A. The Ecology and Evolution of Partial Migration. Oikos 2011, 120, 1764–1775. [Google Scholar] [CrossRef]
- Kerr, L.A.; Secor, D.H.; Piccoli, P.M. Partial Migration of Fishes as Exemplified by the Estuarine-Dependent White Perch. Fish. Res. 2009, 34, 114–123. [Google Scholar] [CrossRef]
- Chapman, B.B.; Hulthén, K.; Brodersen, J.; Nilsson, P.A.; Skov, C.; Hansson, L.-A.; Brönmark, C. Partial Migration in Fishes: Causes and Consequences. J. Fish Biol. 2012, 81, 456–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, J.E. An Alternative View of Smolting in Salmonids. Aquaculture 1994, 121, 105–113. [Google Scholar] [CrossRef]
- Swanson, H.K.; Kidd, K.A.; Reist, J.D. Effects of Partially Anadromous Arctic Charr (Salvelinus alpinus) Populations on Ecology of Coastal Arctic Lakes. Ecosystems 2010, 13, 261–274. [Google Scholar] [CrossRef]
- Rieman, B.E.; Allendorf, F.W. Effective Population Size and Genetic Conservation Criteria for Bull Trout. N. Am. J. Fish. Manag. 2001, 21, 756–764. [Google Scholar] [CrossRef]
- Morita, K.; Yamamoto, S. Effects of Habitat Fragmentation by Damming on the Persistence of Stream-Dwelling Charr Populations. Conserv. Biol. 2002, 16, 1318–1323. [Google Scholar] [CrossRef]
- Tamario, C.; Sunde, J.; Petersson, E.; Tibblin, P.; Forsman, A. Ecological and Evolutionary Consequences of Environmental Change and Management Actions for Migrating Fish. Front. Ecol. Evol. 2019, 7, 271. [Google Scholar] [CrossRef] [Green Version]
- Rieman, B.E.; Lee, D.C.; Thurow, R.F. Distribution, Status, and Likely Future Trends of Bull Trout within the Columbia River and Klamath River Basins. N. Am. J. Fish. Manag. 1997, 17, 1111–1125. [Google Scholar] [CrossRef]
- Branco, P.; Amaral, S.D.; Ferreira, M.T.; Santos, J.M. Do Small Barriers Affect the Movement of Freshwater Fish by Increasing Residency? Sci. Total Environ. 2017, 581–582, 486–494. [Google Scholar] [CrossRef]
- Dunham, J.B.; Rieman, B.E. Metapopulation Structure of Bull Trout: Influences of Physical, Biotic, and Geometrical Landscape Characteristics. Ecol. Appl. 1999, 9, 642–655. [Google Scholar] [CrossRef]
- Rieman, B.E.; Mclntyre, J.D. Demographic and Habitat Requirements for Conservation of Bull Trout. Gen. Tech. Report. 1993, 302, 38. [Google Scholar] [CrossRef]
- Al-Chokhachy, R.; Budy, P. Demographic Characteristics, Population Structure, and Vital Rates of a Fluvial Population of Bull Trout in Oregon. Trans. Am. Fish. Soc. 2008, 137, 1709–1722. [Google Scholar] [CrossRef]
- Howell, P.J.; Colvin, M.E.; Sankovich, P.M.; Buchanan, D.V.; Hemmingsen, A.R. Life Histories, Demography, and Distribution of a Fluvial Bull Trout Population. Trans. Am. Fish. Soc. 2016, 145, 173–194. [Google Scholar] [CrossRef]
- Nelson, M.L.; McMahon, T.E.; Thurow, R.F. Decline of the migratory form in bull charr, Salvelinus confluentus, and implications for conservation. Environ. Biol. Fishes 2002, 64, 321–332. [Google Scholar] [CrossRef]
- Rieman, B.E.; Isaak, D.; Adams, S.; Horan, D.; Nagel, D.; Luce, C.; Myers, D. Anticipated Climate Warming Effects on Bull Trout Habitats and Populations Across the Interior Columbia River Basin. Trans. Am. Fish. Soc. 2007, 136, 1552–1565. [Google Scholar] [CrossRef]
- Al-Chokhachy, R.; Schmetterling, D.; Clancy, C.; Saffel, P.; Kovach, R.; Nyce, L.; Liermann, B.; Fredenberg, W.; Pierce, R. Are Brown Trout Replacing or Displacing Bull Trout Populations in a Changing Climate? Can. J. Fish. Aquat. Sci. 2016, 73, 1395–1404. [Google Scholar] [CrossRef]
- USFWS Recovery Plan for the Coterminous United States Population of Bull Trout (Salvelinus confluentus). 2015. Available online: https://ecos.fws.gov/docs/recovery_plan/Final_Bull_Trout_Recovery_Plan_092915-corrected.pdf (accessed on 1 June 2021).
- Neraas, L.P.; Spruell, P. Fragmentation of Riverine Systems: The Genetic Effects of Dams on Bull Trout (Salvelinus confluentus) in the Clark Fork River System. Mol. Ecol. 2001, 10, 1153–1164. [Google Scholar] [CrossRef]
- DeHaan, P.W.; Bernall, S.R. Spawning Success of Bull Trout Transported above Main-Stem Clark Fork River Dams in Idaho and Montana. N. Am. J. Fish. Manag. 2013, 33, 1269–1282. [Google Scholar] [CrossRef]
- Galloway, B.T.; Muhlfeld, C.C.; Guy, C.S.; Downs, C.C.; Fredenberg, W.A. A Framework for Assessing the Feasibility of Native Fish Conservation Translocations: Applications to Threatened Bull Trout. N. Am. J. Fish. Manag. 2016, 36, 754–768. [Google Scholar] [CrossRef]
- Al-Chokhachy, R.; Moran, S.; McHugh, P.A.; Bernall, S.; Fredenberg, W.; DosSantos, J.M. Consequences of Actively Managing a Small Bull Trout Population in a Fragmented Landscape. Trans. Am. Fish. Soc. 2015, 144, 515–531. [Google Scholar] [CrossRef]
- Thorpe, J.E. Salmon Migration. Sci. Prog. 1988, 72, 345–370. [Google Scholar]
- Dermond, P.; Melián, C.J.; Brodersen, J. Size-Dependent Tradeoffs in Seasonal Freshwater Environments Facilitate Differential Salmonid Migration. Mov. Ecol. 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Dodson, J.J.; Aubin-Horth, N.; Thériault, V.; Páez, D.J. The Evolutionary Ecology of Alternative Migratory Tactics in Salmonid Fishes. Biol. Rev. 2013, 88, 602–625. [Google Scholar] [CrossRef]
- Paul, A.J.; Post, J.R.; Sterling, G.L.; Hunt, C. Density-Dependent Intercohort Interactions and Recruitment Dynamics: Models and a Bull Trout (Salvelinus Confluentus) Time Series. Can. J. Fish. Aquat. Sci. 2000, 57, 1220–1231. [Google Scholar] [CrossRef]
- Ratliff, D.; Spateholts, R.; Hill, M.; Schulz, E. Recruitment of Young Bull Trout into the Metolius River and Lake Billy Chinook, Oregon. N. Am. J. Fish. Manag. 2015, 35, 1077–1089. [Google Scholar] [CrossRef]
- Horn, C.; Tholl, T. Native Salmonid Abundance and Tributary Habitat Restoration Monitoring: Comprehensive Report, 2008–2010; Avista Corporation: Noxon, MT, USA, 2011. [Google Scholar]
- River Design Group. Graves Creek Watershed Assessment and Conceptual Design Report; River Design Group: Whitefish, MT, USA, 2005. [Google Scholar]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis 2020. R Package Version 0.8.30. Available online: https://github.com/fishR-Core-Team/FSA (accessed on 1 June 2021).
- Isermann, D.A.; Knight, C.T. A Computer Program for Age–Length Keys Incorporating Age Assignment to Individual Fish. N. Am. J. Fish. Manag. 2005, 25, 1153–1160. [Google Scholar] [CrossRef]
- Riley, S.C.; Fausch, K.D. Underestimation of Trout Population Size by Maximum-Likelihood Removal Estimates in Small Streams. N. Am. J. Fish. Manag. 1992, 12, 768–776. [Google Scholar] [CrossRef]
- Hijamns, R.J. Geosphere: Spherical Trigonometry 2019. R Package Version 1.5-10. Available online: https://CRAN.R-project.org/package=geosphere (accessed on 1 June 2021).
- Neumann, R.M.; Guy, C.S.; Willis, D.W. Length, Weight, and Associated Indices. In Fisheries Techniques; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 637–676. ISBN 9781934874295. [Google Scholar]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R 2020. R Package Version 3.2-7. Available online: https://github.com/therneau/survival (accessed on 1 June 2021).
- Killick, R.; Fearnhead, P.; Eckley, I.A. Optimal Detection of Changepoints with a Linear Computational Cost. J. Am. Stat. Assoc. 2012, 107, 1590–1598. [Google Scholar] [CrossRef]
- Killick, R.; Eckley, I.A. Changepoint: An R Package for Changepoint Analysis. J. Stat. Softw. 2014, 58, 1–19. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Huntingford, F.A.; Graham, W.D.; Thorpe, J.E. Early Social Status and the Development of Life-History Strategies in Atlantic Salmon. Proc. R. Soc. London. B. Biol. Sci. 1989, 236, 7–19. [Google Scholar] [CrossRef]
- Gross, M.R. Salmon Breeding Behavior and Life History Evolution in Changing Environments. Ecology 1991, 72, 1180–1186. [Google Scholar] [CrossRef]
- ØKland, F.; Jonsson, B.; Jensen, A.J.; Hansen, L.P. Is There a Threshold Size Regulating Seaward Migration of Brown Trout and Atlantic Salmon? J. Fish Biol. 1993, 42, 541–550. [Google Scholar] [CrossRef]
- Forseth, T.; Nesje, T.F.; Jonsson, B.; Harsaker, K. Juvenile Migration in Brown Trout: A Consequence of Energetic State. J. Anim. Ecol. 1999, 68, 783–793. [Google Scholar] [CrossRef]
- Heim, K.C.; Wipfli, M.S.; Whitman, M.S.; Seitz, A.C. Body Size and Condition Influence Migration Timing of Juvenile Arctic Grayling. Ecol. Freshw. Fish 2016, 25, 156–166. [Google Scholar] [CrossRef]
- Zymonas, N. Age Structure, Growth, and Factors Affecting Relative Abundance of Life History Forms of Bull Trout in the Lower Clark Fork River Drainage, Montana and Idaho. Master’s Thesis, Montana State University, Bozeman, MT, USA, 2006. [Google Scholar]
- Downs, C.C.; Horan, D.; Morgan-Harris, E.; Jakubowski, R. Spawning Demographics and Juvenile Dispersal of an Adfluvial Bull Trout Population in Trestle Creek, Idaho. North Am. J. Fish. Manag. 2006, 26, 190–200. [Google Scholar] [CrossRef]
- Enders, E.C.; Boisclair, D. Effects of Environmental Fluctuations on Fish Metabolism: Atlantic Salmon Salmo Salar as a Case Study. J. Fish Biol. 2016, 88, 344–358. [Google Scholar] [CrossRef] [Green Version]
- Morgan, I.J.; McDonald, D.G.; Wood, C.M. The Cost of Living for Freshwater Fish in a Warmer, More Polluted World. Glob. Change Biol. 2001, 7, 345–355. [Google Scholar] [CrossRef]
- Jones, D.A.; Bergman, E.; Greenberg, L. Food Availability in Spring Affects Smolting in Brown Trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2015, 72, 1694–1699. [Google Scholar] [CrossRef] [Green Version]
- Muir, W.D.; Zaugg, W.S.; Giorgi, A.E.; McCutcheon, S. Accelerating Smolt Development and Downstream Movement in Yearling Chinook Salmon with Advanced Photoperiod and Increased Temperature. Aquaculture 1994, 123, 387–399. [Google Scholar] [CrossRef]
- Bottengård, L.; Jørgensen, E.H. Elevated Spring Temperature Stimulates Growth, but Not Smolt Development, in Anadromous Arctic Charr. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Sykes, G.E.; Johnson, C.J.; Shrimpton, J.M. Temperature and Flow Effects on Migration Timing of Chinook Salmon Smolts. Trans. Am. Fish. Soc. 2009, 138, 1252–1265. [Google Scholar] [CrossRef]
- Spence, B.C.; Dick, E.J. Geographic Variation in Environmental Factors Regulating Outmigration Timing of Coho Salmon (Oncorhynchus Kisutch) Smolts. Can. J. Fish. Aquat. Sci. 2014, 71, 56–69. [Google Scholar] [CrossRef]
- Connor, W.; Burge, H.; Yearsley, J.; Bjornn, T. Influence of Flow and Temperature on Survival of Wild Subyearling Fall Chinook Salmon in the Snake River. N. Am. J. Fish. Manag. 2003, 23, 362–375. [Google Scholar] [CrossRef]
- Scheuerell, M.D.; Zabel, R.W.; Sandford, B.P. Relating Juvenile Migration Timing and Survival to Adulthood in Two Species of Threatened Pacific Salmon (Oncorhynchus spp.). J. Appl. Ecol. 2009, 46, 983–990. [Google Scholar] [CrossRef]
- Jakober, M.J.; McMahon, T.E.; Thurow, R.F.; Clancy, C.G. Role of Stream Ice on Fall and Winter Movements and Habitat Use by Bull Trout and Cutthroat Trout in Montana Headwater Streams. Trans. Am. Fish. Soc. 1998, 127, 223–235. [Google Scholar] [CrossRef]
- Johnston, F.D.; Post, J.R.; Mushens, C.J.; Stelfox, J.D.; Paul, A.J.; Lajeunesse, B. The Demography of Recovery of an Overexploited Bull Trout, Salvelinus confluentus, Population. Can. J. Fish. Aquat. Sci. 2007, 64, 113–126. [Google Scholar] [CrossRef]







| Variable | Category | Year-Class | ||
|---|---|---|---|---|
| 2017 | 2018 | 2019 | ||
| Total length (mm) | Small | 151–169 (11) | 100–110 (70) | 105–111 (6) |
| Medium | 170–181 (9) | 111–118 (74) | 112–116 (7) | |
| Large | 182–201 (10) | 119–138 (80) | 116–129 (8) | |
| Relative body Condition (Kn) | Low | 0.91–0.97 (10) | 0.81–0.98 (74) | 0.91–0.99 (8) |
| Medium | 0.98–0.99 (10) | 0.99–1.02 (74) | 1.00–1.03 (6) | |
| High | 1.00–1.10 (10) | 1.03–1.24 (74) | 1.04–1.12 (7) | |
| Age during Summer Sampling (Year-Class) | Summer Sampling (N Tagged) | Density (Mean Number/m2) [95% P.I.] | Autumn Out-Migrants | Spring Out-Migrants |
|---|---|---|---|---|
| Graves Creek 2019/2020 | ||||
| Age 1 (2018) | 486 (451) | 0.0701 (±0.093) | 67 | 86 |
| Age 2 (2017) | 37 (37) | 0.059 (±0.0011) | 22 | 0 |
| Age 3 (2016) | 4 (4) | 0.0007 (±0.0001) | 1 | 0 |
| Total | 527 (492) | 0.0767 (±0.0103) | 90 | 86 |
| Graves Creek 2020/2021 | ||||
| Age 1 (2019) | 283 (251) | 0.0418 (±0.0075) | 5 | 13 |
| Age 2 (2018) | 92 (90) | 0.0138 (±0.0024) | 96 | 9 |
| Age 3 (2017) | 3 (3) | 0.0005 (±0.0001) | 1 | 0 |
| Total | 378 (344) | 0.0561 (±0.0100) | 102 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, M.C.; Guy, C.S.; Oldenburg, E.W.; McMahon, T.E. Individual Characteristics and Abiotic Factors Influence Out-Migration Dynamics of Juvenile Bull Trout. Fishes 2022, 7, 331. https://doi.org/10.3390/fishes7060331
Lewis MC, Guy CS, Oldenburg EW, McMahon TE. Individual Characteristics and Abiotic Factors Influence Out-Migration Dynamics of Juvenile Bull Trout. Fishes. 2022; 7(6):331. https://doi.org/10.3390/fishes7060331
Chicago/Turabian StyleLewis, Madeline C., Christopher S. Guy, Eric W. Oldenburg, and Thomas E. McMahon. 2022. "Individual Characteristics and Abiotic Factors Influence Out-Migration Dynamics of Juvenile Bull Trout" Fishes 7, no. 6: 331. https://doi.org/10.3390/fishes7060331





