In Pursuit of Fish-Free Feeds: A Multi-Species Evaluation
Abstract
1. Introduction
2. Species Evaluated
3. Selection of Dietary Ingredients
4. Fish Holding and Husbandry
5. Data Collection
6. Observations and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, H.S. Some results of feeding experiments with trout fingerlings. Trans. Am. Fish. Soc. 1927, 57, 281–287. [Google Scholar] [CrossRef]
- Davis, H.S. Cheaper trout diets. Progress. Fish-Cult. 1935, 2, 7–10. [Google Scholar] [CrossRef]
- Vetter, H. Meat Substitutes for Feeding Trout by Heinz Vetter, Allgemeine Fischerei-Zeitung, Vol. 63, No. 2, Jan. 15, 1938. Progress. Fish-Cult. 1938, 5, 26–27. [Google Scholar] [CrossRef]
- Tunison, A.V.; Brockway, D.R.; Shaffer, H.B.; Maxwell, J.M.; McKay, C.M.; Palm, C.E.; Webster, D.A. The nutrition of trout. Cortland Hatchery Report No. 12. Fish Res. Bull. 1943, 5, 26. [Google Scholar]
- Leach, G.C. Artificial Propagation of Brook Trout and Rainbow Trout, with Notes on Three Other Species; No. 955; US Government Printing Office: Washington, DC, USA, 1923; p. 74.
- Hayford, C.O.; Davis, N.J.; Davis, H.S. The use of dry foods in the diet of rainbow trout and results of overfeeding. Progress. Fish-Cult. 1936, 3, 7–10. [Google Scholar] [CrossRef]
- Gutsell, J.S. Fingerling trout feeding experiments, Leetown, 1938. Progress. Fish-Cult. 1939, 6, 32–41. [Google Scholar] [CrossRef]
- Gutsell, J.S. Frozen fish in hatchery diets may be dangerous. Progress. Fish-Cult. 1940, 7, 28–32. [Google Scholar] [CrossRef]
- Frick, E.J. Raising of rainbow trout. N. Am. Vet. 1932, 13, 10–14. [Google Scholar]
- Almy, L.H.; Robinson, R.K. Toxic action of ingested linseed meal on trout. J. Biol. Chem. 1920, 43, 97–112. [Google Scholar] [CrossRef]
- Phillips, A.M. Meatless diets and anemia: The development of anemia in trout fed a synthetic diet and its cure by the feeding of fresh beef liver. Progress. Fish-Cult. 1940, 7, 11–13. [Google Scholar] [CrossRef]
- Embody, C.G. Results of some trout feeding experiments carried on in the experimental hatching station of Cornell University. Trans. Am. Fish. Soc. 1918, 48, 26–33. [Google Scholar] [CrossRef]
- Embody, C.G.; Gordon, M. A comparative study of natural and artificial foods of brook trout. Trans. Am. Fish. Soc. 1924, 54, 185–200. [Google Scholar] [CrossRef]
- Davis, H.S. Care and Diseases of Trout; U.S. Department of the Interior: Washington, DC, USA, 1946; Volume 12, p. 98.
- Wolf, L.E. Comparison of yeast and penicillin mat as supplements to dry-meal diets for brown trout. Progress. Fish-Cult. 1951, 13, 117–120. [Google Scholar] [CrossRef]
- Phillips, A.M.; Blazer, G.C., Jr. The nutrition of trout V. Ingredients for trout diets. Progress. Fish-Cult. 1957, 19, 158–167. [Google Scholar] [CrossRef]
- Hublou, W.F.; Wallis, J.; McKee, T.B.; Law, D.K.; Sinnhuber, R.O.; Yu, T.C. Development of the Oregon pellet diet. Fifth progress report on salmon diet experiments. Fish Comm. Or. Res. Briefs 1959, 7, 28–55. [Google Scholar]
- Brockway, D.R. Fish food pellets show promise. Progress. Fish-Cult. 1953, 15, 92–93. [Google Scholar] [CrossRef]
- Jeffries, E.R.; McKee, T.B.; Sinnhuber, R.O.; Law, D.K.; Yu, T.C. Third progress report on spring chinook diet experiments. Fish Comm. Or. Res. Briefs 1954, 5, 32–38. [Google Scholar]
- Schumacher, R.E. Experimental feeding of a pelleted trout food to large fingerling brook, brown and rainbow trout, 1955–1956. Progress. Fish-Cult. 1958, 20, 53–57. [Google Scholar]
- Nielsen, W.E.; Mazuranich, J.J. Dry diets for chinook salmon. Progress. Fish-Cult. 1959, 21, 86–88. [Google Scholar] [CrossRef]
- Grassl, E.F. Pelleted dry rations for trout propagation in Michigan hatcheries. Trans. Am. Fish. Soc. 1956, 86, 307–322. [Google Scholar] [CrossRef]
- Grassl, E.F. Possible value of continuous feeding of medicated dry diets to prevent and control pathogens in hatchery-reared trout. Progress. Fish-Cult. 1957, 19, 85–88. [Google Scholar] [CrossRef]
- Grassl, E.F. Relation of a dry pelleted ration to nutritional anemia in brook and rainbow trout. Progress. Fish-Cult. 1958, 20, 62–65. [Google Scholar] [CrossRef]
- Phillips, A.M., Jr.; Podoliak, H.A.; Poston, H.A.; Livingstone, D.L.; Brooks, H.E.; Pyle, E.E.; Hammer, G.L. Dry Concentrates as Complete Fish Foods; New York State Conservation Department: Albany, NY, USA, 1964; Volume 27, pp. 47–54. [Google Scholar]
- Waite, D.; Buss, K. An automatic feeder for trout. Progress. Fish-Cult. 1963, 25, 52. [Google Scholar] [CrossRef]
- Hardy, R.W. Diet preparation. In Fish Nutrition, 2nd ed.; Halver, J.E., Ed.; Academic Press: San Diego, CA, USA, 1989; pp. 475–548. [Google Scholar]
- Hansen, L. The weak sustainability of the salmon feed transition in Norway—A bioeconomic case study. Front. Mar. Sci. 2019, 6, 764. [Google Scholar] [CrossRef]
- Food and Agricultural Organization. State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Jacobsen, N.S.; Essington, T.E.; Clavelle, T.; Halpern, B.S. Avoiding the ecological limits of forage fish for fed aquaculture. Nat. Sustain. 2018, 1, 298–303. [Google Scholar] [CrossRef]
- McLean, E. Feed ingredients for sustainable aquaculture. In Sustainable Food Science: A Comprehensive Approach; Elsevier Inc.: Amsterdam, The Netherlands, 2023; in press. [Google Scholar]
- Callet, T.; Médale, F.; Larroquet, L.; Surget, A.; Aguirre, P.; Kerneis, T.; Labbé, L.; Quillet, E.; Geurden, I.; Skiba-Cassy, S.; et al. Successful selection of rainbow trout (Oncorhynchus mykiss) on their ability to grow with a diet completely devoid of fishmeal and fish oil, and correlated changes in nutritional traits. PLoS ONE 2017, 12, e0186705. [Google Scholar] [CrossRef]
- Haarstad, A.H.; Lavutich, M.; Strypet, K.; Strøm, E. Multi-commodity price risk hedging in the Atlantic salmon farming industry. Multi-commodity price risk hedging in the Atlantic salmon farming industry. J. Commod. Mark. 2022, 25, 100182. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Feeding farmed salmon: Is organic better? Aquaculture 2007, 272, 399–416. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Meyer, S.; Reckmann, K.; Schroeder, J.P.; Schulz, C. Aspiring for environmentally conscious aquafeed: Comparative LCA of aquafeed manufacturing using different protein sources. J. Clean. Prod. 2013, 52, 225–233. [Google Scholar] [CrossRef]
- Basto-Silva, C.; Valente, L.M.P.; Matos, E.; Brandão, M.; Neto, B. Life cycle assessment of aquafeed ingredients. Int. J. Life Cycle Assess. 2018, 23, 995–1017. [Google Scholar] [CrossRef]
- Glencross, B.D.; Bailey, J.; Berntssen, M.H.G.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2020, 12, 703–758. [Google Scholar] [CrossRef]
- Lewis, S.G.; Alifano, A.; Boyle, M.; Mangel, M. Human rights and the sustainability of fisheries. In Conservation for the Anthropocene Ocean; Levin, P.S., Poe, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 379–396. [Google Scholar]
- Gilman, E.; Perez Roda, A.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P.A.H. Benchmarking global fisheries discards. Sci. Rep. 2020, 10, 14017. [Google Scholar] [CrossRef]
- Al-Masroori, H.; Al-Oufi, H.S.; McLean, E.; Mcllwain, J.L. Catches of lost fish traps (ghost fishing) from fishing grounds near Muscat, Sultanate of Oman. Fish. Res. 2004, 68, 407–414. [Google Scholar] [CrossRef]
- World Trade Organization. Implementing the WTO Agreement on Fisheries Subsidies. Challenges and Opportunities for Developing and Least-Developed Country Members; WTO: Geneva, Switzerland, 2022; p. 23. [Google Scholar]
- Parker, R.W.R.; Blanchard, J.L.; Gardner, C.; Green, B.S.; Hartmann, K.; Tyedmers, P.H.; Watson, R.A. Fuel use and greenhouse gas emissions of world fisheries. Nat. Clim. Chang. 2018, 8, 333–337. [Google Scholar] [CrossRef]
- Boyd, C.E. Overview of aquaculture feeds: Global impacts of ingredient use. In Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 3–25. [Google Scholar]
- Eriksen, H.S. Information Resources on Fish Welfare 1970-20903; AWIC Resource Series No. 20; United States Department of Agriculture: Washington, DC, USA, 2003; p. 435.
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts on the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- McLean, E.; Alfrey, K.; Gatlin, D.M., III; Barrows, F.T. Responses of largemouth bass to fishmeal and fish oil-free diets. Aquac. Res. 2022, 53, 3036–3047. [Google Scholar] [CrossRef]
- Alfrey, K.B.; Gatlin, D.M., III; Barrows, F.T.; McLean, E. Assessment of open-source, fish-free diets for pompano, Trachinotus carolinus (Perciformes, Carangidae), under hyposaline conditions. Aquac. Res. 2022, in press.
- Mai, K.; Zhang, W. Feed developments in mariculture. In Aquaculture in China: Success Stories and Modern Trends, 3rd ed.; Gui, J.F., Tang, Q., Li, Z., Liu, J., De Silva, S.S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 451–462. 677p. [Google Scholar]
- Suehs, B.; Alfrey, K.; Barrows, F.; Gatlin, D.M. III Evaluation of growth performance, condition indices and body composition of juvenile red drum (Sciaenops ocellatus) fed fishmeal- and fish-oil-free diets. Aquaculture 2022, 551, 737961. [Google Scholar] [CrossRef]
- USDA. Livestock and Poultry: World Markets and Trade. 2022. Available online: https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf (accessed on 24 October 2022).
- Hertrampf, J.W.; Piedad-Pascual, F. Handbook on Ingredients for Aquaculture Feeds; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2000; p. 624. [Google Scholar]
- Viyakarn, V.; Watanabe, T.; Aoki, H.; Tsuda, H.; Sakamoto, H.; Okamoto, N.; Iso, N.; Satoh, S.; Takeuchi, T. Use of soybean meal as a substitute for fish meal in a newly developed soft-dry pellet for yellowtail. Nippon. Suisan Gakkaishi 1992, 58, 1991–2000. [Google Scholar] [CrossRef]
- Rhodes, M.A.; Zhou, Y.; Davis, D.A. Use of Dried Fermented Biomass as a Fish Meal Replacement in Practical Diets of Florida Pompano, Trachinotus carolinus. J. Appl. Aquac. 2015, 27, 29–39. [Google Scholar] [CrossRef]
- Rossi, W., Jr.; Moxely, D.; Buentello, A.; Pohlenz, C.; Gatlin, D.M. III Replacement of fishmeal with novel plant feedstuffs in the diet of red drum Sciaenops ocellatus: An assessment of nutritional value. Aquac. Nutr. 2013, 19 (Suppl. S1), 72–81. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Guo, X.; Mi, S.; Hua, X.; Li, N.; Yao, J. Enhanced growth performance, muscle quality and liver health of largemouth bass (Micropterus salmoides) were related to dietary small peptides supplementation. Aquac. Nutr. 2020, 26, 2169–2177. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Olsen, R.E.; Myklebust, R.; Ringø, E. Dietary effect of soybean (Glycine max) products on gut histology and microbiota of fish. In Soybean and Nutrition; El-Shemy, H., Ed.; Tech Europe: Rijeka, Croatia, 2011; pp. 231–250. [Google Scholar]
- Dersjant-Li, Y. The use of soy protein in aquafeeds. In Proceedings of the Avances en Nutrición Acuícola VI. Memorias del VI Simposium Internacional de Nutrición Acuícola, Cancun, Mexico, 3–6 September 2002. [Google Scholar]
- Gyan, W.R.; Ayiku, S.; Yang, Q. Effects of replacing fishmeal with soybean products in fish and crustaceans performance. J. Aquac. Res. Dev. 2019, 10, 573. [Google Scholar]
- Jannathulla, R.; Sravanthi, O.; Moomeen, S.; Gopikrishna, G.; Dayal, J.S. Microbial products in terms of isolates, whole-cell biomass, and live organisms as aquafeed ingredients: Production, nutritional values, and market potential—A review. Aquac. Int. 2021, 29, 623–650. [Google Scholar] [CrossRef]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Replacement of fish meal with fermented plant proteins in the aquafeed industry: A systematic review and meta-analysis. Rev. Aquac. 2022, 1–27. [Google Scholar] [CrossRef]
- Alagawany, M.; Taha, A.E.; Noreldin, A.; El-Tarabily, K.A.; El-Hack, M.E.A. Nutritional applications of species of Spirulina and Chlorella in farmed fish: A review. Aquaculture 2021, 542, 736841. [Google Scholar] [CrossRef]
- Kissinger, K.R.; García-Ortega, A.; Trushenski, J.T. Partial fish meal replacement by soy protein concentrate, squid and algal meals in low fish-oil diets containing Schizochytrium limacinum for longfin yellowtail Seriola rivoliana. Aquaculture 2016, 452, 37–44. [Google Scholar] [CrossRef]
- Guo, H.; Chen, C.; Yan, X.; Li, Y.; Wen, X.; You, C.; Monroig, Ó.; Tocher, D.R.; Wang, S. Effects of different dietary oil sources on growth performance, antioxidant capacity and lipid deposition of juvenile golden pompano Trachinotus ovatus. Aquaculture 2021, 530, 735923. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, X.; Jiao, L.; Shen, Y.; Luo, J.; Zhu, T.; Zhao, W.; Gen, Z.; Zhou, Q.; Jin, M. Effects of different lipid sources on growth performance, fatty acids composition in tissue and expression of genes related to lipid metabolism in largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 23, 101013. [Google Scholar] [CrossRef]
- Meigs, H.; Barrows, F.T.; Sims, N.A.; Alfrey, K. Testing Diets without Fishmeal and Fish Oil for Kampachi. Responsible Seafood Advocate 24 August 2020. 2020. Available online: https://www.globalseafood.org/advocate/testing-diets-without-fishmeal-and-fish-oil-for-kampachi/ (accessed on 14 October 2022).
- Stuart, K.R.; Barrows, F.T.; Silbernagel, C.; Alfrey, K.; Rotstein, D.; Drawbridge, M.A. Complete replacement of fish oil and fish meal in the diet of juvenile California yellowtail Seriola dorsalis. Aquac. Res. 2020, 52, 655–665. [Google Scholar] [CrossRef]
- McLean, E.; Fredriksen, L.; Alfrey, K.; Craig, S.R.; Barrows, F.T. Performance of largemouth bass Micropterus salmoides (Lacépède, 1802), fed fishmeal- and fish oil-free diets. J. Fish. Aquat. Stud. 2020, 8, 6–10. [Google Scholar] [CrossRef]
- McLean, E.; Fredriksen, L.; Alfrey, K.; Tlusty, M.F.; Barrows, F.T. Growth, integrity, and consumer acceptance of largemouth bass, Micropterus salmoides (Lacépède, 1802), fed marine resource-free diets. Int. J. Fish. Aquat. Stud. 2020, 8, 365–369. [Google Scholar] [CrossRef]
- Riche, M.; Barrows, F.T.; Nilles, Z.; Alfrey, K.B.; Wills, P.S. Replacement of Fish Oil with a High DHA Algal Oil in a Fishmeal-Free Diet Fed to Florida pompano Trachinotus carolinus; Harbor Branch Oceanographic Institute: Fort Pierce, FL, USA, 2022; to be submitted. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride−methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Archibeque, S.L.; Lunt, D.K.; Gilbert, C.D.; Tume, R.K.; Smith, S.B. Fatty acid indices of stearoyl-CoA desaturase do not reflect actual stearoyl-CoA desaturase enzyme activities in adipose tissues of beef steers finished with corn-, flaxseed-, or sorghum-based diets. J. Anim. Sci. 2005, 83, 1153–1166. [Google Scholar] [CrossRef]
- Sheehan, D.C.; Hrapchak, B.B. Theory and Practice of Histotechnology, 2nd ed.; Battelle Press: Columbus, OH, USA, 1987; p. 481. [Google Scholar]
- Gomes, E.F.; Rema, P.; Kaushik, S.J. Replacement of fish meal by plant proteins in the diet of rainbow trout (Oncorhynchus mykiss): Digestibility and growth performance. Aquaculture 1995, 130, 177–186. [Google Scholar] [CrossRef]
- Kissil, G.W.; Lupatsch, I.; Higgs, D.A.; Hardy, R.W. Dietary substitution of soy and rapeseed protein concentrates for fish meal, and their effects on growth and nutrient utilization in gilthead seabream Sparus aurata L. Aquac. Res. 2000, 31, 595–601. [Google Scholar] [CrossRef]
- Barrows, F.T.; Gaylord, T.G. Changing technologies, ingredients and formulations to replace fish meal in salmonid diets. In Nutritional Biotechnology in the Food and Feed Industry; Lyons, T.P.T., Jacques, K., Eds.; Nottingham University Press: Nottingham, UK, 2006; pp. 307–324. [Google Scholar]
- Lunger, A.N.; McLean, E.; Gaylord, T.G.; Kuhn, D.; Craig, S.R. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Silva, J.; Espe, M.; Conceição, L.; Dias, J.; Valente, L. Senegalese sole juveniles (Solea senegalensis Kaup, 1858) grow equally well on diets devoid of fish meal provided the dietary amino acids are balanced. Aquaculture 2009, 296, 309–317. [Google Scholar] [CrossRef]
- Davidson, J.; Barrows, F.T.; Kenney, P.B.; Good, C.; Schroyer, K.; Summerfelt, S.T. Effects of feeding a fishmeal-free versus a fishmeal-based diet on post-smolt Atlantic salmon Salmo salar performance, water quality, and waste production in recirculation aquaculture systems. Aquac. Eng. 2016, 74, 38–51. [Google Scholar] [CrossRef]
- Lorez, E.K.; Sabioni, R.E.; Volkoff, H.; Cyrino, J.E.P. Growth performance, health, and gene expression of appetite-regulating hormones in Dourado Salminus brasiliensis, fed vegetable-based diets supplemented with swine liver hydrolysate. Aquaculture 2022, 548, 737640. [Google Scholar] [CrossRef]
- Yadav, A.K.; Rossi, W., Jr.; Habte-Tsion, H.-M.; Kumar, V. Impacts of dietary eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) level and ratio on the growth, fatty acids composition and hepatic-antioxidant status of largemouth bass (Micropterus salmoides). Aquaculture 2020, 529, 735683. [Google Scholar] [CrossRef]
- Fracalossi, D.M.; Craig-Schmidt, M.C.; Lovell, R.T. Effect of dietary lipid sources on production of leukotriene B by head kidney of channel catfish held at different water temperatures. J. Aquat. Anim. Health 1995, 6, 242–250. [Google Scholar] [CrossRef]
- Luzzana, U.; Scolari, M.; Campo Dall’Orto, B.; Caprino, F.; Turchini, G.; Orban, E.; Sinesio, F.; Valfre, F. Growth and product quality of European eel (Anguilla tilizat) as affected by dietary protein and lipid sources. J. Appl. Ichthyol. 2003, 19, 74–78. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, B.; Liu, K.; Dong, X.; Yang, Q.; Chi, S.; Liu, H.; Zhang, S.; Wang, H. Effects of different dietary lipids on growth, body composition and lipid metabolism-related enzymes and genes in juvenile largemouth bass, Micropterus salmoides. Aquac. Nutr. 2019, 25, 1318–1326. [Google Scholar] [CrossRef]
- Habte-Tsion, H.M.; Kolimadu, G.D.; Rossi, W.; Filer, K.; Kumar, V. Effects of Schizochytrium and micro-minerals on immune, antioxidant, inflammatory and lipid-metabolism status of Micropterus salmoides fed high- and low-fishmeal diets. Sci. Rep. 2020, 10, 7457. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011; p. 375. [Google Scholar]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef]
- Xu, H.; Turchini, G.M.; Francis, D.M.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 50, 101064. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; Du, Z.-Y.; Olsen, R.E.; Ringø, E.; Tocher, D.R. The lipids. In Fish Nutrition, 4th ed.; Hardy, R.W., Kaushik, S.J., Eds.; Academic Press: London, UK, 2022. [Google Scholar]
- Tacon, A.G.J.; Lemos, D.; Metian, M. Fish for health: Improved nutritional quality of cultured fish for human consumption. Rev. Fish. Sci. Aquac. 2020, 28, 449–458. [Google Scholar] [CrossRef]
- Fiorella, K.J.; Okronipa, H.; Baker, K.; Heilpern, S. Contemporary aquaculture: Implications for human nutrition. Curr. Opin. Biotechnol. 2021, 70, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.S.; Ostenfeld, T.H.; Rønsholdt, B.; McLean, E. Manipulation of end-product quality of rainbow trout with finishing diets. Aquac. Nutr. 2000, 6, 17–23. [Google Scholar] [CrossRef]
- Parés-Sierra, G.; Durazo, E.; Ponce, M.A.; Badillo, D.; Correa-Reyes, G.; Viana, M.T. Partial to total replacement of fishmeal by poultry by-product meal in diets for juvenile rainbow trout (Oncorhynchus mykiss) and their effect on fatty acids from muscle tissue and the time required to retrieve the effect. Aquac. Res. 2014, 45, 1459–1469. [Google Scholar] [CrossRef]
- Rønsholdt, B.; McLean, E. Quality characteristics of fresh rainbow trout as perceived by the Danish processing industry. Aquac. Int. 1999, 7, 117–127. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, A.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Hasan, M.R.; Subasinghe, R.P. Use of fishery resources as feed inputs to aquaculture development: Trends and policy implications. In FAO Fisheries Circular 1018; FAO: Rome, Italy, 2006. [Google Scholar]
- Volpato, J.P.; Ribeiro, L.B.; Torezan, G.B.; da Silva, I.C.; de Oliveira Martins, I.; Genova, L.; de Oliveira, N.T.E.; Carvalho, S.T.; de Oliveira Carvalho, P.L.; Vasconcellos, R.S. Characterization of the variations in the industrial processing and nutritional variables of poultry by-product meal. Poult. Sci. 2022, 101, 101926. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Steffens, W. Replacing fish meal with poultry by-product meal in diets for rainbow trout, Oncorhynchus mykiss. Aquaculture 1994, 124, 27–34. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Chungu, P.; Fotedar, R.; Howieson, J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PLoS ONE 2019, 14, e0215025. [Google Scholar] [CrossRef]
- Pham, H.D.; Siddik, M.A.B.; Phan, U.V.; Le, H.M.; Rahman, M.A. Enzymatic tuna hydrolysate supplementation modulates growth, nutrient utilisation and physiological response of pompano (Trachinotus blochii) fed high poultry-by product meal diets. Aquac. Rep. 2021, 21, 100875. [Google Scholar] [CrossRef]
- Woodgate, S.L.; Wan, A.H.L.; Hartnett, F.; Wilkinson, R.G.; Davis, S.L. The tilization of European processed animal proteins as safe, sustainable and circular ingredients for global aquafeeds. Rev. Aquac. 2022, 14, 1572–1596. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Fishmeal alternative protein sources for aquaculture feeds. In Feeds for the Aquaculture Sector; Springer Briefs in Molecular Science; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Hemre, G.-I. Effects of replacing fish meal and oil with plant resources in on-growing diets for Atlantic cod Gadus morhua L. Aquac. Nutr. 2013, 19, 641–650. [Google Scholar] [CrossRef]
- Seong, T.; Kitagima, R.; Haga, Y.; Satoh, S. Non-fish meal, non-fish oil diet development for red sea bream, Pagrus major, with plant protein and graded levels of Schizochytrium sp.: Effect on growth and fatty acid composition. Aquac. Nutr. 2020, 26, 1173–1185. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part I: Effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids 2021, 53, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Chu, J.H.; Kirby, R.; Sheen, S.S.; Chien, A. The effects of replacing fish meal protein with a mixture of poultry by-product meal and fermented soybean meal on the growth performance and tissue nutritional composition of Asian seabass (Lates calcarifer). Aquac. Res. 2021, 52, 4105–4115. [Google Scholar] [CrossRef]
- Saxena, P.; Khanna, N. Animal feed formulation: Mathematical programming techniques. CAB Rev. 2014, 9, 035. [Google Scholar]
- Uyeh, D.D.; Pamulapati, T.; Mallipeddi, R.; Park, T.; Asem-Hiablie, S.; Woo, S.; Kim, J.; Kim, Y.; Ha, Y. Precision animal feed formulation: An evolutionary multi-objective approach. Anim. Feed Sci. Technol. 2019, 256, 114211. [Google Scholar] [CrossRef]
- Bailey, C.A. Chapter 21—Precision poultry nutrition and feed formulation. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: London, UK, 2020; pp. 367–378. [Google Scholar]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage centers as a histological indicator of immune function in fish and other poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef]
- Bansemer, M.S.; Forder, R.E.; Howarth, G.S.; Suitor, G.M.; Bowyer, J.; Stone, D.A. The effect of dietary soybean meal and soy protein concentrate on the intestinal mucus layer and development of subacute enteritis in yellowtail kingfish (Seriola lalandi). Aquac. Nutr. 2014, 21, 300–310. [Google Scholar] [CrossRef]
- Hermann, R.; Boissinger, K.; Krandick, L. Price premia for sustainability characteristics in foods: Measurement matters! In Proceedings of the System Dynamics and Innovation in Food Networks 2018, Innsbruck, Austria, 5–9 February 2018; pp. 28–37. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Tulli, F.; Messina, M.; Tibaldi, E.; Guillou, C. Stable isotope ratio analysis as a tool to discriminate between rainbow trout (O. mykiss) fed diets based on plant or fish-meal proteins. Rapid Commun. Mass Spectrom. 2008, 22, 3706–3710. [Google Scholar] [CrossRef] [PubMed]
- Schwägele, F. Traceability from a European perspective. Meat Sci. 2005, 71, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Van der Zanden, M.J.; Hulshof, M.; Ridgway, M.S.; Rasmussen, J.B. Application of stable isotope techniques to trophic studies of age-0 smallmouth bass. Trans. Am. Fish. Soc. 1998, 127, 729–739. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Polunin, N.V.C. Contributions of stable-isotope data to elucidating food webs of Mediterranean rocky littoral fishes. Oecologia 2000, 122, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Schlechtriem, C.; Focken, U.; Becker, K. Stable isotopes as a tool for nutrient assimilation studies in larval fish feeding on live food. Aquat. Ecol. 2004, 38, 93–100. [Google Scholar] [CrossRef]
- Preston, N.P.; Smith, D.M.; Kellaway, D.M.; Bunn, S.E. The use of enriched 15N as an indicator of the assimilation of individual protein sources from compound diets for juvenile Penaeus monodon. Aquaculture 1996, 147, 249–259. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Le Vay, L. Natural stable isotopes as indicators of the relative contribution of soy protein and fish meal to tissue growth in Pacific white shrimp (Litopenaeus vannamei) fed compound diets. Aquaculture 2009, 291, 115–221. [Google Scholar] [CrossRef]
- Wertz, A.; Mazumder, D.; Carter, C.G.; Codabaccus, M.B.; Fitzgibbon, Q.P.; Smith, G.S. Application of stable isotope analysis to evaluate the assimilation of protein sources in juvenile slipper lobsters (Thenus australiensis). Aquacultuire 2022, 560, 738570. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Zapata, D.B.; Lazo, J.P.; Herzka, S.Z.; Viana, M.T. The effect of substituting fishmeal with poultry by-product meal in diets for Totoaba macdonaldi juveniles. Aquac. Res. 2016, 47, 1778–1789. [Google Scholar] [CrossRef]
- Tran, L.H.; Nhut, T.C.; Alfrey, K.B.; Barrows, F.T.; Kuhn, D.; McLean, E. Performance of Pacific whiteleg shrimp fed a fishmeal and fish oil-free diet under commercial conditions. Int. J. Fish. Aquat. Stud. 2022, 10, 33–41. [Google Scholar] [CrossRef]
- McLean, E.; Tran, L.H.; Craig, S.R.; Alfrey, K.; Barrows, F.T. Complete replacement of fishmeal by soybean and poultry meals in whiteleg shrimp feeds: Growth and tolerance to EMS/AHPND and WSSV challenge. Aquaculture 2020, 527, 735383. [Google Scholar] [CrossRef]
- Anonymous. Winners of Global Seafood Industry’s Contest F3 Challenge Announced. 2022. Available online: https://www.aquafeed.com/newsroom/news/winners-of-global-seafood-industrys-contest-f3-challenge-announced/?utm_source=Aquafeed&utm_campaign=0a2c65f74e-EMAIL_CAMPAIGN_2019_06_26_19_COPY_01&utm_medium=email&utm_term=0_0e7f7c0399-0a2c65f74e-100222 (accessed on 6 October 2022).
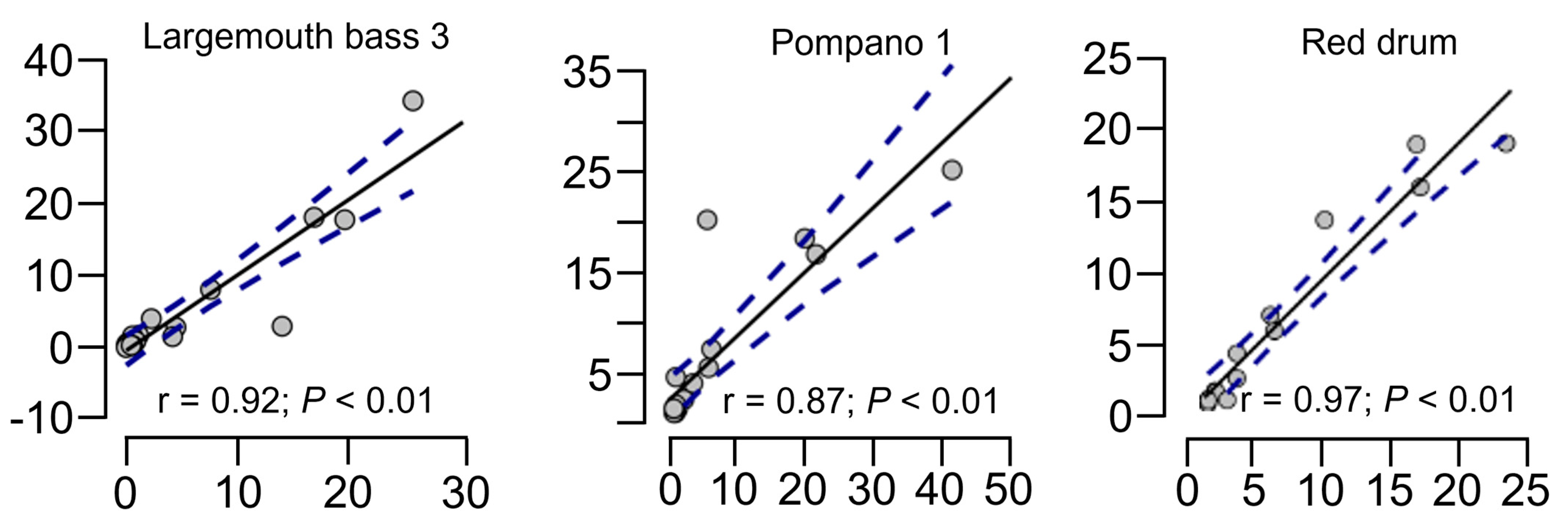
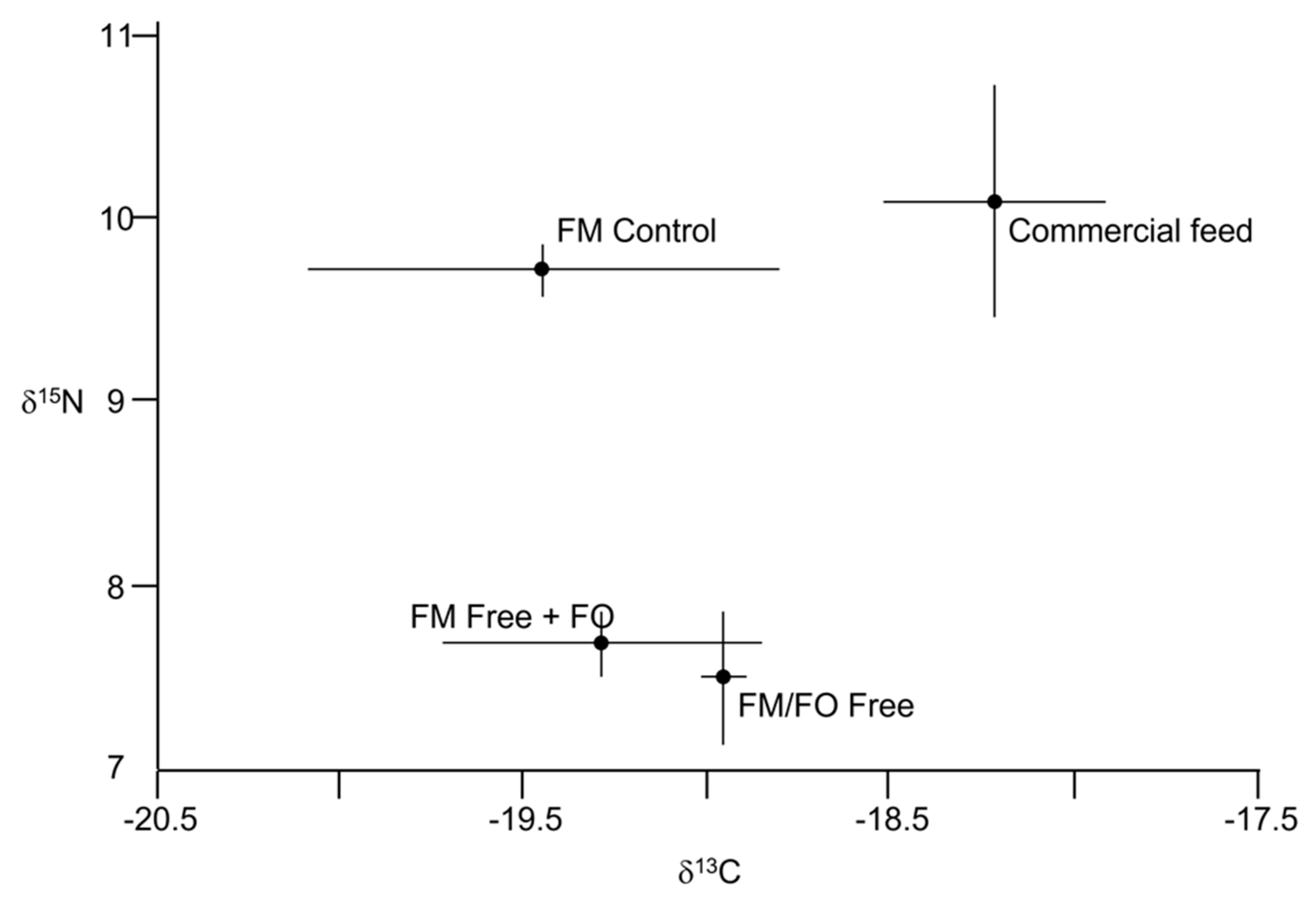
| Ingredient | Kampachi [65] | Yellowtail [66] | Largemouth Bass 1 [67] | Largemouth Bass 2 [68] | Largemouth Bass 3 [46] | Red Drum [49] | Pompano 1 [47] | Pompano 2 [69] |
|---|---|---|---|---|---|---|---|---|
| Poultry by-product meal | 36.12 | 23.12 | 25.62 | 25.62 | 28.80 | 28.8 | 36.12 | 23.12 |
| Wheat, whole ground | 20.53 | 16.75 | 20.43 | 22.7 | 18.41 | 18.41 | 17.77 | 16.75 |
| Corn gluten meal | - | - | 8.16 | 8.16 | - | - | - | - |
| Corn protein concentrate | 13.56 | 7.14 | - | - | - | - | 8.22 | 7.14 |
| Non-GMO soybean meal | - | - | 11 | 11 | - | - | - | - |
| Soy protein concentrate | 7.86 | - | 17.93 | - | 24.32 | 24.32 | 5.96 | - |
| MrFeed Pro50 | - | - | - | 15 | 12.5 | 12.5 | 12.5 | - |
| Algae meal | - | - | - | 6 | - | - | - | - |
| Spirulina | - | 30 | - | - | - | - | - | 30 |
| Algal oil, Veramaris | 5.32 | 10.80 | - | - | 2.28 | 2.28 | 2.13 | 10.80 |
| Flax oil | 2.71 | - | - | - | - | 4.52 | 4.86 | - |
| Non-GMO soy oil | - | - | 4.73 | 2.7 | - | - | - | - |
| Canola oil | 2.38 | - | - | - | 5.42 | 0.9 | 1.32 | - |
| Fish oil—Menhaden | - | - | 3 | - | - | - | - | - |
| Dicalcium phosphate | 3.1 | 4.16 | - | - | - | - | 3.10 | 4.16 |
| Monocalcium phosphate | - | - | 1.97 | 1.35 | 1.8 | 1.8 | - | - |
| Lysine-HCL | 2.67 | 2.68 | 1.66 | 1.97 | 1.62 | 1.62 | 2.27 | 2.68 |
| Taurine | 2 | 2 | 1 | 1 | - | - | 2 | 2 |
| DL-Methionine | 0.69 | 0.64 | 0.64 | 0.64 | 0.74 | 0.74 | 0.77 | 0.64 |
| Threonine | 0.46 | 0.31 | 0.31 | 0.31 | 0.21 | 0.21 | 0.38 | 0.31 |
| Choline CL | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.60 | 0.60 |
| Lecithin | - | - | 2 | 2 | 2 | 2 | - | - |
| Stay-C | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.20 | 0.20 |
| Vitamin Premix ARS 702 | 1.5 | 1.5 | 0.5 | 0.5 | 1 | 1 | 1.50 | 1.50 |
| Trace min premix ARS 1520 | 0.10 | 0.10 | 0.25 | 0.25 | 0.10 | 0.10 | 0.10 | 0.10 |
| Trace min premix F3 | 0.20 | - | - | - | - | - | 0.20 | - |
| TOTAL | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| System | # of Fish/Tank | Start Weight (g) | Study Length (d) | Temperature (°C) | DO2 (mg L−1) | Salinity (g L−1) | Daily Feed Schedule | |
|---|---|---|---|---|---|---|---|---|
| Kampachi | Tanks | 30 → 15 | 282 | 84 | - | - | - | 2× → 1× to satiety |
| Yellowtail | RAS | 15 | 20 | 64 | 22 | 10-12 | 34.5 | 5–10% body wt |
| Largemouth bass 1 | RAS | 20 | 25 | 84 | 28 | 7.7 | 3.1 | 3× to satiety |
| Largemouth bass 2 | RAS | 60–64 | 48 | 126 | 28 | 8.2 | 3.7 | 3× using feed tables |
| Largemouth bass 3 | RAS | 20 | 15.2 | 70 | 28 | 6.0 | 1.2 | 2× to satiety |
| Red drum | RAS | 15 | 3 | 56 | 27.6 | 6.9 | 5.4 | 2× to satiety |
| Pompano 1 | RAS | 10 | 15 | 84 | 26.6 | 7.4 | 3 | 2× for 5 min |
| Pompano 2 | RAS | 20 | 4.1 | 84 | 28 | 8.0 | 34 | 4× up to 5% body wt |
| Ingredient | Kampachi [65] | Yellowtail [66] | Largemouth Bass 1 [67] | Largemouth Bass 2 [68] | Largemouth Bass 3 [46] | Red Drum [49] | Pompano 1 [47] | Pompano 2 [69] |
|---|---|---|---|---|---|---|---|---|
| Wt gain (%) | 419↓ | 633.6↓ | 201.4 | 149.3 | 398 | 666↓ | 243.1↓ | 1149 |
| FE | - | - | - | - | - | 1.09 | 0.52 | - |
| FCR | 1.31 | 1.33 | 1.28 | 1.95↑ | 0.89 | - | - | 1.6 |
| PER | - | - | - | - | 2.34 | - | - | 1.2↓ |
| Survival (%) | - | 100 | 99 | 84↓ | 100 | 90 | - | 100 |
| Fillet yield (%) | 60.9 | - | - | - | 32.3 | 31.5 | 31.2 | - |
| HSI (%) | - | - | 1.49 | 1.66 | 3.0 | 1.99 | 2.60 | - |
| IPF ratio (%) | - | - | - | - | 3.0 | 0.39↓ | 0.01 | - |
| K factor | - | - | 1.16 | 1.19 | 1.29 | 1.34 | 1.59 | |
| VSI (%) | 5.7↓ | - | 4.55 | 1.93 | - | - | - | - |
| Proximate composition | ||||||||
| Moisture | - | 70.9 | - | - | 68.8 | 74.9 | 68.6 | - |
| Protein | - | 20.98 | 45.4 | 41.5 | 17.9 | 17.4 | 18.1 | - |
| Lipid | - | 7.37↓ | 14.7 | 15 | 8.8 | 3.87↓ | 9.72 | - |
| Ash | - | 2.46↑ | 6.96 | 7.39 | 4.0↓ | 3.90 | 3.29 | - |
| Largemouth Bass 3 | Pompano 1 | Red Drum | ||||
|---|---|---|---|---|---|---|
| F3 Feed | F3 Fillet | F3 Feed | F3 Fillet | F3 Feed | F3 Fillet | |
| C14:0 | 1.10 | 1.72↓ | 1.32 | 2.07↓ | 1.12 | 0.87↓ |
| C14:1 | 0.05 | 0.31 | - | 0.58 | - | - |
| C16:0 | 16.74 | 18.13 | 15.85 | 21.42 | 17.3 | 17.6↓ |
| C16:1 | 2.23 | 4.02↓ | 2.96 | 3.40↓ | 2.13 | 2.65↓ |
| C18:0 | 4.42 | 2.81 | 4.51 | 5.65 | 5.90 | 5.72 |
| C18:1n9 | 25.55 | 34.35↑ | 24.28 | 41.32 | 20.80 | 24.60↑ |
| C18:2n6 | 19.47 | 17.80↑ | 17.44 | 19.74 | 20.7 | 17.3↑ |
| C18:3n3 | 13.89 | 2.97↑ | 19.29 | 5.46 | 14.7 | 9.81↑ |
| C20:0 | 0.42 | 0.31 | 0.51 | 0.58 | 0.48 | 0.33 |
| C20:1n9 | 0.55 | 1.62 | 0.36 | 1.13 | - | - |
| C20:2n6 | 0.07 | 0.57 | 0.04 | 0.71 | 0.12 | 0.28 |
| C20:3n3 | - | 0.36 | - | 0.81 | - | - |
| C20:4n6 | 0.86 | 0.89↑ | 0.83 | 0.87 | 1.06 | 0.95 |
| C20:5n3 | 4.13 | 1.52↓ | 3.57 | 0.87↓ | 4.10 | 2.66↓ |
| C22:0 | 0.31 | 0.31 | 0.32 | 0.58 | 0.37 | 0.32 |
| C22:1 | - | - | - | 0.58 | - | - |
| C22:6n3 | 7.51 | 8.09↓ | 6.36 | 6.08↓ | 7.14 | 5.39 |
| C24:0 | 0.56 | 0.31 | 0.39 | 0.58 | - | - |
| C24:1n9 | 0.44 | 0.31 | 0.43 | 0.58 | 0.41 | 1.85 |
| Total ɷ-3 Isomers | 25.53 | 22.53 | 29.22 | 19.58 | 26.0 | 17.9 |
| Total ɷ-6 Isomers | 20.40 | 18.62 | 18.31 | 16.64 | 21.9 | 18.6 |
| EPA/DHA | 0.55 | 0.19 | 0.56 | 0.14 | 0.57 | 0.49 |
| n3:n6 | 0.78 | 0.83 | 0.63 | 0.85 | 1.19 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, K.B.; McLean, E.; Barrows, F.T. In Pursuit of Fish-Free Feeds: A Multi-Species Evaluation. Fishes 2022, 7, 336. https://doi.org/10.3390/fishes7060336
Campbell KB, McLean E, Barrows FT. In Pursuit of Fish-Free Feeds: A Multi-Species Evaluation. Fishes. 2022; 7(6):336. https://doi.org/10.3390/fishes7060336
Chicago/Turabian StyleCampbell, Kelly B., Ewen McLean, and Frederic T. Barrows. 2022. "In Pursuit of Fish-Free Feeds: A Multi-Species Evaluation" Fishes 7, no. 6: 336. https://doi.org/10.3390/fishes7060336
APA StyleCampbell, K. B., McLean, E., & Barrows, F. T. (2022). In Pursuit of Fish-Free Feeds: A Multi-Species Evaluation. Fishes, 7(6), 336. https://doi.org/10.3390/fishes7060336







